Abstract
Human opsonins directed against specific meningococcal outer membrane structures in sera obtained during meningococcal disease were quantified with a recently developed antigen-specific, opsonin-dependent phagocytosis and oxidative burst assay. Outer membrane vesicles (OMVs) and PorA (class 1) and PorB (class 3) proteins purified from mutants of the same strain (44/76; B:15:P1.7.16) were adsorbed to fluorescent beads, opsonized with acute- and convalescent-phase sera from 40 patients with meningococcal disease, and exposed to human leukocytes. Flow cytometric quantitation of the resulting leukocyte phagocytosis products (PPs) demonstrated that disease-induced serum opsonins recognized meningococcal OMV components and both porins. The PPPorA and PPPorB values induced by convalescent-phase sera correlated positively with the PPOMV values. However, the PPPorB values were higher than the PPPorA values in convalescent-phase sera (medians [ranges] of 754 [17 to 1,057] and 107 [4 to 458], respectively) (P < 0.0001) and correlated positively with higher levels of immunoglobulin G against PorB than against PorA as evaluated by enzyme-linked immunosorbent assay. Extensive individual variations in the anti-OMV and antiporin serum opsonic activities between patients infected by serotypes and serosubtypes homologous and heterologous to the target antigens were observed. Simultaneously measured oxidative burst activity correlated with the opsonophagocytosis, an indication that both of these important steps in the in vitro phagocytic elimination of meningococci are initiated by opsonins directed against OMV components, including PorA and PorB. In conclusion, human patient opsonins against meningococcal OMV components and in particular PorB epitopes were identified by this new method, which might facilitate selection of opsonin-inducing meningococcal antigens for inclusion in future vaccines.
Whereas the majority of studies concerning the immune response following meningococcal disease and vaccination have focused on the role of human serum bactericidal activity against meningococci, some reports strongly indicate that phagocytic killing of meningococci is an important host defense mechanism, particularly against serogroup B meningococci (6, 32, 36, 38). Effective uptake and ingestion of meningococci are dependent on the deposition of opsonins (complement and antibodies) on epitopes exposed on the bacterial surfaces (8, 35, 43). Increasing human serum opsonic activity has been demonstrated during the course of meningococcal disease and after vaccination with a complex serogroup B outer membrane vesicle (OMV) preparation, using whole meningococci as target antigens in functional assays (1, 13, 14, 18, 24, 27, 39). Furthermore, patient serum opsonic activity has been shown to correlate positively with levels of immunoglobulin G (IgG) antibody to meningococcal PorA and PorB proteins (17). However, functional assays for direct identification of bacterial antigens that are recognized by serum opsonins have not been available.
The aim of the present study was to evaluate whether functional opsonins produced in response to meningococcal disease are directed against meningococcal outer membrane components and, if so, to determine whether patient opsonins recognize PorA and PorB proteins. We employed a recently developed functional assay that quantifies antigen-specific serum opsonic activity, as reflected by induction of phagocytosis and oxidative burst mechanisms in human leukocytes (25, 26). Fluorescent polystyrene beads coated with OMVs and purified porins from mutants of the same meningococcal strain (44/76; B:15:P1.7,16) were opsonized with acute- and convalescent-phase sera from 40 surviving patients infected by a variety of meningococcal strains. The leukocyte phagocytosis and oxidative burst induced by opsonized antigen-coated beads were quantified by flow cytometry (FCM), visualized by confocal laser scanning microscopy (CLSM), and correlated to serum anti-OMV and antiporin Ig levels as measured by enzyme-linked immunosorbent assays (ELISAs).
MATERIALS AND METHODS
Patients.
Serum samples were obtained from 40 survivors (22 females and 18 males; age, 14 to 58 years; median age, 18) of meningococcal disease on admission to Haukeland Hospital, University of Bergen, Bergen, Norway, between admission and 6 weeks later (intermediate samples, available between days 3 and 24; median day, 13; n = 38) (Table 1) and at 6 weeks after admission. The patients were allocated into five groups (groups I to V) according to the serogroups, serotypes, and serosubtypes of meningococcal strains isolated from cerebrospinal fluid or blood (Table 1). Four clinical disease categories were employed (Table 1) (18). Six patients had been injected twice with an OMV vaccine produced from strain 44/76 in a large-scale vaccine trial (5, 10), and patient 4 was immunized with the Meningococcal Polysaccharide A+C Vaccine (Pasteur-Merieux Serums and Vaccins, Lyon, France), 1 to 4 years prior to disease (Table 1).
TABLE 1.
Clinical characteristics of patients with meningococcal disease (n = 40)
| Group and patient no.a | Meningococcal strain | Disease categoryb | Time of preadmission symptoms (h) | Intermediate sampling day |
|---|---|---|---|---|
| I (n = 8) | ||||
| 1 | B:15:P1.7,16 | 2 | 12 | 15 |
| 2 | B:15:P1.7,16 | 1 | 12 | 10 |
| 3 | B:15:P1.7,16 | 1 | 18 | 16 |
| 4 (V−A+C [1]) | B:15:P1.7,16 | 3 | 20 | 12 |
| 5 | B:15:P1.7,16 | 4 | 11 | 16 |
| 6 | B:15:P1.7,16 | 1 | 25 | 10 |
| 7 | B:15:P1.7,16 | 4 | 24 | 9 |
| 8 | B:15:P1.7,16 | 2 | 10 | 17 |
| II (n = 7) | ||||
| 9 | C:15:P1.7,16 | 4 | 20 | |
| 10 | C:15:P1.7,16 | 3 | 20 | 24 |
| 11 | C:15:P1.7,16 | 4 | 48 | 17 |
| 12 | C:15:P1.7,16 | 1 | 16 | 8 |
| 13 | C:15:P1.7,16 | 1 | 24 | 5 |
| 14 | C:15:P1.7,16 | 2 | 16 | 7 |
| 15 | C:15:P1.7,16 | 3 | 30 | 4 |
| III (n = 10) | ||||
| 16 | B:15:P1.2 | 3 | 23 | 13 |
| 17 | B:15:P1.2,5 | 4 | 36 | 5 |
| 18 | B:15:P1.12,13 | 2 | 12 | 24 |
| 19 | B:15:P1.12,13 | 2 | 11 | 15 |
| 20 | B:15:P1.12 | 4 | 24 | 18 |
| 21 | B:15:P1.12 | 4 | 18 | 5 |
| 22 (V−B [3]) | B:15:P1.12 | 1 | 14 | 13 |
| 23 (V−B [3]) | B:15:P1.12 | 3 | 18 | 8 |
| 24 (V−B [3]) | B:15:P1.12 | 1 | 14 | 10 |
| 25 (V−B [1]) | B:15:P1.12 | 1 | 26 | 3 |
| IV (n = 6) | ||||
| 26 | C:2a:P1.2 | 4 | 20 | 6 |
| 27 | C:2a:P1.2 | 2 | 11 | 13 |
| 28 | C:2a:P1.2 | 4 | 11 | 14 |
| 29 | C:2a:P1.2 | 2 | 28 | 4 |
| 30 (V−B [4]) | C:2a:P1.2 | 2 | 20 | 15 |
| 31 (V−B [4]) | C:2a:P1.2 | 2 | 10 | 17 |
| V (n = 9) | ||||
| 32 | B:NT:P1.12 | 4 | 18 | 13 |
| 33 | B:NT:P1.16 | 1 | 24 | 13 |
| 34 | B:NT:P1.3 | 4 | 20 | 8 |
| 35 | B:NT:NT | 3 | 30 | 14 |
| 36 | B:4:NT | 4 | 10 | 16 |
| 37 | B:4:P1.12 | 2 | 25 | 9 |
| 38 | B:8:P1.15 | 1 | 18 | |
| 39 | B:19:P1.15 | 4 | 16 | 8 |
| 40 | Microscopyc | 3 | 11 | 14 |
V−A+C, immunized with meningococcal polysaccharide A+C vaccine; V−B, immunized with meningococcal serogroup B OMV vaccine. Numbers in brackets indicate years since vaccination.
1, meningitis; 2, septicemia with shock; 3, meningitis and septicemia with shock; 4, septicemia without shock and with or without meningitis.
Gram-negative diplococci in cerebrospinal fluid.
Sera from five persons without a history of meningococcal disease and reaction mixtures without serum were included as controls. Control admission and convalescent-phase sera were obtained from a patient with pneumococcal meningitis and a patient with varicella-zoster meningoencephalitis. All sera had normal complement activity (as determined by 50% hemolytic complement units) and were stored at −70°C until used.
Fluorochromes and buffers.
Polystyrene microspheres with incorporated red fluorescent dye (Fluoresbrite Plain Microspheres PCRed; Polysciences Inc., Warrington, Pa.) and with a size similar to that of meningococci (1 μm in diameter) were used for the opsonophagocytosis studies. The oxidative burst substrate dihydrorhodamine 123 (DHR 123) (Molecular Probes, Eugene, Oreg.) was used to measure leukocyte oxidative burst activity. DHR 123 is converted intracellularly to green fluorescent rhodamine 123 (R-123) by reactive oxygen intermediates (37). Dulbecco’s phosphate-buffered saline (DPBS) (pH 7.4) (25) was supplemented with 5 × 10−3 M glucose and 5 mg of bovine serum albumin (BSA) (Boehringer GmbH, Mannheim, Germany) per ml (DPBS-GA), as well as with 9 × 10−4 M CaCl2 · 2H2O and 5 × 10−4 M MgSO4 · H2O (DPBS-GACM).
Antigens.
OMVs from Neisseria meningitidis 44/76 (B:15:P1.7,16) were prepared as for vaccine production (10). Meningococcal PorA (class 1) and PorB (class 3) outer membrane proteins were purified by detergent extraction and column chromatography from mutant variants of strain 44/76 (44/76Δ3Δ4 and 44/76Δ1Δ4, lacking PorB and PorA, respectively, as well as RmpM [class 4 protein]) (15, 16). The mutant strains were employed to minimize contamination with nonporin proteins, and gel electrophoresis demonstrated negligible lipopolysaccharide contamination (data not shown). PorA and PorB proteosomes were prepared as described previously (47).
Antigen adsorption to beads.
Meningococcal OMV and PorA and PorB proteosomes were adsorbed to fluorescent polystyrene beads as described previously (25). In brief, after two washes in borate buffer (0.1 M boric acid, pH 8.5), 500 μl of beads (4.55 × 1010 beads/ml) were incubated with an excess of each antigen (600 μg) with end-over-end rotation at room temperature (20°C) overnight (20 h). The degree of antigen adsorption to the bead surfaces was determined by using the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.) (25). Remaining active sites on the bead surfaces were blocked with 2% BSA in 0.1 M boric acid (pH 8.5) to avoid adsorption of nonspecific serum proteins during the subsequent incubation with human serum. The antigen-coated beads were suspended in storage buffer (25) and stored at 4°C until used.
Leukocytes.
Leukocytes were separated from freshly drawn, heparinized venous blood from one healthy nonsmoker by an erythrocyte-lysing method, as described previously (25). The leukocyte suspension was adjusted to 1.25 × 107 nonlymphocytes (monocytes and polymorphonuclear leukocytes, i.e., the potentially phagocytosing cells) per ml in DPBS-GA.
FCM analysis of phagocytosis and oxidative burst assay.
Polystyrene beads (20 μl; 2.5 × 108 beads/ml) coated with meningococcal OMVs or PorA or PorB proteosomes and control beads coated with BSA were opsonized with patient serum (5 μl; 5% of final incubation volume) in 96-well microtiter plates (96 WELL Polypropylene Cluster, U-bottom with polystyrene lid; Costar Corporation, Cambridge, Mass.) for 7.5 min with shaking at 37°C in the presence of 20 μl of DHR/123 (10 μg/ml) and 35 μl of DPBS-GACM per well. Donor leukocytes (20 μl; 1.25 × 107 nonlymphocytes/ml, which provides a bead/nonlymphocyte ratio of 20:1) were added to each well, and the incubation was continued for 7.5 min. Phagocytosis was terminated by addition of 200 μl of ice-cold PBS with 0.02% EDTA to each well. The samples were kept briefly on ice and diluted 1/5 prior to a 30-s FCM analysis (Epics XL-MCL; Coulter Corporation, Harpenden, England) (25).
An argon laser operating at 488 nm produced excitation in the fluorochromes. The green R-123 fluorescence was collected between 505 and 545 nm (FL1 channel), and the red bead fluorescence was collected between 560 and 590 nm (FL2 channel). Electronic color compensations eliminated spectral overlaps between the fluorochromes. The FCM coincidence rate was repeatably 1 to 2% (25, 26). The FCM was calibrated daily with fluorescent beads (DNA-Check, EPICS Alignment Fluorospheres; Coulter Corporation, Hialeah, Fla.).
FCM parameters.
Lymphocytes and nonlymphocytes were discriminated and quantified by combined measurements of forward-angle light scatter and side-angle light scatter, and nonlymphocytes were gated to separate forward-angle light scatter versus log fluorescence cytograms and analyzed for associated R-123 (FL1) and bead (FL2) fluorescence. The percentage of phagocytosing nonlymphocytes was defined as the percentage of nonlymphocytes with associated bead fluorescence. The mean number of beads per phagocytosing cell was calculated by dividing the mean bead fluorescence associated with gated nonlymphocytes by the fluorescence of single beads (25, 26). The phagocytosis product (PP) was defined as the percentage of phagocytosing nonlymphocytes multiplied by the mean number of beads per phagocytosing cell, and the PP values were denoted with the antigen employed (i.e., PPOMV when OMVs were adsorbed to beads). Oxidative burst activity was reflected by the mean nonlymphocyte R-123 fluorescence.
ELISA.
Serum IgG antibodies to OMVs were quantified as described previously (29, 30). In brief, twofold dilutions of sera were applied to OMV-coated microtiter plates (4 μg of protein/ml in 0.1 M Tris-HCl [pH 8.6] with 100 μl/well at 4°C for at least 24 h) and incubated for 2 h at 37°C. Immunosorbance-purified biotinylated sheep anti-human IgG antibody mixed with streptavidin and alkaline phosphatase-biotin conjugate at optimal dilutions were added, and 2-p-nitrophenyl phosphate (Sigma Chemical Co., St. Louis, Mo.) was used as substrate. After a 30-min incubation at room temperature, the absorbances were read at 405 nm (Emax microplate reader; Molecular Devices, Sunnyvale, Calif.). The levels of anti-OMV IgG antibodies were determined from the standard curve of a standard serum by using SOFTmax four-parameter analysis (Molecular Devices).
The amounts of serum IgA, IgM, and IgG main and subclass antibody-recognizing epitopes on purified PorA and PorB were determined as described previously (15, 16, 47), with secondary antibodies and/or conjugates from Sigma Chemical Co. IgG was quantified in micrograms per milliliter against a separate ELISA with wells coated with Fab-specific anti-human IgG and dilutions of an IgG standard. IgG subclass titrations were performed with mouse anti-human IgG1, IgG2, IgG3, and IgG4 as secondary antibodies and alkaline phosphatase-conjugated goat anti-mouse IgG as the conjugate. Twofold serial dilutions of sera started at 1:50 for IgG subclass analyses and at 1:100 for IgA and IgM analyses, and the levels were calculated as the reciprocal serum dilutions that gave an A405 of 1.0 after 1 h of incubation.
CLSM.
Immediately after incubation of opsonized antigen-coated beads and leukocytes as described for FCM, CLSM (MRC 1000; Bio-Rad, Hemel Hempstead, United Kingdom) was performed to visualize phagocytosis and R-123 formation (25, 26).
Statistical methods.
The FCM results are presented as means of triplicate measurements. Wilcoxon’s signed rank test was used to determine differences between data. A P value of <0.05 was considered statistically significant. Correlations were evaluated by Spearman’s rank correlation coefficient. SPSS 7.5.1 for Windows and SigmaStat software were used.
RESULTS
Phagocytosis.
The amount of patient opsonins that recognized the complex OMV antigen and the purified PorA and PorB proteins increased during meningococcal disease, as reflected by enhanced phagocytosis of opsonized antigen-coated beads by human leukocytes (Fig. 1). Serum opsonic responses were demonstrated by increases in both the percentage of phagocytosing nonlymphocytes and the mean number of beads per cell, as shown in the summary of FCM parameters obtained with the porin-coated beads opsonized with sera from patients in groups I to V (Table 2). However, since both of these parameters are required to describe the total opsonophagocytosis, the product of these two parameters (the PP) was used to reflect the opsonic activities.
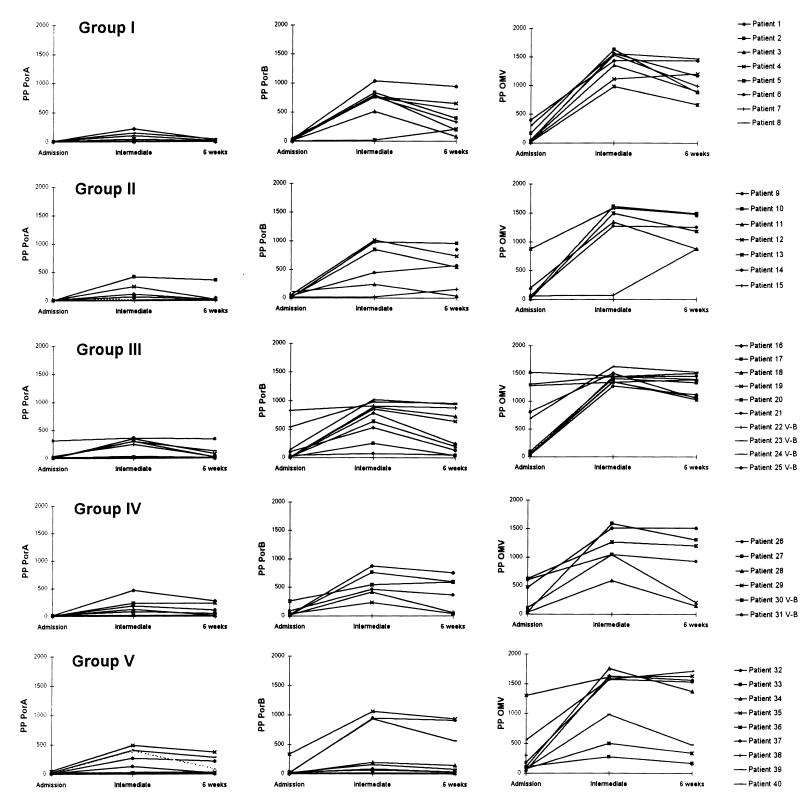
FIG. 1.
Serum opsonic activities of individual patients in groups I to V (Table 1) against meningococcal PorA and PorB and OMVs from 44/76 (B:15:P1.7,16) strains during meningococcal disease, as reflected by leukocyte PPs (the percentage of phagocytosing nonlymphocytes multiplied by the number of opsonized, antigen-coated beads per cell). The antigen-specific opsonic activities were measured in sera obtained on admission to hospital (admission), between admission to hospital and 6 weeks later (intermediate), and 6 weeks after admission (6 weeks).
TABLE 2.
Phagocytosis and oxidative burst activities mediated by anti-PorA and anti-PorB serum opsonins in meningococcal disease patients
| Parameter | Patient group | Median (range) for beads coated with:
|
|||||
|---|---|---|---|---|---|---|---|
| PorA proteosomes
|
PorB proteosomes
|
||||||
| Admissiona | Intermediateb | 6 weeksc | Admission | Intermediate | 6 weeks | ||
| % Phagocytosing nonlymphocytes | I | 8 (3–11) | 23 (4–81) | 10 (5–32) | 12 (4–40) | 95 (16–96) | 88 (44–95) |
| II | 11 (4–30) | 44 (7–92) | 15 (8–91) | 22 (5–51) | 91 (12–96) | 94 (23–96) | |
| III | 10 (6–87) | 58 (13–91) | 15 (6–88) | 19 (9–96) | 95 (44–97) | 88 (27–97) | |
| IV | 7 (5–13) | 70 (15–94) | 46 (9–93) | 18 (7–81) | 92 (77–95) | 91 (27–94) | |
| V | 10 (7–34) | 73 (11–95) | 24 (10–92) | 15 (7–89) | 56 (14–97) | 44 (10–97) | |
| Beads/phagocytosing nonlymphocyte (mean no.) | I | 1.3 (1.2–1.4) | 1.4 (1.3–2.7) | 1.3 (1.2–1.5) | 1.4 (1.3–1.5) | 8.0 (1.3–10.8) | 4.1 (1.7–9.8) |
| II | 1.3 (1.3–1.4) | 1.7 (1.4–4.5) | 1.3 (1.2–3.9) | 1.5 (1.4–2.0) | 7.0 (1.4–2.0) | 6.1 (1.5–9.9) | |
| III | 1.4 (1.3–3.6) | 2.1 (1.3–4.0) | 1.3 (1.3–3.9) | 1.5 (1.3–8.6) | 8.6 (1.6–10.5) | 4.9 (1.4–9.9) | |
| IV | 1.3 (1.3–1.5) | 2.2 (1.7–4.9) | 1.6 (1.3–2.9) | 1.5 (1.4–3.2) | 5.5 (3.0–9.3) | 5.2 (1.5–8.0) | |
| V | 1.3 (1.2–1.4) | 2.7 (1.2–5.1) | 1.4 (1.2–4.0) | 1.4 (1.3–3.7) | 4.5 (1.6–10.9) | 1.5 (1.3–9.6) | |
| PPd | I | 10 (4–14) | 33 (5–219) | 13 (6–45) | 17 (5–60) | 759 (21–1,026) | 356 (75–931) |
| II | 15 (5–42) | 88 (11–414) | 20 (10–355) | 31 (7–102) | 642 (17–1,008) | 567 (35–950) | |
| III | 12 (8–313) | 144 (17–360) | 20 (8–343) | 26 (12–826) | 817 (70–1,019) | 440 (38–950) | |
| IV | 9 (7–12) | 152 (20–461) | 76 (12–270) | 26 (10–259) | 503 (231–874) | 473 (41–752) | |
| V | 12 (10–48) | 199 (15–485) | 34 (13–368) | 21 (9–329) | 169 (20–1,057) | 79 (13–931) | |
| Oxidative burste | I | 0.2 (0.2–0.2) | 0.3 (0.2–3.9) | 0.2 (0.2–0.4) | 0.2 (0.2–0.3) | 13.8 (0.2–18.6) | 4.9 (0.4–17.1) |
| II | 0.2 (0.2–2.2) | 0.2 (0.2–9.2) | 0.2 (0.2–7.7) | 0.3 (0.2–2.8) | 7.7 (0.2–18.3) | 10.3 (0.2–18.3) | |
| III | 0.2 (0.2–6.6) | 2.2 (0.2–7.2) | 0.2 (0.2–6.3) | 0.3 (0.2–11.0) | 13.4 (0.4–18.8) | 6.6 (0.3–19.1) | |
| IV | 0.2 (0.1–0.2) | 1.4 (0.2–6.2) | 0.8 (0.2–4.8) | 0.2 (0.2–1.8) | 6.7 (2.0–12.1) | 6.5 (0.3–11.0) | |
| V | 0.2 (0.2–0.2) | 1.8 (0.2–8.9) | 0.3 (0.2–7.9) | 0.2 (0.2–2.4) | 5.9 (0.2–20.3) | 0.3 (0.2–18.6) | |
Sera obtained on admission to hospital.
Sera obtained on day 4 to 24 (median, day 13) (Table 1).
Sera obtained 6 weeks after admission to hospital.
Percent phagocytosing nonlymphocytes multiplied by mean number of beads per phagocytosing cell.
Mean nonlymphocyte R-123 fluorescence.
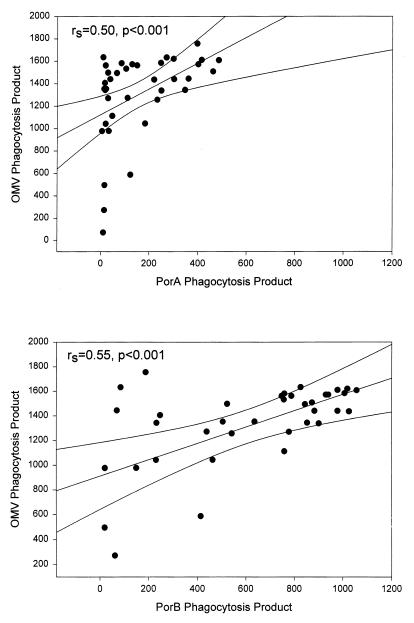
Increases in anti-OMV opsonic activity were detected in 38 (95%) of the patients during the course of disease, and the highest PPOMV values were registered with intermediate sera (median PPOMV, 1,438; range, 72 to 1756; n = 38) (Fig. 1; Table 2). Increased PPPorB values were observed in 37 intermediate sera (97%) with a median PPPorB of 754 (range, 17 to 1,057). In contrast, only 22 intermediate sera (58%) induced increased PPPorA values, with a lower median PPPorA of 107 (range, 4 to 485). The difference in the magnitudes of the PPPorA and the PPPorB values obtained with intermediate sera was statistically significant (P < 0.0001). However, both the PPPorA and the PPPorB values were positively correlated with the PPOMV values (Fig. 2).
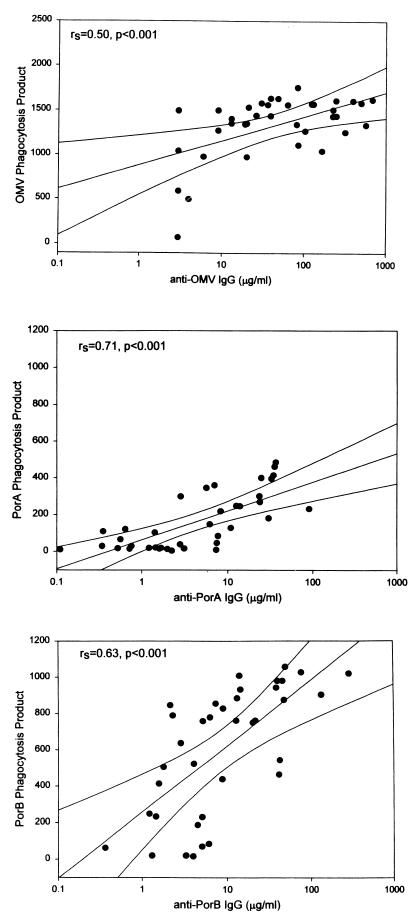
FIG. 2.
Patient serum opsonic activities against meningococcal OMVs, PorA, and PorB, using antigen-coated beads as targets for functional patient opsonins before exposure to human leukocytes and FCM to reflect the resulting leukocyte PPs (the percentage of phagocytosing nonlymphocytes multiplied by the number of beads per cell). Spearman rank correlation coefficients are given for the plots of PPPorA versus PPOMV (upper panel) and PPPorB versus PPOMV (lower panel) induced by patient sera obtained between admission to hospital with meningococcal disease and 6 weeks later (intermediate sera; n = 38). Simple regression lines with 99% confidence intervals are shown.
The patients were grouped according to the main serogroups, serotypes, and serosubtypes of the disease-causing strains (Table 1). Patients in groups I and II were infected by meningococcal strains with the serotype (serotype 15) and serosubtype (P1.7,16) homologous to those of the strain from which the target antigens were extracted, whereas strains with heterologous serotypes and/or serosubtypes were isolated from patients in groups III to V. No statistical differences in the serum opsonic activity were found between the groups; however, considerable interindividual variations in both the anti-OMV and the antiporin opsonic responses were demonstrated in patient sera within each group (Fig. 1; Table 2).
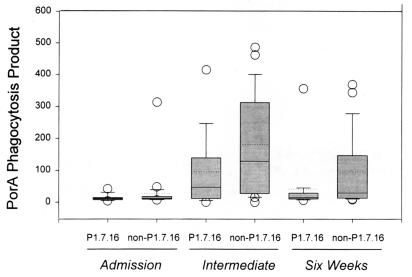
Convalescent-phase sera from patients infected by non-serosubtype P1.7,16 strains induced 2.8-fold (intermediate sera)- and 2-fold (6-week sera)-higher median PPPorA values than sera from patients with P1.7,16 strains (Fig. 3). However, no statistically significant differences were found between the PPPorA values induced by serosubtype P1.7,16 versus non-P1.7,16 convalescent-phase sera (P = 0.27 and P = 0.21 for intermediate and 6-week sera, respectively).
FIG. 3.
Box plots of leukocyte PPPorA values induced by sera from meningococcal disease patients infected with a meningococcal serosubtype homologous or heterologous to that of the strain from which the PorA target antigen was extracted (P1.7,16). The upper and lower levels of the boxes represent the 75th and 25th percentiles, and the error bars represent the 90th and 10th percentiles. The open circles denote outliers. The solid lines represent the median values, and the dotted lines represent the means.
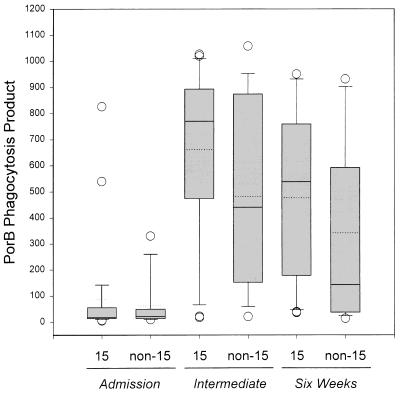
Convalescent-phase sera from patients infected by serotype 15 meningococci induced 1.8-fold (intermediate sera)- and 3.8-fold (6-week sera)-higher median PPPorB values than sera from patients infected by other strains (Fig. 4). However, no statistically significant differences between the PPPorB values induced by serotype 15 versus non-serotype 15 convalescent-phase sera were found (P = 0.20 and P = 0.13 for intermediate and 6-week sera, respectively).
FIG. 4.
Box plots of leukocyte PPPorB values induced by sera from meningococcal disease patients infected with a meningococcal serotype homologous or heterologous to that of the strain from which the PorB target antigen was extracted (serotype 15). The upper and lower levels of the boxes represent the 75th and 25th percentiles, and the error bars represent the 90th and 10th percentiles. The open circles denote outliers. The solid lines represent the median values, and the dotted lines represent the means.
Small amounts of serum opsonins against all employed antigens were detected in most of the patients on admission to hospital (Fig. 1). The highest opsonic activities were registered in intermediate samples, and these were significantly higher than those measured with admission sera (P < 0.001 for PPOMV, PPPorA, and PPPorB values). Anti-OMV, anti-PorA, and anti-PorB opsonic activities were still significantly increased in 6-week sera (P < 0.0001 for PPOMV, PPPorA, and PPPorB values, compared with admission values), whereas a decline was registered between intermediate and 6-week samplings (P < 0.0001 for all values).
Six patients had been vaccinated with a serogroup B meningococcal OMV vaccine (strain 44/76; B:15:P1.7,16) 1 to 4 years prior to infection caused by B:15:P1.12 (n = 4) and C:2a:P1.2 (n = 2) strains (Table 1). Whereas the vaccinee admission sera induced higher PPOMV values (median PPOMV, 749; range, 603 to 1,306) than admission sera from nonvaccinated patients (P = 0.028), no differences in the PPOMV values induced by convalescent-phase sera were found. No statistically significant differences in the PPPorA and PPPorB values between vaccinated and nonvaccinated patients were found (data not shown). As for the other patients, the PP values induced by vaccinee intermediate sera were higher than those obtained with their admission sera (P = 0.028 for PPOMV, PPPorA, and PPPorB values).
Oxidative burst.
The oxidative burst responses were reflected by the formation of R-123 (Table 2). The mean nonlymphocyte R-123 fluorescence after stimulation with opsonized OMV- and porin-coated beads corresponded to the PP values with admission sera (r = 0.88 [P < 0.01], r = 0.34 [P < 0.05], and r = 0.84 [P < 0.01] for anti-OMV, anti-PorA, and anti-PorB activities, respectively), intermediate sera (r = 0.83, 0.91, and 0.95 [P < 0.01] for anti-OMV, anti-PorA, and anti-PorB activities, respectively), and 6-week sera (r = 0.97, 0.80, and 0.99 [P < 0.01] for anti-OMV, anti-PorA, and anti-PorB activities, respectively).
Control admission and convalescent-phase sera induced low PP and oxidative burst responses with all antigens, which were similar to those observed with admission sera from nonvaccinated meningococcal disease patients (data not shown). Sera from healthy controls induced highly variable PPOMV values (median PPOMV, 55; range 6 to 1,167) and R-123 activity (median, 0.17; range, 0.14 to 7.40) and low PPPorA and PPPorB values and R-123 activities (median PPPorA, 7 [range, 5 to 14], median PPPorB, 6 [range, 4 to 10]; and mean R-123 fluorescence, <0.18 with all sera). Control beads coated with BSA and unopsonized antigen-coated beads induced low PP values and R-123 formation (PP values of <10 and mean R-123 fluorescence of <0.20).
ELISA.
IgG antibodies recognizing OMV epitopes were detected in sera from all but one patient (no. 33) during meningococcal disease. The anti-OMV IgG levels in 38 available intermediate sera (median, 39 μg/ml; range, <2.5 to 681 μg/ml) were significantly higher than those in admission sera (median, 4 μg/ml; range <2.5 to 59 μg/ml) and 6-week sera (median, 22 μg/ml; range, <2.5 to 384 μg/ml) (P < 0.001 for both). The patient anti-OMV IgG levels corresponded to the anti-OMV opsonic activity, as reflected by PPomv values (r = 0.68, 0.50, and 0.72 [P < 0.01] for all admission, intermediate, and 6-week sera, respectively) (results for intermediate sera for PPomv versus anti-OMV IgG are shown in Fig. 5).
FIG. 5.
Correlation plots between levels of IgG against meningococcal PorA, PorB, and OMVs and PP values induced by antigen-specific opsonins in patient sera obtained between admission to hospital with meningococcal disease and 6 weeks later (intermediate sera).
IgG antibodies recognizing PorA and PorB proteins were detected in sera from all patients during meningococcal disease, and the highest levels were found in intermediate serum samples (Table 3). The anti-PorB IgG levels (median, 0.4 μg/ml [range, 0.1 to 47.4 μg/ml]; median, 22.7 μg/ml [range, 0.3 to 457.7 μg/ml]; and median, 6.8 μg/ml [range, 0.4 to 290.3 μg/ml] for all admission, intermediate, and 6-week sera, respectively) were higher than the anti-PorA IgG levels (median, 0.2 μg/ml [range, 0.1 to 4.7 μg/ml]; median, 3.1 μg/ml [range, 0.1 to 90.9 μg/ml]; and median, 1.7 μg/ml [range, 0.1 to 53.5 μg/ml] for all admission, intermediate, and 6-week sera, respectively) (P < 0.001 for all). The antiporin IgG levels were correlated to the antiporin opsonic activity (r = 0.25 [P < 0.12], r = 0.71 [P < 0.01], and r = 0.75 [P < 0.01] for anti-PorA IgG versus PPPorA values and r = 0.70, 0.63, and 0.88 [P < 0.01] for anti-PorB IgG versus PPPorB values, using admission, intermediate, and 6-week sera, respectively) (values for intermediate sera are shown in Fig. 5).
TABLE 3.
Meningococcal disease patient intermediate sera with antiporin (PorA and PorB) IgG, IgA, and IgM antibodies, as evaluated by ELISA
| Ig | PorA
|
PorB
|
||
|---|---|---|---|---|
| n | Median (range)a | n | Median (range)a | |
| IgG | 38 | 3.1 (0.1–90.9) | 38 | 22.7 (0.9–457.7) |
| IgG1 | 22 | 339 (58–5,200) | 32 | 1,125 (74–31,500) |
| IgG2 | 4 | 147 (87–370) | 5 | 100 (59–100) |
| IgG3 | 11 | 151 (50–501) | 21 | 661 (60–12,300) |
| IgG4 | 0 | 0 | ||
| IgA | 10 | 344 (124–1,740) | 24 | 449 (150–1,800) |
| IgM | 36 | 430 (100–3,200) | 37 | 1,600 (100–5,040) |
Only for sera with detectable Ig levels. IgG is quantified as micrograms per milliliter; IgA, IgM, and IgG subclass titers are presented as reciprocals of serum dilutions.
The antiporin IgG responses were mainly of the IgG1 and/or IgG3 subclass (Table 3). More intermediate sera exhibited detectable IgG1 and IgG3 antibody levels against the PorB protein than against PorA (Table 3), and the median IgG1 and IgG3 levels against PorB were higher than those against PorA (P < 0.05 for differences in IgG1 and IgG3 levels in admission, intermediate, and 6-week sera). IgG2 antibodies recognizing PorA and PorB were observed in only four and five sera, respectively (Table 3). Antiporin IgG4 was not found (Table 3).
Antiporin IgM responses were detected in sera from the majority of patients during meningococcal disease, and the anti-PorB IgM responses were more pronounced than the anti-PorA responses (P < 0.001 for admission, intermediate, and 6-week samples). The highest antiporin IgM titers were found in intermediate sera (Table 3), but the antiporin IgM levels did not correlate with the antiporin opsonic activity (r < 0.35 [P > 0.05] for all sera).
Four admission sera had detectable levels of anti-PorB IgA (data not shown), whereas anti-PorA and anti-PorB IgA were found in 10 and 24 intermediate sera, respectively (Table 3). No association between the IgA levels and the opsonic activities was found.
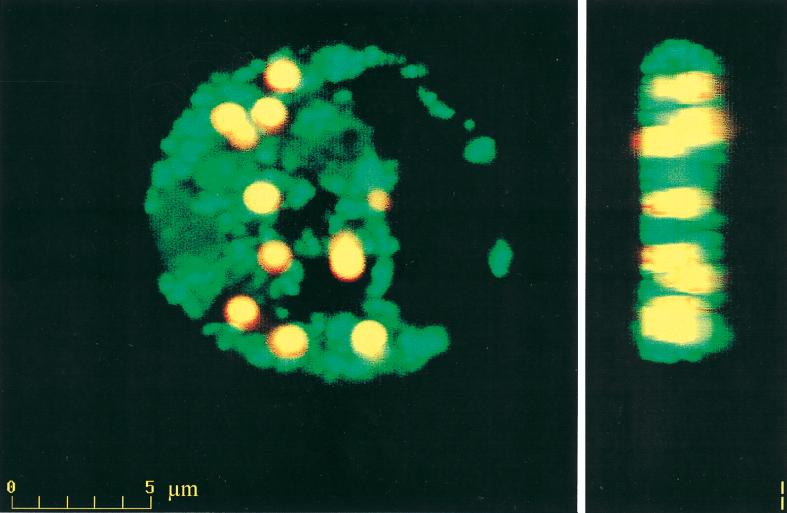
CLSM.
Phagocyte internalization of opsonized OMV- and porin-coated beads and the intracellular R-123 oxidative responses were visualized by CLSM, as shown with PorA-coated beads opsonized with intermediate serum from patient no. 35 (Fig. 6). The cell nucleus appeared black against the green R-123 in the cytoplasm, in which the opsonized antigen-coated beads were located. Control beads coated with BSA and unopsonized antigen-coated beads were not phagocytosed and did not initiate R-123 formation (not shown).
FIG. 6.
CLSM front- and side-view images of phagocytosed PorA-coated beads and green R-123 oxidative burst fluorescence.
DISCUSSION
We have demonstrated that human serum opsonins produced in response to meningococcal disease are directed against meningococcal outer membrane components and, for the first time, have specifically identified opsonins that recognize meningococcal PorA and PorB displayed in a purified form on the surface of beads. Significant increases in both phagocytosis and oxidative burst activity were observed with PorA- and PorB-coated beads opsonized with patient sera obtained during meningococcal disease (Fig. 1; Table 2). The results indicate that opsonins directed against these structures are induced by natural infection.
Previously, increasing amounts of human serum opsonins during meningococcal disease and after OMV vaccination have been demonstrated by using ethanol-fixed bacteria in functional opsonophagocytosis assays (1, 13, 14, 18, 24, 39), and such serum opsonic activity in patients has been shown to correlate with levels of IgG antibody to purified meningococcal PorA and PorB (17). To directly characterize the antigen specificity of the opsonic activities, we recently replaced the bacteria with antigen-coated beads (25, 26). Phagocytosis and oxidative burst activity induced by opsonized OMV-coated beads correlated with results obtained with opsonized ethanol-fixed meningococci (25). The epitopes exposed on OMV, PorA, and PorB may be different from those exposed on live bacteria (1a, 1b). In the present study, the vesicle and proteosome formulations were employed to present the antigens in as close to the native conformation as presently possible. The antigen specificity of the antiporin opsonic activity was further ensured by the extraction of porins from mutant strains lacking potentially contaminating proteins. Accordingly, the assay should reflect serum opsonic activities to defined bacterial structures.
The patient anti-PorA and anti-PorB opsonic activities correlated with the anti-OMV opsonic activity (Fig. 2), which suggests that patient opsonins recognize porin epitopes on the vesicles. However, higher anti-PorB opsonic activities than anti-PorA opsonic activities were observed (Fig. 1 to 4), and these were further found to correlate with higher IgG levels against PorB than against PorA in intermediate sera (Fig. 5). Higher IgG antibody levels against class 3 (PorB) than against class 1 (PorA) during meningococcal infections have previously been reported (17). The results suggest that PorB epitopes are more immunogenic than PorA during meningococcal disease. A strong immune response to PorB was also observed after three doses of an OMV vaccine, in contrast to two doses (34, 45). Thus, viable bacteria seem to induce a more rapid immune response to PorB than the OMV vaccine.
The PorA and PorB proteins were recognized by serum opsonins produced in patients infected by meningococci with serogroups, serotypes, and serosubtypes both homologous and heterologous to the strain from which the target antigens were extracted (Fig. 1; Table 1). With convalescent-phase sera, the median PPPorB values were markedly higher after infections with homologous serotypes (Fig. 4), whereas the median PPPorA values were higher after infections with heterologous serosubtypes (Fig. 3). However, no statistically significant differences were found when all anti-PorA and anti-PorB opsonic activities induced by meningococci of homologous versus heterologous serosubtypes and serotypes were compared. This may be explained by considerable interindividual differences in the PPPorA and PPPorB values (Fig. 1, 3, and 4). However, the presence of cross-reactive patient opsonic activities concurs with the serotype- and serosubtype-independent IgG responses observed after meningococcal disease (15, 16, 20, 28). Thus, the production of antiporin opsonic antibodies during meningococcal disease may at least in part be initiated by non-serotype and non-serosubtype-specific epitopes shared by various meningococcal outer membrane components.
In accordance with previous findings, the disease-induced antiporin IgG antibodies were primarily of subclasses IgG1 and IgG3 (15, 16). These are known to trigger complement activation and Fc receptor binding (2, 7, 40), which implies that the observed antiporin opsonic responses were due primarily to antigen-specific IgG1 and IgG3 antibodies.
Modest amounts of serum IgA antibodies recognizing PorA and PorB proteins were detected. Previous studies have proposed that a high fraction of IgA recognizing meningococcal components increases the disease susceptibility and that circulatory IgA can block the serum bactericidal activity and possibly also the opsonic activity of antimeningococcal antibodies (12, 22, 23). However, Haneberg et al. recently found that vaccinee IgA antibodies did not reduce the serum bactericidal activities (19). In the present study, only four admission sera had detectable antiporin (PorB) IgA antibodies, and no association was found between the IgA levels during disease and the magnitude of the phagocytic responses. Accordingly, the antiporin IgA antibodies did not seem to affect the opsonophagocytosis.
Phagocyte internalization of opsonized OMV-coated beads and initiation of intracellular oxidative burst mechanisms have previously been demonstrated by CLSM (25, 26). CLSM generates serial sections through individual phagocytes and projects fluorescent images from separate sections into one, which visualizes the total number of attached and internalized beads and the locations of beads and R-123 within individual phagocytes. The CLSM image supports the FCM results by confirming that both phagocytosis and oxidative burst are initiated by patient opsonins against bead-associated meningococcal porins (Fig. 6).
The higher anti-OMV opsonic activity observed in admission sera from previously OMV-vaccinated patients may be due to prevailing serum opsonins prior to infection but is most probably due to a rapid secondary immune response. Previous studies have shown that even though both IgG antibodies to OMV and species-specific bactericidal and opsonic antibodies were induced after similar two-dose OMV vaccinations, the anti-OMV IgG levels were significantly decreased after one year (13, 21, 24, 34, 46). Low levels of anti-OMV opsonic antibodies prior to infection may accordingly explain the occurrence of vaccine failures among the included patients. The finding of a suboptimal protective efficacy of 57% during a 29-month observation period (5) has intensified investigations concerning the OMV immunization scheme and routes and the search for the protective antigens for inclusion in improved vaccines (3, 4, 11, 19, 29, 31, 34, 41, 42, 45, 48).
The mechanisms responsible for the development of protective immunity to meningococcal disease remain unclear (3, 4, 31). Ross et al. demonstrated that serogroup B meningococci are more resistant to complement-mediated bactericidal activity of normal serum than serogroup A and C meningococci but are susceptible to phagocytic killing by polymorphonuclear leukocytes after opsonization (36). Furthermore, since genetic variations in the phagocyte Fcγ receptors have been shown to influence the susceptibility to meningococcal disease and since low opsonic activity against meningococci in admission sera is associated with serious and fatal meningococcal disease (6, 18), opsonophagocytosis seems to be of importance in the defense against meningococcal disease. Accordingly, knowledge about the opsonic activity of antibodies to meningococcal structures in natural infections is of value in the complex process of selecting future meningococcal vaccine constituents.
Immunocompetent survivors of meningococcal disease appear to acquire immunity to infection with heterologous serogroups, an indication that subcapsular antigens can generate cross-protective immune responses (3, 9). Our functional in vitro model with purified meningococcal antigens adsorbed to beads seems to facilitate dissection of subcapsular components that are recognized by disease-induced serum opsonins and accordingly may contribute to the identification of opsonin-inducing antigens for inclusion in future vaccines. Serum opsonins produced in response to a variety of disease-causing strains recognized OMV components and in particular PorB epitopes and initiated phagocytosis and oxidative burst activity, which suggests that these antigens are attractive meningococcal vaccine candidates.
ACKNOWLEDGMENTS
The serotyping of N. meningitidis was performed at the National Institutes of Public Health (NIPHs) in Oslo, Norway, and in Birmingham, United Kingdom. We thank Lisbeth Meyer Næss at NIPH, Oslo, Norway, for advice concerning the anti-OMV ELISA. We thank Eduardo Ramirez for assistance with the CLSM, which was provided by the FFS-Medical Research Center, University of Bergen, Bergen, Norway. The complement hemolytic activity was measured by the Department of Microbiology and Immunology, Haukeland Hospital, Bergen, Norway.
This study was supported by grants from Nina’s Memorial Fund, Norway.
REFERENCES
- 1.Aase A, Bjune G, Høiby E A, Rosenqvist E, Pedersen A K, Michaelsen T. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect Immun. 1995;63:3531–3536. doi: 10.1128/iai.63.9.3531-3536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Aase A, Høiby E A, Michaelsen T. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand J Immunol. 1998;47:388–396. doi: 10.1046/j.1365-3083.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 1b.Aase A, Høiby E A, Kolberg J, Rosenqvist E, Michaelsen T. Most antibodies to PorB do not bind to viable meningococci, but bind strongly to ethanol-killed bacteria. In: Nassif X, Quintin-Millet M-J, Taha M-K, editors. Abstracts of the Eleventh International Pathogenic Neisseria Conference, 1 to 6 November 1999, Nice, France. Paris, France: Editions E.D.K.; 1998. p. 284. [Google Scholar]
- 2.Aase A, Michaelsen T E. Opsonophagocytosis activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand J Immunol. 1994;39:581–587. doi: 10.1111/j.1365-3083.1994.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 3.Ala’Aldeen D A A, Griffiths E. Vaccines against meningococcal diseases. In: Ala’Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. Chichester, England: John Wiley & Sons Ltd.; 1995. pp. 1–39. [Google Scholar]
- 4.Ala’Aldeen D A A, Cartwright K A V. Neisseria meningitidis: vaccines and vaccine candidates. J Infect. 1996;33:153–157. doi: 10.1016/s0163-4453(96)92081-2. [DOI] [PubMed] [Google Scholar]
- 5.Bjune G, Høiby E A, Grønnesby J K, Arnesen Ø, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 6.Bredius R G M, Derkx B H F, Fijen A P, de Wit T P M, de Haas M, Weening R S, van de Winkel J G J, Out T A. Fcγ receptor IIa (CD32) polymorphism in fulminant meningococcal shock in children. J Infect Dis. 1994;170:848–853. doi: 10.1093/infdis/170.4.848. [DOI] [PubMed] [Google Scholar]
- 7.Burton D R, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy. 1986;50:510–516. [PubMed] [Google Scholar]
- 8.Ehlenberger A G, Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977;145:357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueroa J E, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm L O, Lindbak A K, Møgster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–80. [PubMed] [Google Scholar]
- 11.Fusco P C, Michon F, Tai J Y, Blake M S. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J Infect Dis. 1997;175:364–372. doi: 10.1093/infdis/175.2.364. [DOI] [PubMed] [Google Scholar]
- 12.Griffiss J M. Mechanisms of host immunity. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons; 1995. pp. 35–70. [Google Scholar]
- 13.Guttormsen H-K, Bjerknes R, Halstensen A, Næss A, Høiby E A, Solberg C O. Cross-reacting serum opsonins to meningococci after vaccination. J Infect Dis. 1993;167:1314–1319. doi: 10.1093/infdis/167.6.1314. [DOI] [PubMed] [Google Scholar]
- 14.Guttormsen H-K, Bjerknes R, Næss A, Lehmann V, Halstensen A, Sørnes S, Solberg C O. Cross-reacting serum opsonins in patients with meningococcal disease. Infect Immun. 1992;60:2777–2783. doi: 10.1128/iai.60.7.2777-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guttormsen H-K, Wetzler L M, Næss A. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect Immun. 1993;61:4734–4742. doi: 10.1128/iai.61.11.4734-4742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttormsen H-K, Wetzler L M, Solberg C O. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect Immun. 1994;62:1437–1443. doi: 10.1128/iai.62.4.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttormsen H-K, Wetzler L M, Solberg C O. Serum opsonins induced during the course of meningococcal disease correlate with anti-outer membrane protein antibodies. In: Evans J S, Yost S E, Maiden M C J, Feavers I M, editors. Neisseria 94, Proceedings of the Ninth International Pathogenic Neisseria Conference, 26 to 30 September 1994, Winchester, England. 1994. pp. 54–65. [Google Scholar]
- 18.Halstensen A, Sjursen H, Vollset S E, Frøholm L O, Næss A, Matre R, Solberg C O. Serum opsonins to serogroup B meningococci in meningococcal disease. Scand J Infect Dis. 1989;21:267–276. doi: 10.3109/00365548909035696. [DOI] [PubMed] [Google Scholar]
- 19.Haneberg B, Dalseg R, Wedege E, Høiby E A, Haugen I L, Oftung F, Andersen S R, Naess L M, Aase A, Michaelsen T E, Holst J. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harthug S, Rosenqvist E, Høiby E A, Gedde-Dahl T W, Frøholm L O. Antibody response in group B meningococcal disease determined by enzyme-linked immunosorbent assay with serotype 15 outer membrane antigen. J Clin Microbiol. 1986;24:947–953. doi: 10.1128/jcm.24.6.947-953.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Høiby E A, Rosenqvist E, Frøholm L O, Bjune G, Feiring B, Nøkleby H, Rønnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 1991;14:147–155. [PubMed] [Google Scholar]
- 22.Kaythy H, Jousimies-Somer H, Peltola H, Makela P H. Antibody response to capsular polysaccharides of groups A and C Neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J Infect Dis. 1981;143:32–41. doi: 10.1093/infdis/143.1.32. [DOI] [PubMed] [Google Scholar]
- 23.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann A K, Halstensen A, Næss A, Vollset S E, Sjursen H, Bjune G. Immunization against serogroup B meningococci. Opsonin response in vaccinees as measured by chemiluminescence. APMIS. 1991;99:769–772. [PubMed] [Google Scholar]
- 25.Lehmann A K, Halstensen A, Holst J, Bassøe C-F. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J Immunol Methods. 1997;200:55–68. doi: 10.1016/s0022-1759(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann A K, Halstensen A, Bassøe C-F. Flowcytometric quantitation of human opsonin-dependent phagocytosis and oxidative burst responses to meningococcal antigens. Cytometry. 1998;33:6–13. doi: 10.1002/(sici)1097-0320(19981201)33:4<406::aid-cyto3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann V, Solberg C O. Group B meningococcal opsonins in serum measured by polymorphonuclear leukocyte chemiluminescence. APMIS. 1980;88:227–231. doi: 10.1111/j.1699-0463.1980.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 28.Mandrell R E, Zollinger W D. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect Immun. 1989;57:1590–1598. doi: 10.1128/iai.57.5.1590-1598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer Næss L, Oftung F, Aase A, Wetzler L M, Sandin R, Michaelsen T E. Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. Infect Immun. 1998;66:959–965. doi: 10.1128/iai.66.3.959-965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer Næss L, Rosenqvist E, Høiby E A, Michaelsen T E. Quantitation of IgG subclass antibody responses after immunization with a group B meningococcal outer membrane vesicle vaccine, using monoclonal mouse-human chimeric antibodies as standards. J Immunol Methods. 1996;196:41–49. doi: 10.1016/0022-1759(96)00108-1. [DOI] [PubMed] [Google Scholar]
- 31.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Høiby E A, Rosenqvist E, Holst J, Nøkleby H, Sotolongo F, Sierra G, Huergo C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 32.Raff H V, Devereux D, Shuford W, Abbot-Brown D, Maloney G. Human monoclonal antibody with protective activity for Escherichia coli K1 and Neisseria meningitidis group B infections. J Infect Dis. 1988;157:118–126. doi: 10.1093/infdis/157.1.118. [DOI] [PubMed] [Google Scholar]
- 33.Rosenqvist E, Høiby E A, Bjune G, Bryn K, Closs O, Feiring B, Klem A, Nøkleby H, Frøholm L O. Human antibody responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. NIPH Ann. 1991;14:169–179. [PubMed] [Google Scholar]
- 34.Rosenqvist E, Høiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross G D. Complement and complement receptors. Curr Opin Immunol. 1989;2:50–62. doi: 10.1016/0952-7915(89)90097-6. [DOI] [PubMed] [Google Scholar]
- 36.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 37.Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger M, Greenberg R, Levy J, Kaythy H, Levy R. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J Infect Dis. 1994;170:449–453. doi: 10.1093/infdis/170.2.449. [DOI] [PubMed] [Google Scholar]
- 39.Sjursen H, Bjerknes R, Halstensen A, Næss A, Frøholm L O, Rosenqvist E, Solberg C O. Serum opsonins to group B meningococci. Acta Pathol Microbiol Immunol Scand. 1987;95:283–289. doi: 10.1111/j.1699-0463.1987.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 40.Turner M, Owen M. Antigen receptor molecules. In: Roitt I, Brostoff J, Male D, editors. Immunology. London, United Kingdom: Mosby-Year Book Europe Limited; 1993. pp. 4.1–4.20. [Google Scholar]
- 41.Van der Ley P, Poolman J T. Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect Immun. 1992;60:3156–3161. doi: 10.1128/iai.60.8.3156-3161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Voort E R, van Dijken H, Kuipers B, van der Biezen J, van der Ley P, Meylis J, Claassen I, Poolman J. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect Immun. 1997;65:5184–5190. doi: 10.1128/iai.65.12.5184-5190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van de Winkel J G J, Capel P J A. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 44.Wedege E, Bjune G, Frøholm L O, Høiby E A, Rosenqvist E. Immunoblotting studies of vaccinee and patient sera from a Norwegian serogroup B meningococcal vaccine trial. NIPH Ann. 1991;14:183–186. [PubMed] [Google Scholar]
- 45.Wedege E, van der Voort E R, Kuipers B, Bolstad K, van Duken H, Poolman J T. Induced antibody specificities with the Norwegian outer membrane vesicle vaccine in Icelandic teenagers: comparison of bactericidal antibody levels using isogenic strains with specific antibody levels determined by densitometric immunoblot analysis. In: Nassif X, Quintin-Millet M-J, Taha M-K, editors. Abstracts of the Eleventh International Pathogenic Neisseria Conference, 1 to 6 November 1998, Nice, France. Paris, France: Editions E.D.K.; 1998. p. 176. [Google Scholar]
- 46.Wedege E, Høiby E A, Rosenqvist E, Bjune G. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect Immun. 1998;66:3223–3231. doi: 10.1128/iai.66.7.3223-3231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wetzler L M, Blake M S, Barry K, Gotschlich E C. Gonococcal porin evaluation: comparison of por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J Infect Dis. 1992;166:551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- 48.Zollinger W D. New and improved vaccines against meningococcal disease. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 469–488. [Google Scholar]