Abstract
Background
The zoonotic bacterium Francisella tularensis, the causative agent of tularaemia, can be transmitted to humans via multiple routes, including through contact with infected animals, contaminated water or arthropod vectors. Ticks have not previously been described as transmitting the disease in Sweden. Recently, Ixodid tick species have expanded their latitudinal and altitudinal range in Sweden to areas where the disease is endemic. Tularaemia is a cause of growing concern, spreading to new areas in Sweden and infecting hares and humans.
Objectives
To establish whether ticks could be a potential arthropod vector in the transmission of F. tularensis subsp. holarctica in Sweden.
Methods
Ticks were collected from northern Sweden and screened for F. tularensis. A follow‐up study with ticks collected from F. tularensis‐positive hares was performed. Ticks were analysed using real‐time PCR and a pathological examination was performed on the hares.
Results
F. tularensis subsp. holarctica was identified in ticks from one cat and three F. tularensis‐infected hares. Two hares had skin lesions associated with tick bites with intralesional F. tularensis bacteria.
Conclusions
F. tularensis subsp. holarctica was isolated from ticks collected from the hares and cat, the first such reports in ticks in Sweden. Identification of the bacteria at the tick bite site and the more chronic character of the skin lesions compared to those of inner organs suggest that the ticks infected the hares. The cat showed no clinical signs of disease, suggesting that its tick was indeed the vector. These new findings suggest that ticks play a role in the transmission of F. tularensis to human and animal hosts in Sweden.
Keywords: cat, climate change, Francisella tularensis, hare, northern hemisphere, tularaemia
Tularaemia is a cause of growing concern, spreading to new areas in Sweden and infecting hares and humans. This study reveal that Francisella tularensis subsp. holarctica has been isolated from ticks in Sweden for the first time. The bacterium has been detected in ticks collected from both hare and cat. The potential role of ticks in the transmission of F. tularensis to human and animal hosts deserves further investigations.

1. Introduction
Tularaemia is a vector‐borne zoonosis caused by Francisella tularensis, a bacterium with a broad host range that includes invertebrates, mammals and birds. F. tularensis is categorised into the subspecies F. tularensis subsp. tularensis (Type A) and F. tularensis subsp. holarctica (Type B), which differ with respect to the severity of illness in humans and animals and its geographical distribution (Michelet et al. 2014; Sjöstedt 2007). In the northern hemisphere, Type B is the most common subspecies. It is less virulent than Type A, which is found almost exclusively in North America (Svensson et al. 2005). F. tularensis subsp. holarctica can be transmitted to humans via multiple routes, including through contact with infected animals, contaminated water or arthropod vectors (Hestvik et al. 2015), which are reflected in the different clinical presentations of the disease (Plymoth et al. 2024; Sjöstedt 2007). The disease presents itself in different forms; ulceroglandular, oropharyngeal, oculoglandular, pneumonic and typhoid/septic form. The clinical presentation depends on the subspecies of the bacteria and the route of the infection. In Sweden, the ulceroglandular and pneumonic forms dominate, and the ulceroglandular form is most prevalent with around 65% of the cases. It is characterized by a typical ulcer with enlarged regional lymph nodes. Usually, patients also exhibit nonspecific symptoms such as prolonged fever, severe fatigue and myalgia (Plymoth et al. 2024). Two principal life cycles of tularaemia are described in the literature: a terrestrial life cycle and an aquatic life cycle (Maurin and Gyuranecz 2016). Both life cycles are suspected to be present in association with F. tularensis subsp. holarctica in Europe (Maurin and Gyuranecz 2016). In Sweden and Finland, the aquatic life cycle is the most predominant environment (Maurin and Gyuranecz 2016), and mosquitos are considered to play a major role in transmission of the disease (Dryselius et al. 2019; Sormunen et al. 2021). To the best of our knowledge, outbreaks among humans and hares have not been reported to be linked to tick‐bites in the Nordic countries. However, Sharma et al. (2023) have confirmed that the transmission cycle of F. tularensis varies in different ecosystems. Both Types A and B are associated with diverse life cycles in different animal hosts and arthropod vectors and can also coexist. Type B tularaemia is more frequently associated with the aquatic cycle, although outbreaks of tick‐borne tularaemia involving subspecies holarctica have been reported (Maurin and Gyuranecz 2016). The simultaneous presentation of the terrestrial and aquatic life cycles has been shown in Ukraine where F. tularensis was found in water, mammals and ticks (Hightower et al. 2014).
The clinical presentation varies depending on the route of infection. Among animals, the disease is frequent in hares and rodents. In Sweden, hares have previously been described as primarily showing acute fatal infections (Hestvik et al. 2017); thus, it is considered that they die promptly after infection and therefore are not able to maintain the infection for prolonged periods. In Sweden, the number of infected humans and hares fluctuates greatly from year to year. Figure 1A–D shows the geographical distribution of human cases of F. tularensis subsp. holarctica in Sweden between 2018 and 2021 (Public Health Agency of Sweden 2023). In the same period, 118 necropsied hares were diagnosed with F. tularensis subsp. holarctica via the national general wildlife disease surveillance programme at the Swedish Veterinary Agency (SVA) (Figure 2).
FIGURE 1.
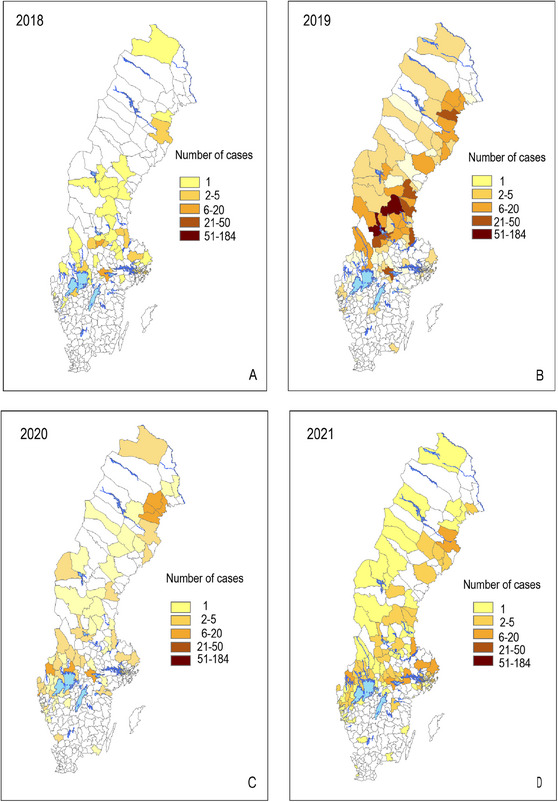
Geographical distribution of human cases of Francisella tularensis subsp. holarctica in Sweden in: (A) 2018, (B) 2019, (C) 2020 and (D) 2021.
FIGURE 2.
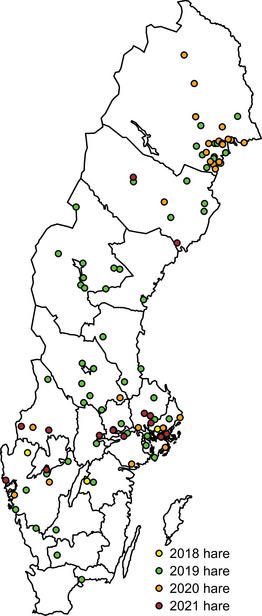
Geographical distribution of reported Francisella tularensis subsp. holarctica‐positive hares (n = 118) between 2018 and 2021 in Sweden.
Ticks transmit several pathogens of medical and veterinary importance (De la Fuente et al. 2017; Heyman et al. 2010). In Europe's temperate and cold regions, the hard tick Ixodes ricinus is the most important vector of tick‐borne diseases in terms of both animal and human health, for example, tick‐borne encephalitis virus, Borrelia spp., Anaplasma phagocytophilum, Rickettsia spp., Neoehrlichia mikurensis and Babesia spp. (Springer et al. 2020). F. tularensis has been detected in different tick species in several European countries (Guryčová et al. 1995; Hestvik et al. 2015), but to the authors’ knowledge, it has not been reported in I. ricinus in Sweden. However, several European studies have shown evidence that the I. ricinus tick species is a biological vector of F. tularensis subsp. holarctica (Hestvik et al. 2015). Detection of F. tularensis DNA in questing I. ricinus ticks has been confirmed in the Czech Republic (Hubalek and Halouzka 1997), France (Reis et al. 2011), Serbia (Milutinović et al. 2008) and Slovakia (Guryčová et al. 1995), in nymph stages of I. ricinus in Switzerland (Wicki et al. 2000) and in I. ricinus ticks collected from vertebrate hosts in Germany (Franke et al. 2010). However, the detected prevalence rate of F. tularensis in I. ricinus ticks is low, at between 0% and 3%, as summarized by Hestvik et al. (2015). It is known that the bacterium can be present and replicate in the ticks’ midgut and salivary glands, and transstadial transmission has also been demonstrated experimentally (Petersen, Mead, and Schriefer 2009; Reif et al. 2011). Furthermore, ticks can transmit the infection during a blood meal (Reif et al. 2011). An adult female tick may feed on their host for up to seven days, which exceeds the normal incubation period of tularaemia. I. ricinus occurs in a wide variety of habitats throughout Europe as far north as 66°N and is known to be the most common tick species in Fennoscandia (Hvidsten et al. 2020; Omazic et al. 2023). Global environmental changes, such as shifts towards a warmer and wetter climate with milder winters and less snow cover, are likely to improve tick survival and affect their distribution and abundance (Jore et al. 2014). Furthermore, scenario models show that climate change this century will probably increase the geographic range of I. ricinus as vegetation communities and mammals associated with high tick densities expand their geographic ranges due to a prolonged vegetation period (Hvidsten et al. 2020; Jaenson and Lindgren 2011). Compared to mosquitoes (that have a shorter lifespan), ixodid ticks like I. ricinus could be important for the long‐term persistence of endemic foci of tularaemia in northern Europe since they have longer developmental cycles and can act as reservoir of tick‐borne pathogens for years (Sonenshine and Roe 2014). All these factors together have contributed to the ticks’ expanded distribution in Fennoscandia and to a shift in their latitudinal and altitudinal distribution (Hvidsten et al. 2020; Jaenson and Lindgren 2011; Jore et al. 2014). This northward expansion of ticks and tick‐borne pathogens in Sweden is a considerable public health concern.
The focus of the present study was to investigate whether ticks might be involved in the transmission of F. tularensis subsp. holarctica in Sweden. Molecular analyses of F. tularensis subsp. holarctica were performed in ticks collected from (i) a citizen science study in Sweden and (ii) F. tularensis subsp. holarctica‐positive hares.
2. Materials and Methods
Ticks (n = 3131) were collected from municipalities in the provinces located north of the river Dalälven in Sweden (above latitude 60°N) in a citizen science study conducted between June and October 2018 (Omazic et al. 2023; Grandi et al. 2024) (manuscript in progress). SVA (Uppsala, Sweden) issued a press release in early June 2018 to inform the public in the study area about the study. Information about the collection was also disseminated with the help of national media channels and social media. Citizens living in or visiting the study area could send in ticks found either on themselves or on domestic and wild animals between June and October in 2018. The tick samples were sent by post to SVA, where all the ticks were registered with information about the municipalities from where they had been submitted and the host species from which they were collected. Individual ticks were stored at −80°C pending molecular analysis of tick‐borne pathogens. Morphological and molecular identification of I. ricinus and Ixodes persulcatus in the collected ticks was performed at SVA and is described in Omazic et al. (2023).
2.1. High‐Throughput Microfluidic Real‐Time PCR
A subset of collected ticks (n = 1087) was screened for microorganisms using chips with 48.48 dynamic arrays in a Bio‐Mark real‐time PCR system (Fluidigm, San Francisco, California, USA), enabling 48 samples to be analysed in 48 PCR reactions simultaneously on each chip (Gondard et al. 2018; Michelet et al. 2014). Primers and probes targeting the two gene regions tul4 and fopA were applied for detection of F. tularensis subsp. holarctica. Microfluidic real‐time PCR results were confirmed by a real‐time PCR assay for the detection of F. tularensis subsp. holarctica, targeting the junction between ISFtu2 and the flanking 3’‐region (Larsson et al. 2009). Samples with a Ct‐value ≤ 38 were considered positive. Samples with a clear curve and a Ct‐value above 38 but below 45 were considered suspected positive.
2.2. Follow‐Up Study
Two to five ticks were collected from the head region of three randomly selected F. tularensis subsp. holarctica‐positive European brown hares (Lepus europaeus) from the national general wildlife disease surveillance programme at SVA in Sweden between 2020 and 2021. Ticks from each animal were pooled and then analysed using real‐time PCR as described above. A hare was defined as F. tularensis subsp. holarctica‐positive when testing positive either using PCR or the immunohistochemistry of inner organs. A necropsy of each hare was followed by a histopathologic examination of the collected tissue samples fixed in 4% formalin. Fixed tissues were prepared and stained with Mayer's haematoxylin and eosin, in accordance with standard procedures. To visualise F. tularensis, immunohistochemistry was performed, following in‐house standard protocols, on prepared tissue samples of inner organs and skin at the site of the tick bite. A monoclonal primary antibody clone BM2369M, formerly clone FB11 (Meridian Life Science Inc., Nordic Biosite AB, Täby, Sweden) directed against F. tularensis sp. lipopolysaccharide antigen, diluted 1:6000, was used.
3. Results
3.1. Real‐Time PCR of Ticks
Of the 1087 ticks (collected from the citizen science sampling), one I. ricinus female tick tested positive for F. tularensis subsp. holarctica (Figure 3). This tick was collected in Mora municipality in 2018 and had been found on a cat. According to the owner, the cat showed no symptoms of disease at the time the tick was found and removed. Two of the three hares diagnosed with tularemia carried ticks that were postitive for F. tularenisis subsp. holaritica (ID A and ID B in Table 1; Figure 3) and one of the hares carried ticks that were suspected positive (ID C in Table 1; Figure 3). The ticks collected from the hares were identified as I. ricinus (engorged) females.
FIGURE 3.
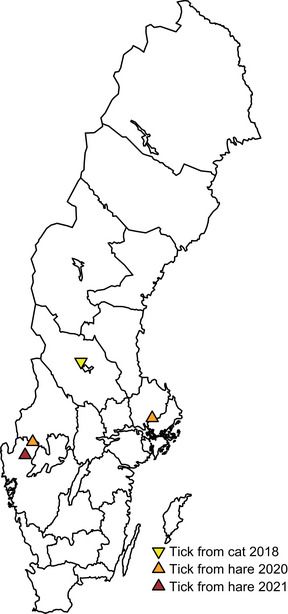
Locations of the tick positive for Francisella tularensis subsp. holarctica found attached to a cat and of the F. tularensis subsp. holarctica‐positive hares that were found to have carried ticks positive for F. tularensis subsp. holarctica.
TABLE 1.
Ticks collected from Francisella tularensis subsp. holarctica‐positive hares via the national general wildlife disease surveillance programme at the Swedish Veterinary Agency in Sweden from 2020 to 2021.
| ID | Year of collection | Age | Origin (municipality) | Francisella tularensis in hares a |
Francisella tularensis in ticks b (Ct value) |
|---|---|---|---|---|---|
| A | 2020 | Adult | Säffle | Positive | 24.0 |
| B | 2020 | Adult | Knivsta | Positive | 28.4 |
| C | 2021 | Adult | Bengtsfors | Positive | 40.1 |
A hare was defined as Francisella tularensis subsp. holarctica‐positive when it either tested positive using PCR or the immunohistochemistry of inner organs.
Conventional real‐time PCR assay: Samples are considered positive below a cut‐off (Ct) value of 38.
3.2. Pathology in Hares
All three hares were infected with F. tularensis subsp. holarctica at the time of death. Two of the hares (ID A and ID B) died of an acute systemic infection caused by F. tularensis, whereas the third hare (ID C) died from blunt trauma.
ID A was an adult female European hare (L. europaeus) found dead, with mild autolytic changes, in October 2020 in Säffle municipality (Table 1; Figure 3). The hare was emaciated and heavily infested with ticks in the head and neck region. A focal skin reaction associated with regional lymphadenopathy was seen at the site of the tick bites. The hare presented with splenomegaly. Microscopically, multifocal severe necrosis was observed in multiple internal organs (bone marrow, liver, spleen, lung and parotid lymph node) with limited inflammatory reaction and a multifocal presence of intralesional bacteria suggestive of F. tularensis. The most pronounced lesions were detected in the lymph node. At the site of the tick bites, the skin presented with focal necrosis surrounded by a granulomatous inflammatory reaction. F. tularensis antigen was identified by immunohistochemistry in inner organs and skin, mainly associated with areas of necrosis (Figure 4).
FIGURE 4.
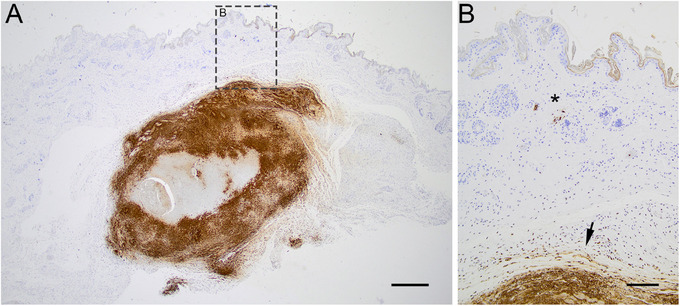
Microphotograph of the immunohistochemistry visualizing the bacteria Francisella tularensis (brown stain) in the skin at the location of a tick bite in a F. tularensis subsp.‐holarctica positive hare (ID A). (A) Section of skin at the site of the tick bite. F. tularensis visualized in lytic lesion in the subcutis, bar = 500 µm. (B) Close‐up of (A), bacteria visualized in the cytoplasm of vascular endothelial cell (asterisk) and lytic lesion in the subcutis (arrow), bar = 200 µm.
ID B was an adult male European hare found dead, with rigor mortis, in May 2020 in Knivsta municipality (Table 1; Figure 3). The hare was in good body condition, with a few ticks attached to its neck. The hare presented with splenomegaly. Microscopically, multifocal severe necrosis was observed in multiple internal organs (bone marrow, liver and spleen) with limited inflammatory reaction and a multifocal presence of intralesional bacteria suggestive of F. tularensis. In the skin of the neck, multiple focal areas of necrosis were observed in association with the tick bites. F. tularensis antigen was identified by immunohistochemistry in the inner organs and skin, mainly associated with areas of necrosis (Figure 4).
ID C was an adult male European hare found dead, with mild autolytic changes, in October 2021 in Bengtsfors municipality (Table 1; Figure 3). The hare was emaciated and had general muscle atrophy. Ticks were present in the head region. The diaphragm was acutely ruptured, with displacement of the liver and intestines to the thoracic cavity. Microscopically, purulent meningitis and acute hepatitis were observed. F. tularensis was identified by immunohistochemistry in the meninges and choroid plexus. Molecular analysis (PCR) of the inner organs (pooled sample of lung, liver, bone marrow and spleen) tested positive for F. tularensis subsp. holarctica.
4. Discussion
To the best of the authors’ knowledge, this is the first time that F. tularensis subsp. holarctica has been reported in I. ricinus ticks in Sweden. Generally, the transmission of F. tularensis subsp. holarctica in Sweden is considered to be through the aquatic life cycle (Maurin and Gyuranecz 2016), with mosquitos a major biological vector of the disease (Dryselius et al. 2019; Sormunen et al. 2021). This is reflected in the predominance of the ulceroglandular form of the disease, as highlighted in a recent study from Sweden (Plymoth et al. 2024). Both ticks and hares are part of the terrestrial life cycle of F. tularensis. However, it is likely that the terrestrial and aquatic life cycles can be present simultaneously in different regions in one country and/or on different occasions (Sharma et al. 2023). Previously, hares have not been considered to be part of the reservoir of F. tularensis subsp. holarctica in Sweden, as the acute fatal infection and death promptly after infection may prevent them being able to maintain the infection for prolonged periods. At present, the pattern for the disease among hares is changing in Sweden, with infected hares now observed in large parts of the country and more frequent reports of cases with chronic lesions (Hestvik et al. 2017). Moreover, antibodies against F. tularensis subsp. holarctica have been reported in European hares and mountain hares (Lepus timidus) found dead with lesions corresponding to tularaemia in Sweden (Averhed et al. 2022), which may suggest a chronic manifestation of the disease since seroconversion generally appears after 3 weeks (Kreizinger et al. 2016).
Altogether, the findings of the present study suggest that I. ricinus ticks can be part of the disease transmission of F. tularensis subsp. holarctica in Sweden. Results from the citizen science study in 2018 revealed one tick infected with F. tularensis attached to a cat. The cat showed no clinical signs of tularaemia, which indicates that the cat did not have septicaemia, and it is therefore likely that the tick acted as a carrying vector of tularaemia, regardless of its host infection status. Although both Types A and B subspecies have been isolated from cats, Type A is responsible for most tularaemia cases in cats (Larson et al. 2014). F. tularensis subsp. holarctica has previously been reported in clinically healthy cats (Kittl et al. 2020). Case reports from both Norway and Sweden describe humans infected by F. tularensis subsp. holarctica after cat bites (Petersson and Athlin 2017; Yaqub, Bjørnholt, and Enger 2004). Transmission of tularaemia from cats to humans caused by both F. tularensis subsp. holarctica and F. tularensis subsp. tularensis has also been described in North America from both sick and healthy cats (Capellan and Fong 1993; Woods et al. 1998). Furthermore, studies performed in Sweden by Eliasson et al. (2002) showed independent associations between humans acquiring tularaemia and owning a cat. Cats are at high risk of exposure to tularaemia predominantly due to their hunting behaviour (Larson et al. 2014), they may eat infected pray, and they are highly exposed to vectors when moving through vegetation associated with high tick densities. Additionally, in 2019, 1 year after the tick was collected from the cat, Sweden experienced its largest outbreak of tularaemia in over 50 years, with Dalarna county (which includes the municipality of Mora) worst affected by the tularaemia outbreak, according to the Public Health Agency of Sweden (Dryselius et al. 2019). Previous studies together with the present findings strongly indicate that cats can increase the risk of human infection both by bringing ticks into the household and through bites and scratches.
Two of the three tularaemic hares (ID A and ID B) carried ticks positive for F. tularensis subsp. holarctica, and one of the hares (ID C) carried a tick that was suspected positive. The hares carrying positive ticks were diagnosed with acute tularaemia as the cause of death. Interestingly, hare ID A and hare ID B had a concurrent skin reaction, with visualized intralesional tularaemia bacteria at the site of the tick bite(s). Hestvik et al. (2017) previously described similar skin lesions at the site of tick bites with several foci of necrosis in the dermis and subcutis in two European hares, and the authors discussed the possibility of transmission of the bacteria via tick bites but did not analyse the ticks. In hare ID A, a subacute inflammatory reaction was observed to be linked to foci of necrosis and bacteria in the skin compared with more acute lesions in the inner organs (Figure 4A,B). This observation, together with most severe necrotic lesions seen in the regional lymph node (parotid), suggests that the tick bite is the portal of entry. The hare exhibited a disease pattern consistent with the most common form of tularaemia, the ulceroglandular form, affecting skin at the portal of entry and regional lymph nodes, potentially progressing into systemic disease (Maurin and Gyuranecz 2016; Sjöstedt 2007). This information indicates that the bacteria were transmitted from the tick to the hare, causing a local skin inflammation with lymphogenous spread to the regional lymph node, and further causing a fatal septicaemia. A similar case has previously been described by Park et al. (2009), where immunohistochemistry was performed on both the skin of a Japanese hare (Lepus brachyurus) and the tick attached to the hare. A bacterial antigen was detected not only in the tick intestines, but in the haemocoel of the tick as well. The hare displayed skin granuloma on its external ear, with regional lymphadenopathy and acute necrosis in the inner organs. The authors concluded that the hare acquired the tularaemia infection from the tick and not vice versa (Park et al. 2009), as in the present study.
The ticks that tested positive for F. tularensis subsp. holarctica in the present study were I. ricinus adult females. F. tularensis subsp. holarctica has been found in I. ricinus in several European countries (Reis et al. 2011), but biological studies demonstrating that the bacteria can be transmitted by ixodid ticks are scarce. In particular, the vectorial capacity of I. ricinus can currently only be hypothesized, and it is not clear if the present findings are due to transmission of the infection from the tick to the animal host or vice versa. In the present study, the pathology of the hare was consistent with the tick bite as the port of bacterial entry, which suggests that F. tularensis subsp. holarctica can be transmitted by I. ricinus ticks. Additionally, the presence of F. tularensis subsp. holarctica was observed in a tick on a clinically healthy cat, which further supports the role of I. ricinus as a potential vector of the bacteria.
In Sweden, human cases (and outbreaks) of tularaemia have mainly been reported in northern Sweden, but cases in the central and southern parts of the country have been increasing in recent years (Figure 1). The same pattern can be seen in infected hares: In the last two decades, bacterial infection of F. tularensis in hares has been shown to be more common in areas south of the previous endemic region in northern Sweden (Andersson et al. 2022). Mosquitos are considered the main vector for tularaemia, and outbreaks are suggested to be dependent on mosquito prevalence in boreal forest regions (Rydén et al. 2012). The species of mosquitos (Aedes cinereus) that are often considered to spread tularaemia prefer forests habitat. This habitat is present in a larger degree (13 times as common) in one of the studied northern locations compared to the collection spot in southern Sweden (Schäfer and Lundström 2001). Forest and wetland habitats are present in whole Sweden, that is, forests cover 68% and wetlands cover 22% of the total area, and forests are predominant in northern Sweden (Sweden 2023). In southern and central Sweden, ticks are more abundant and might play a role as a potential vector of transmission of the tularaemia bacteria. Simultaneously, ongoing climatic and environmental changes are favouring the spread of ticks towards the northern parts of Sweden. A recent study by Omazic et al. (2023) confirmed that I. ricinus and I. persulcatus have enlarged their distributional area in northern Sweden compared with previous reports. The increasing number of ticks might become important in the future transmission of F. tularensis subsp. holarctica in Sweden. The prevalence of tularaemia in ticks in Europe is low, and in several countries, including Sweden, largely unknown. Mosquitos are considered the major vector for F. tularensis subsp. holartica in Sweden with a relatively high presence of the bacterium in mosquito species sampled in endemic areas (Thelaus et al. 2014). In the present study, tularaemia has only been demonstrated in a few ticks indicating a low prevalence. This suggests that tularaemia transmission in animals and humans through tick bites might be quite rare in Sweden. However, our study revealed for the first time in Sweden that we have tularaemia positive ticks and that they can infect hares which indicates that ticks may play a role in the transmission of F. tularensis subsp. holarctica in Sweden. This knowledge is important as an information base for future F. tularensis subsp. holarctica surveillance in the country. Further studies are needed in order to investigate the role of ticks in the transmission of F. tularensis subsp. holarctica between animals and humans, and between infected animals, during an outbreak.
5. Conclusions
F. tularensis subsp. holarctica has been isolated from ticks in Sweden for the first time. The bacterium was detected in ticks collected from three hares and a cat. Immunohistochemical identification of the bacteria at the site of the tick bite, together with associated subacute skin lesions and subsequent acute disseminated disease, suggests that the hares acquired the infection from the tick. The cat showed no clinical signs of disease, which suggests a possible transmission of the bacterium from the tick to the cat. Hares have not previously been considered to be part of the transmission of F. tularensis subsp. holarctica in Sweden, mainly due to the acute nature of the infection and rapid death. However, the disease pattern has recently changed in Sweden, and hares may now also be diagnosed with chronic infections. Furthermore, this is confirmed by the presence of antibodies, which enables them to be an important part of the transmission cycle of the bacteria. Finally, the findings of this study suggest that ticks may play a role in the transmission of F. tularensis to human and animal hosts in Sweden, which merits further investigation.
Author Contributions
Ellinor Spörndly‐Nees: conceptualization, investigation, resources, visualization, writing–original draft. Tomas N. Gustafsson: conceptualization, funding acquisition, writing–review and editing. Anna Omazic: conceptualization, data curation, investigation, funding acquisition, project administration, validation, writing–original draft. Elina Thorsson: investigation, resources, visualization, writing–review and editing. Giulio Grandi: conceptualization, funding acquisition, writing–review and editing.
Ethics Statement
An ethics statement is not applicable as this study was not classified as a human or animal experiment. Ticks were sent in voluntarily by people living in or visiting the study area. Their personal data were only used to locate the geographical area and host information. SVA processed the data in accordance with Data Protection Regulation (EU) 2016/679. The senders were informed that SVA reserves the right to use the submitted ticks and the accompanying metadata for research purposes as part of its activities. The hares were found dead and were sent to the SVA through the national general wildlife disease surveillance programme, therefore no ethical approval was needed.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70094.
Acknowledgements
Sincere thanks to the members of the public in northern Sweden who provided us with ticks during the 2018 tick season; to laboratory staff at the Swedish Veterinary Agency (SVA), Uppsala, Sweden for undertaking the administration and storage of the tick sample collection; to Nadin Idris Nuru for preliminary work (master's thesis) related to the study; to Sara Moutailler and Clémence Galon at ANSES, Laboratoire de Santé Animale, École Nationale Vétérinaire d'Alfort, INRAE, Maisons‐Alfort, France for performing the high‐throughput microfluidic real‐time amplification; to Tomas Jinnerot and Karin Ullman at SVA for performing the confirmation real‐time PCR assay for the detection of Francisella tularensis subsp. holarctica; to Ulrika Larsson Petterson at SVA for laboratory work with sectioning and immunohistochemistry on hare tissue; to Gunnar Andersson of SVA for kindly providing maps of the geographical distribution of reported F. tularensis subsp. holarctica‐positive hares and the location of ticks positive for F. tularensis subsp. holarctica found in the present study; to Marika Hjertqvist of the Public Health Agency of Sweden for kindly providing maps with information about notified human tularaemia cases in Sweden; to Gete Hestvik at SVA who contributed valuable feedback after reviewing the manuscript; and to Gete Hestvik and Henrik Uhlhorn for performing autopsies on two of the hares.
Funding: This work was funded by the County Council of Norrbotten (NLL‐933177) and Umeå University through a regional agreement with the County Council of Norrbotten (ALF Universitets‐ST). The study was partially supported by the Swedish Research Council (2018‐03830) [TICKBIOCON—Ticks from animals and their microbiome: interaction between pathogens and endosymbionts as a potential target for biocontrol tools] and the following foundations: the Jan Skogsborg Foundation [The incidence of ticks and tick‐borne diseases is increasing in Sweden—Increased risk of disease for dogs too?] and the Albert Hjärre Foundation [What infectious agents do ticks carry with them when they spread north in Sweden?] and The Swedish Environmental Protection Agency, The Swedish name of the organization is Naturvårdsverket.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Andersson, E. , Andersson M., Axén C., et al. 2022. Surveillance of Infectious Diseases in Animals and Humans in Sweden. Uppsala: National Veterinary Institute (SVA). https://www.sva.se/media/huzaulyj/tularaemia.pdf. [Google Scholar]
- Averhed, G. , Bröjer C., Höök E., et al. 2022. Wildlife Disease Surveillance in Sweden 2022. Uppsala: National Veterinary Institute, SVA. https://www.sva.se/media/qdym2uml/sva‐rapport‐86‐2023‐sjukdoms%C3%B6vervakning‐av‐vilda‐djur‐i‐sverige‐2022.pdf. [Google Scholar]
- Capellan, J. , and Fong I. W.. 1993. “Tularemia From a Cat Bite: Case Report and Review of Feline‐Associated Tularemia.” Clinical Infectious Diseases 16, no. 4: 472–475. 10.1093/clind/16.4.472. [DOI] [PubMed] [Google Scholar]
- De la Fuente, J. , Antunes S., Bonnet S., et al. 2017. “Tick‐Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick‐Borne Diseases.” Frontiers in Cellular and Infection Microbiology 7: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryselius, R. , Hjertqvist M., Mäkitalo S., et al. 2019. “Large Outbreak of Tularaemia, Central Sweden, July to September 2019.” Eurosurveillance 24, no. 42: 1900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson, H. , Lindbäck J., Nuorti J. P., Arneborn M., Giesecke J., and Tegnell A.. 2002. “The 2000 Tularemia Outbreak: A Case‐Control Study of Risk Factors in Disease‐Endemic and Emergent Areas, Sweden.” Emerging Infectious Diseases 8, no. 9: 956–960. 10.3201/eid0809.020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, J. , Fritzsch J., Tomaso H., Straube E., Dorn W., and Hildebrandt A.. 2010. “Coexistence of Pathogens in Host‐Seeking and Feeding Ticks Within a Single Natural Habitat in Central Germany.” Applied and Environmental Microbiology 76, no. 20: 6829–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondard, M. , Michelet L., Nisavanh A., et al. 2018. “Prevalence of Tick‐Borne Viruses in Ixodes ricinus Assessed by High‐Throughput Real‐Time PCR.” Pathogens and Disease 76, no. 8: fty083. [DOI] [PubMed] [Google Scholar]
- Grandi, G. , Han S., Ullman K., et al. 2024. High‐throughput screening for tick‐borne pathogens in ixodid ticks collected through crowdsourcing in northern Sweden. Unpublished manuscript, Department of Animal Biosciences, Swedish University of Agricultural Sciences. Uppsala, Sweden. [Google Scholar]
- Guryčová, D. , Kocianova E., Výrosteková V., and Řeháček J.. 1995. “Prevalence of Ticks Infected With Francisella tularensis in Natural Foci of Tularemia in Western Slovakia.” European Journal of Epidemiology 11: 469–474. [DOI] [PubMed] [Google Scholar]
- Hestvik, G. , Uhlhorn H., Södersten F., et al. 2017. “Tularaemia in European Brown Hares (Lepus europaeus) and Mountain Hares (Lepus timidus) Characterized by Histopathology and Immunohistochemistry: Organ Lesions and Suggestions of Routes of Infection and Shedding.” Journal of Comparative Pathology 157, no. 2–3: 103–114. [DOI] [PubMed] [Google Scholar]
- Hestvik, G. , Warns‐Petit E., Smith L., et al. 2015. “The Status of Tularemia in Europe in a One‐Health Context: A Review.” Epidemiology & Infection 143, no. 10: 2137–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman, P. , Cochez C., Hofhuis A., et al. 2010. “A Clear and Present Danger: Tick‐Borne Diseases in Europe.” Expert Review of Anti‐Infective Therapy 8, no. 1: 33–50. [DOI] [PubMed] [Google Scholar]
- Hightower, J. , Kracalik I. T., Vydayko N., Goodin D., Glass G., and Blackburn J. K.. 2014. “Historical Distribution and Host‐Vector Diversity of Francisella tularensis, the Causative Agent of Tularemia, in Ukraine.” Parasites & Vectors 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek, Z. , and Halouzka J.. 1997. “Mosquitoes (Diptera: Culicidae), in Contrast to Ticks (Acari: Ixodidae), Do Not Carry Francisella tularensis in a Natural Focus of Tularemia in the Czech Republic.” Journal of Medical Entomology 34, no. 6: 660–663. [DOI] [PubMed] [Google Scholar]
- Hvidsten, D. , Frafjord K., Gray J. S., et al. 2020. “The Distribution Limit of the Common Tick, Ixodes ricinus, and Some Associated Pathogens in North‐Western Europe.” Ticks and Tick‐Borne Diseases 11, no. 4: 101388. [DOI] [PubMed] [Google Scholar]
- Jaenson, T. G. , and Lindgren E.. 2011. “The Range of Ixodes ricinus and the Risk of Contracting Lyme Borreliosis Will Increase Northwards When the Vegetation Period Becomes Longer.” Ticks and Tick‐Borne Diseases 2, no. 1: 44–49. [DOI] [PubMed] [Google Scholar]
- Jore, S. , Vanwambeke S. O., Viljugrein H., et al. 2014. “Climate and Environmental Change Drives Ixodes ricinus Geographical Expansion at the Northern Range Margin.” Parasites & Vectors 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittl, S. , Francey T., Brodard I., et al. 2020. “First European Report of Francisella tularensis Subsp. holarctica Isolation From a Domestic Cat.” Veterinary Research 51, no. 1: 109. 10.1186/s13567-020-00834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreizinger, Z. , Erdélyi K., Felde O., et al. 2016. “Comparison of Virulence of Francisella tularensis Ssp. holarctica Genotypes B. 12 and B. FTNF002‐00.” BMC Veterinary Research 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, M. A. , Fey P. D., Hinrichs S. H., and Iwen P. C.. 2014. “ Francisella tularensis Bacteria Associated With Feline Tularemia in the United States.” Emerging Infectious Diseases 20, no. 12: 2068–2071. 10.3201/eid2012.131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, P. , Forsman M., Byström M., et al. 2009. Validering av multiplex realtids PCR för harmonisering av molekylär detektion av riskklass 3 bakterier inom FBD . Forum för beredskapsdiagnostik. https://rib.msb.se/filer/pdf/27318.pdf.
- Maurin, M. , and Gyuranecz M.. 2016. “Tularaemia: Clinical Aspects in Europe.” Lancet Infectious Diseases 16, no. 1: 113–124. 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- Michelet, L. , Delannoy S., Devillers E., et al. 2014. “High‐Throughput Screening of Tick‐Borne Pathogens in Europe.” Frontiers in Cellular and Infection Microbiology 4: 103. 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović, M. , Masuzawa T., Tomanović S., Radulović Ž., Fukui T., and Okamoto Y.. 2008. “ Borrelia burgdorferi Sensu Lato, Anaplasma phagocytophilum, Francisella tularensis and Their Co‐Infections in Host‐Seeking Ixodes ricinus Ticks Collected in Serbia.” Experimental and Applied Acarology 45: 171–183. [DOI] [PubMed] [Google Scholar]
- Omazic, A. , Han S., Albihn A., et al. 2023. “Ixodid Tick Species Found in Northern Sweden‐Data From a Frontier Area.” Ticks and Tick‐Borne Diseases 14, no. 6: 102244. [DOI] [PubMed] [Google Scholar]
- Park, C.‐H. , Nakanishi A., Hatai H., et al. 2009. “Pathological and Microbiological Studies of Japanese Hare (Lepus brachyurus angustidens) Naturally Infected With Francisella tularensis Subsp. holarctica .” Journal of Veterinary Medical Science 71, no. 12: 1629–1635. [DOI] [PubMed] [Google Scholar]
- Petersen, J. M. , Mead P. S., and Schriefer M. E.. 2009. “ Francisella tularensis: An Arthropod‐Borne Pathogen.” Veterinary Research 40, no. 2: 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson, E. , and Athlin S.. 2017. “Cat‐Bite‐Induced Francisella tularensis Infection With a False‐Positive Serological Reaction for Bartonella quintana .” JMM Case Reports 4, no. 2: e005071. 10.1099/jmmcr.0.005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymoth, M. , Lundqvist R., Nystedt A., Sjöstedt A., and Gustafsson T. N.. 2024. “Targeting Tularemia: Clinical, Laboratory, and Treatment Outcomes From an 11‐Year Retrospective Observational Cohort in Northern Sweden.” Clinical Infectious Diseases 78, no. 5: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif, K. E. , Palmer G. H., Ueti M. W., et al. 2011. “ Dermacentor andersoni Transmission of Francisella tularensis Subsp. novicida Reflects Bacterial Colonization, Dissemination, and Replication Coordinated With Tick Feeding.” Infection and Immunity 79, no. 12: 4941–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, C. , Cote M., Paul R. E., and Bonnet S.. 2011. “Questing Ticks in Suburban Forest Are Infected by at Least Six Tick‐Borne Pathogens.” Vector‐Borne and Zoonotic Diseases 11, no. 7: 907–916. [DOI] [PubMed] [Google Scholar]
- Rydén, P. , Björk R., Schäfer M. L., et al. 2012. “Outbreaks of Tularemia in a Boreal Forest Region Depends on Mosquito Prevalence.” Journal of Infectious Diseases 205, no. 2: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, M. , and Lundström J. O.. 2001. “Comparison of Mosquito (Diptera: Culicidae) Fauna Characteristics of Forested Wetlands in Sweden.” Annals of the Entomological Society of America 94, no. 4: 576–582. [Google Scholar]
- Sharma, R. , Patil R. D., Singh B., et al. 2023. “Tularemia—A Re‐Emerging Disease With Growing Concern.” Veterinary Quarterly 43, no. 1: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt, A. 2007. “Tularemia: History, Epidemiology, Pathogen Physiology, and Clinical Manifestations.” Annals of the New York Academy of Sciences 1105, no. 1: 1–29. [DOI] [PubMed] [Google Scholar]
- Sonenshine, D. E. , and Roe R. M.. 2014. Biology of Ticks. Oxford: Oxford University Press. [Google Scholar]
- Sormunen, J. , Pakanen V., Elo R., Mäkelä S., and Hytönen J.. 2021. “Absence of Francisella tularensis in Finnish Ixodes ricinus and Ixodes persulcatus Ticks.” Ticks and Tick‐Borne Diseases 12, no. 6: 101809. [DOI] [PubMed] [Google Scholar]
- Springer, A. , Glass A., Topp A.‐K., and Strube C.. 2020. “Zoonotic Tick‐Borne Pathogens in Temperate and Cold Regions of Europe—A Review on the Prevalence in Domestic Animals.” Frontiers in Veterinary Science 7: 604910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson, K. , Larsson P., Johansson D., Byström M., Forsman M., and Johansson A.. 2005. “Evolution of Subspecies of Francisella tularensis .” Journal of Bacteriology 187, no. 11: 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistikmyndigheten . 2023. Marken i Sverige . Accessed September 5, 2024. https://www.scb.se/hitta-statistik/sverige-i-siffror/miljo/marken-i-sverige/.
- Thelaus, J. , Andersson A., Broman T., et al. 2014. “ Francisella tularensis Subspecies holarctica Occurs in Swedish Mosquitoes, Persists Through the Developmental Stages of Laboratory‐Infected Mosquitoes and is Transmissible During Blood Feeding.” Microbial Ecology 67: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki, R. , Sauter P., Mettler C., et al. 2000. “Swiss Army Survey in Switzerland to Determine the Prevalence of Francisella tularensis, Members of the Ehrlichia Phagocytophila Genogroup, Borrelia Burgdorferi Sensu Lato, and Tick‐Borne Encephalitis Virus in Ticks.” European Journal of Clinical Microbiology and Infectious Diseases 19: 427–432. [DOI] [PubMed] [Google Scholar]
- Woods, J. P. , Crystal M. A., Morton R. J., and Panciera R. J.. 1998. “Tularemia in Two Cats.” Journal of the American Veterinary Medical Association 212, no. 1: 81–83. [PubMed] [Google Scholar]
- Yaqub, S. , Bjørnholt J. V., and Enger A. E.. 2004. “Tularemi Etter Kattebitt.” Tidskrift Norsk Lageforening 124, no. 24: 3197–3198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


