Abstract
Background
Neurofibromatosis type 1 (NF1) is a common genetic disorder of phenotypic variability with age-dependent penetrance. This study describes the diagnosis, clinical characterization, management, and outcomes of a large patient cohort with plexiform neurofibroma (PN) treated with selumetinib in a real-world clinical setting.
Methods
This single-center observational study consecutively enrolled patients with NF1-PN treated with selumetinib from April 2018 to 2023. Data on clinical features, tumor types and locations, and results from genetic tests were recorded at baseline; details of disease management with selumetinib and surgical intervention and disease evolution including imaging data and evaluations of pain and function were documented.
Results
Overall, 54 patients with a median age (range) of 16.4 (4.5–58.0) years were enrolled. Most had cutaneous manifestations (88.9%), including cutaneous neurofibromas and PN. Patients underwent [18F]fluorodeoxyglucose (FDG)-PET/CT imaging before treatment to rule out malignant lesions. Initial evaluations included directed magnetic resonance imaging (MRI), which facilitated future comparisons and allowed for the assessment of PN resectability. Pharmacological treatment with selumetinib (with surgery, without surgery) resulted in the following proportion of patients achieving stable disease (58.8%, 54.3%), partial response (29.4%, 28.6%), and improved pain (58.8%, 37.1%), deformity (17.6%, 20.0%), and functional (17.6%, 20.0%) outcomes, respectively.
Conclusions
Results from this study demonstrate that NF1-PN can be managed effectively with selumetinib with surgical intervention in some patients. Most patients achieved tumor stability and improved symptom control, and the majority of patients continue under treatment. Effective diagnosis and management were achieved through individualized utility of FDG-PET/CT and MRI imaging and targeted resource allocation.
Keywords: neurofibromatosis type 1, plexiform neurofibroma, selumetinib
Lay Summary
Neurofibromatosis type 1 (NF1) is a genetic condition that causes tumors to grow on nerves. Selumetinib is a medication approved for treating tumors called plexiform neurofibroma (PN), which are common in people with NF1. The authors of this study wanted to describe their experience with using selumetinib. To do this they reviewed the medical records (including imaging, blood tests, surgeries and symptoms) of patients with NF1 who were treated with selumetinib over several years at their hospital. Their results showed that most patients’ tumors stopped growing, and some even shrank. Most patients also reported relief from symptoms like pain and improvements in their physical abilities.
Key Points.
Most patients with NF1 present with cutaneous neurofibromas and PN.
Selumetinib with or without surgery led to positive outcomes and tumor stability.
FDG-PET/CT and MRI can be used on an individual basis to detect and monitor disease.
Importance of the Study.
Neurofibromatosis type 1 (NF1) is a common autosomal dominant disorder associated with cutaneous, ophthalmological, neurological, and musculoskeletal manifestations. Real-world clinical evidence of disease heterogeneity, diagnostic practice, and patient management is sparse but could help to improve multidisciplinary processes and refine resource allocation. In this study, MRI was used for baseline measure of tumor burden, and then selectively for patients at high risk of malignant transformation. For these patients, imaging can help to detect and characterize plexiform neurofibroma (PN) and malignant tumors, improving disease management. In this study, selumetinib was shown to effectively control disease in most patients, and was suitable for long-term treatment, including in patients transitioning from childhood to adulthood. Surgical intervention in addition to pharmacological treatment can stabilize tumor size, and reduce NF1-associated pain and functional impairment.
Neurofibromatosis type 1 (NF1) is one of the most common, autosomal dominant disorders, with an estimated birth incidence of 1 in 2000–3500 individuals.1–3 According to the revised NIH guidelines, 2 diagnostic criteria must be satisfied to reach a diagnosis of NF1, either 2 clinical features (except if only café-au-lait macules and freckling are present) or 1 symptom and a parent with NF1.1,2 Symptoms of NF1 are highly heterogeneous, with various typical ages of onset; emergence of symptoms later in life may result in delayed diagnosis.1,2,4,5
NF1 is a neurocutaneous condition characterized by café-au-lait macules, intertriginous freckling, Lisch nodules, neurofibromas, and increased risk of benign and malignant tumorigenesis, such as optic pathway gliomas (OPGs) or malignant peripheral nerve sheath tumors (MPNSTs).1,2 Neurofibromas (tumors of Schwann cell origin that can affect the peripheral or central nervous system) can be classified into various types, which include cutaneous and plexiform neurofibromas (PN).1–3 Whilst cutaneous manifestations may cause disfigurement and discomfort, PN are associated with significant morbidity and carry the risk of malignant transformation.1–3 NF1 is also associated with neurocognitive, musculoskeletal, and cardiovascular manifestations.1,2
Between 30% and 50% of patients with NF1 develop PN, which can involve multiple cell types, be of any size and location along the nerve, and range from localized and nodular to diffuse in appearance.2,3 PN are commonly associated with symptoms including pain, sensory impairment, autonomic dysfunction and motor dysfunction, particularly if the PN interferes with nerve function.1,6 PN may undergo malignant transformation to MPNST (reported in 8%–13% of patients), which is associated with poor prognosis.1–3 Ultimately, NF1 with PN (NF1-PN) can negatively impact quality of life (QoL), placing a burden on both patients and caregivers.6
Surgical removal was the most common treatment option for PN; however, the surgical risk may outweigh the clinical benefit for some patients, rendering the PN inoperable.3 Therefore, pharmacological interventions demonstrating effective disease control may benefit patients.3,7
NF1 is caused by loss-of-function variants of NF1, a tumor suppressor gene, which encodes the protein neurofibromin.7,8 Neurofibromin binds to and inhibits RAS, but when neurofibromin-mediated inhibition is disrupted, the RAS/RAF/MEK/ERK pathway becomes constitutively active, resulting in uncontrolled cell proliferation.8 Selumetinib selectively inhibits MEK1/2, inhibiting cell proliferation via the neurofibromin pathway.9
Based upon results from the Phase 2 SPRINT trial, selumetinib is approved by the U.S. Food and Drug Administration (aged ≥2 years), the European Medicines Agency (aged ≥3 years), Japan (aged ≥3 years), and China (aged ≥3 years) for pediatric patients with NF1 and symptomatic, inoperable PN.9–13 The recommended dose of selumetinib is individualized based on 25 mg/m2 of body surface area, rounded to the nearest achievable 5 mg or 10 mg dose (between 30–100 mg per day taken over 2 oral doses).9–14
This case series aims to describe a large, heterogeneous cohort of patients with NF1-PN who received selumetinib treatment. Additionally, it explores how clinical and radiological parameters can be interpreted to inform patient management given the heterogeneity in presentation and disease course of NF1.
Methods
Patients with NF1 with inoperable PN associated with significant or potentially significant morbidity, treated with selumetinib per individualized dosing (based on 25 mg/m2 of body surface area) from April 2018 to April 2023, were observed in a real-world case series for up to 5 years. Patients were enrolled consecutively from the Francisco Gentil Portuguese Institute of Oncology of Lisbon, and the patient, their parent, or caregiver provided written consent to participate in this study. Patients were treated per institutional protocol (no screening or additional study assessments were performed before surgical or pharmacological treatment), selumetinib was initiated at study enrollment. Access to selumetinib was gained with individually submitted requests for adults and children who started treatment before EMA approval through a compassionate use program.
Patient demographics, medical history and comorbidities, manifestations of NF1 and associated symptoms, and details and location of PN were recorded at baseline. The molecular diagnosis and pretreatment procedures, including magnetic resonance imaging (MRI), and integrated PET and CT with [18F]fluorodeoxyglucose (FDG-PET/CT), and surgery were also documented at baseline before selumetinib initiation where available.
Clinical evaluations were performed 2–4 weeks after enrollment and every 3 months thereafter per standard multidisciplinary clinical practice. Cardiological assessment was performed every 6 months; ophthalmological assessment every 6 months for pediatric patients aged ≤6 years, annually for older patients, and more frequently if symptoms were observed. Genetic consultation was performed for any patients who had not been previously evaluated. Whole body FDG-PET/CT was performed either to exclude malignant transformation before initiating selumetinib treatment or when malignant transformation was suspected in patients receiving treatment. Images were acquired 90 minutes after a weight-based dose of radiotracer, and an adjusted CT acquisition protocol was implemented for pediatric patients. Patient preparation and image reconstruction were performed according to the European Nuclear Medicine Guidelines v2.0.15 Non-volumetric MRI analysis per World Health Organization (WHO) criteria was performed annually, or every 4 months if abnormalities were observed with FDG-PET/CT, and whole-body images were used to define tumor distribution.16 Treatment outcomes, including imaging parameters, subjective symptom evaluations, and adverse events were recorded, as well as any changes to selumetinib treatment dose and schedule. Tumor response was categorized as either partial response, stability, or progression independently of the tumor volume removed surgically for patients who underwent partial or complete resections. Specific volumetric WHO data were not described due to the irregular nature and variability of lesions, which preclude accurate quantitative volumetric measurements, rather tumor response was classified per those criteria, and is described here.
Pain was assessed using a numerical rating scale and the Wong-Baker Faces Pain Rating Scale. Functional assessment was performed using the Medical Research Council Manual Muscle Testing Scale, and deformity was subjectively assessed at the clinician’s discretion. The impact of disease on daily activities was assessed using the KPS Scale (KPSS). Safety was graded according to the NCI Common Terminology Criteria for Adverse Events v5.0.
Patients were treated according to a refined institutional protocol, and their case data were electronically recorded by the clinician at study visits, recorded, and analyzed based on verbatim clinical notes to more generalized categories to analyze patterns and trends across patient groups.
Results
Patient Characteristics
Overall, 60 patients were enrolled in this study, and 6 were subsequently excluded from the analysis due to inclusion in an alternative registry.17 The median age (range) of the 54 patients included in this analysis was 16.4 (4.5–58.0) years, and 23 patients (42.6%) were female. The study population included 37 patients enrolled at <18 years of age, with 29 patients (53.7%) still <18 years of age at study completion and 8 patients (14.8%) transitioning from childhood to adulthood during the course of the study; 17 patients (31.5%) enrolled at ≥18 years of age. Baseline demographics are described in Table 1.
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | Value (N = 54) |
|---|---|
| Age, years | |
| Mean (SD) | 18.3 (10.0) |
| Median (range) | 16.4 (4.5–58.0) |
| Sex, n (%) | |
| Male | 31 (57.4) |
| Molecular diagnosis, n (%) | |
| NF1 | 53 (98.1) |
| NF2 | 1 (1.9) |
| Family history, n (%) | |
| De novo | 30 (55.6) |
| Positive family history | 21 (38.9) |
| Unknown | 3 (5.6) |
| Previous PN therapy, n (%) | |
| Yes | 8 (14.8) |
| No | 46 (85.2) |
| Baseline, pretreatment assessments | |
| Histology, recorded, n (%) | 22 (40.7) |
| Whole-body MRI, recordeda, n (%) | 13 (24.1) |
| KPSS, median (min, max) | 80 (40, 90) |
aMRI not performed for 38 patients and unknown for 3 patients.
Abbreviations: KPSS, KPS Scale; NF, neurofibromatosis.
Age of NF1 diagnosis was available for 52 patients, with 44 (84.6%) diagnosed <5 years of age, 7 (13.5%) diagnosed between ages 5–16 years, and 1 (1.9%) diagnosed at age 20. As per the eligibility criteria, all patients had PN; 33 patients (61.1%) were diagnosed with PN when aged <5 years, 13 (24.1%) were aged 5–16 years, and 8 (14.8%) were aged >16 years (Figure 1).
Figure 1.
Age of patients at diagnosis.
Data included for patients with available data (n = 28, NF1 diagnosis; n = 54, PN diagnosis).
Abbreviations: NF1, neurofibromatosis type 1; PN, plexiform neurofibroma.
Disease Characterization
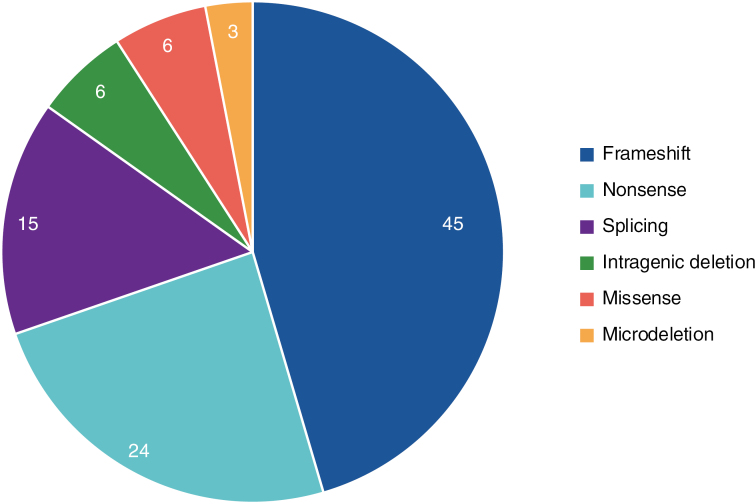
Molecular testing and clinical examination confirmed that 52/53 patients had NF1. One patient presented with probable confined mosaic NF1 and 1 patient with atypical presentation has a neurofibromatosis type 2-related schwannomatosis (NF2-SWN). Variant categories are described in Figure 2 and described verbatim in Supplementary Table 1.
Figure 2.
Percentage of neurofibromin variant categories.
Data included for patients with available data (n = 33). Categories of genetic variants were grouped. The categories of 21 variants were unknown. One variant was found in NF2 (Supplementary Table 1).
The symptomatic characterization of the patient cohort is outlined in Table 2. The most common manifestations were cutaneous, observed in 48 patients (88.9%), including café-au-lait spots, intertriginous freckling, and cutaneous neurofibromas. Over half of all patients (30, 55.6%) had ophthalmological manifestations, primarily Lisch nodules and optic pathway tumors, and 9 patients (16.7%) had a history of symptomatic OPG. Supplementary Figure 1 shows the location of OPGs. A total of 26 patients (48.1%) had neurological manifestations, which included pain, alterations in sensitivity, and decreased muscle strength. Neurodevelopmental abnormalities were observed in 25 patients (46.3%), including learning difficulties, intellectual disabilities or developmental delay, attention deficit hyperactivity disorder, and autism, with multiple patients experiencing more than 1 condition concurrently. A total of 19 patients (35.2%) had scoliosis, and an additional 8 (14.8%) had other musculoskeletal deformities. Less common manifestations included cardiological manifestations in 4 patients (7.4%), and 31% of patients had other comorbidities. No endocrinological manifestations were observed.
Table 2.
Baseline Clinical Characterization
| Symptoms | Patients, n (%); (N = 54) |
|---|---|
| Cutaneous manifestationsa | 48 (88.9) |
| Café-au-lait spots | 48 (88.9) |
| Intertriginous freckling | 8 (14.8) |
| Cutaneous neurofibromas | 5 (9.3) |
| None | 1 (1.9) |
| Unknown | 5 (9.3) |
| Ophthalmic manifestationsa | 30 (55.6) |
| Optic nerve pathway tumor | 21 (38.8) |
| Lisch nodules | 17 (31.5) |
| Visual field deficit | 2 (3.7) |
| Other | 3 (5.6) |
| None | 16 (29.6) |
| Unknown | 8 (14.8) |
| OPGa | 21 (38.8) |
| OPG present | 21 (38.8) |
| OPG symptomatic | 9 (16.7) |
| None | 33 (61.1) |
| Unknown | 0 (0.0) |
| Growth featuresa | 16 (29.6) |
| Macrocephaly | 6 (11.1) |
| Short stature | 5 (9.3) |
| Relative macrocephaly | 4 (7.4) |
| Other | 2 (3.7) |
| None | 26 (48.1) |
| Unknown | 12 (22.2) |
| Musculoskeletal manifestationsa | 27 (50.0) |
| Scoliosis | 21 (38.9) |
| Pectus abnormalities | 2 (3.7) |
| Right sphenoid wing dysplasia | 1 (1.9) |
| Tibial pseudarthrosis | 1 (1.9) |
| Other | 2 (3.7) |
| None | 23 (42.6) |
| Unknown | 4 (7.4) |
| Cardiovascular manifestationsa | 4 (7.4) |
| Hypertension | 4 (7.4) |
| Hypertension and renal artery stenosis | 1 (1.9) |
| None | 40 (74.1) |
| Unknown | 10 (18.5) |
| Neurodevelopmental manifestationsa | 25 (46.3) |
| ADHD | 11 (20.4) |
| Developmental delay/Intellectual disability | 9 (16.7) |
| Learning difficulty | 5 (9.3) |
| Language or speech disorder | 3 (5.6) |
| Autism spectrum disorders | 2 (3.7) |
| None | 23 (42.6) |
| Unknown | 6 (11.1) |
| Neurological manifestationsa | 26 (48.1) |
| Decreased muscle strength | 4 (7.4) |
| Tetraparesis | 4 (7.4) |
| Seizures | 4 (7.4) |
| Incontinence | 4 (7.4) |
| Reflex abnormalities | 2 (3.7) |
| Neuroimaging abnormalities | 2 (3.7) |
| Other | 8 (14.8) |
| None | 27 (50.0) |
| Unknown | 1 (1.9) |
| Tumorsa | 54 (100.0) |
| PN | 54 (100.0) |
| MPNST | 3 (5.6) |
| ANNUBP | 2 (3.7) |
| Non-OPG astrocytomab | 3 (5.6) |
| Breast cancer | 1 (1.9) |
aCategories not mutually exclusive; therefore, multiple manifestations may be present for a single patient and the numbers of individual manifestations may exceed the total number of patients with that category of manifestations.
bPilocytic cerebral low-grade glioma (2 instances) and cerebellar low-grade glioma (1 instance).
Abbreviations: ADHD, attention deficit hyperactivity disorder; ANNUBP, atypical neurofibromatous neoplasm with unknown biological potential; MPNST, malignant peripheral nerve sheath tumor; OPG, optic pathway glioma; PN, plexiform neurofibroma.
All patients (n = 54; 100%) had PN, 2 of whom (3.7%) also had atypical neurofibromatous neoplasm with unknown biological potential (ANNUBP). Overall, 3 patients (5.6%) developed MPNST (2 from ANNUBP), and 4 (7.4%) had additional tumors, including 3 with biopsy-proven low-grade gliomas (2 pilocytic instances in the cerebral hemispheres, 1 cerebellar instance) and 1 with breast cancer. The localization of PN varied with patient age, with facial, orbital, and limb PN being more frequent in patients aged <10 years (Supplementary Figure 2).
Disease Management
Patients received selumetinib at a mean (range) starting dose of 31.7 (10–50) mg twice daily based on 25 mg/m2 of body surface area, for a median (range) treatment duration of 38.3 (0.3–58.9) months. The most common reasons influencing treatment initiation were deformity for 48 patients (88.9%), pain for 25 (46.3%), and functional impairment for 14 (25.9%). Supplementary Table 2 describes the full details of the treatment rationale.
The dermatological safety profile of selumetinib was consistent with that observed in clinical trials. Gastrointestinal symptoms were observed less frequently in this patient cohort, whereas cardiovascular events, particularly valve disease, and ophthalmological adverse events were observed more frequently than in published studies (Table 3).9
Table 3.
Adverse Events
| Adverse event | Patients, n (%); (N = 54) | CTCAE grade, n (%) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | NR | ||
| Dermatologicala | 53 (98.1) | ||||
| Acneiform rash | 31 (57.4) | 2 (6.4) | 28 (90.3) | 0 | 1 (3.2) |
| Paronychia | 26 (48.1) | 2 (7.7) | 20 (76.9) | 4 (15.4) | 0 |
| Xeroderma/dry skin | 17 (31.5) | 14 (82.4) | 2 (11.8) | 0 | 1 (5.9) |
| Alopecia | 16 (29.6) | 12 (75.0) | 0 | 0 | 4 (25.0) |
| Hair color faded | 7 (13.0) | 7 (100.0) | 0 | 0 | 0 |
| Rash (maculopapular and other) | 7 (13.0) | 1 (14.3) | 5 (71.4) | 0 | 1 (14.3) |
| Other | 22 (40.7) | ||||
| None/not recorded | 1 (1.9) | ||||
| Cardiovasculara | 24 (44.4) | ||||
| Ejection fraction decrease | 8 (14.8) | 0 | 8 (100.0) | 0 | 0 |
| Mitral valve disease | 14 (25.9) | 14 (100.0) | 0 | 0 | 0 |
| Tricuspid valve disease | 7 (13.0) | 5 (71.4) | 0 | 0 | 0 |
| Other | 6 (11.1) | ||||
| None/not recorded | 30 (55.6) | ||||
| Ophthalmological | 8 (14.8) | ||||
| Photophobia | 4 (7.4) | 2 (50.0) | 0 | 0 | 2 (50.0) |
| Vision decreased | 2 (3.7) | 1 (50.0) | 1 (50.0) | 0 | 0 |
| Blurred vision | 2 (3.7) | 2 (100.0) | 0 | 0 | 0 |
| None/not recorded | 46 (85.2) | ||||
| Laboratorya | 38 (70.4) | ||||
| CPK increase | 28 (51.9) | 18 (64.3) | 4 (14.3) | 3 (10.7) | 3 (10.7) |
| Neutrophil count decrease | 7 (13.0) | 2 (28.6) | 5 (71.4) | 0 | 0 |
| ALT and AST increasedb | 6 (11.1) | 4 (66.7) | 1 (16.7) | 0 | 1 (16.7) |
| Rash and pruritus | 1 (1.9) | 0 | 0 | 0 | 1 (100.0) |
| Other | 10 (18.5) | ||||
| None/not recorded | 16 (26.9) | ||||
| Other | 32 (59.3) | ||||
aCategories not mutually exclusive; therefore, multiple events could occur for a single patient and the number of individual events may exceed the total number of patients with that category of events.
bOne patient experienced prolonged treatment interruption secondary to ALT and AST abnormalities.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; NR, not reported.
All patients experienced adverse events. These were successfully managed by dose reduction or interruption for 20 patients (37.0%); 8/19 (42.1%) and 5/8 (62.5%) of whom experienced a second and third dose reduction, respectively. Supplementary Table 3 outlines the reasons for treatment suspensions. Only 2 patients discontinued therapy, both due to paronychia; one resumed treatment to treat disease progression and one remained off treatment as her condition remained stable with no recurrence of pain.
Surgical intervention was performed at operable sites for 31/54 evaluable patients (57.4%). The majority were debulking procedures (21/31, 67.7%), with 4 (12.9%) biopsies, 1 (3.2%) to treat the malignancy, and 1 (3.2%) surgery unrelated to PN. The rationale for surgery was not recorded for the remaining 4 (7.4%) patients. The date of surgery was available for 49 instances across 19 patients, with some patients having surgery more than once; 8 patients had surgery before selumetinib treatment; 5 during treatment only and 6 both before and during treatment (Figure 3).
Figure 3.
Treatment effects. (A) Observed tumor activity based on subjective evaluation of imaging data according to use and sequencing of surgery alongside treatment with selumetinib;a (B) Observed symptom improvements according to use and sequencing of surgery alongside treatment with selumetinib.b
aImaging data based on final MRI evaluation and neuroradiological assessment.
bSymptom categories are not mutually exclusive per patient.
cFor pain, improvement is defined as a ≥2-point reduction in the numerical pain scale over 2 consecutive visits, resolution of the absence of pain.
dIncontinence improvement (3 instances, 2 resolved), stability (2 instances), respiratory function improvement (2 instances), aesthetic improvement (2 instances), dysphagia improvement (2 instances), dysarthria improvement (2 instances), exercise tolerance improvement (2 instances), sensory symptom resolution, discomfort improvement, decrease of lower limb diameter, improved social interaction, scoliosis improvement, muscular strength improvement, neurofibroma growth, functional paresis decline.
Disease Evolution
Forty-six patients (85.2%) underwent FDG-PET/CT to exclude malignancy; focal MRI was used to characterize the target lesion before commencing treatment for 53/54 patients (98.1%). Whole-body MRI was used to assess disease state before selumetinib treatment in 13 patients (24.1%), and again following treatment in 3 (23.1%) of those patients. An additional 6 patients underwent post-treatment whole-body MRI only. The patient who was not assessed with localized MRI pretreatment began treatment before the assessment to avoid treatment delay.
Of the 19 patients who underwent surgery and 35 who did not, follow-up imaging data were available for 46 patients, enabling classification of tumor response (Figure 3). One patient had progressive tumor growth (2.2%), 30 had stable tumors (65.2%), and 15 had a partial response (32.6%; Figure 3A). Tumor response in patients who did or did not undergo surgery was as follows. Progression was recorded for 1/19 patients who underwent surgery (5.3%) versus none who did not undergo surgery. This patient underwent 2 partial resection surgeries while receiving selumetinib and was diagnosed with MPNST at the time of the second surgery. A total of 20/35 patients who did not undergo surgery (57.1%) had stable disease versus 10/19 patients who underwent surgery (52.6%). Partial response was observed for 10 patients who did not undergo surgery (28.6%) and 5 patients who underwent surgery (26.3%), some were observed after 12 months, and others after 24 months of treatment.
The timing of surgery impacted tumor response as follows. For 5 patients who underwent surgery whilst receiving selumetinib, partial response was observed for 1 patient, stability for 1 patient, and progression (tumor size increase) for 1 patient who died. Data were missing for 2 patients. For the 8 patients who underwent surgery before treatment, partial response was observed for 2 patients, stability for 5, and tumor progression was not observed. Data were missing for 1 patient.
Following surgical intervention, symptom control improved; pain measures improved for 10 patients (52.6%), functionality improved for 3 (15.8%), deformity improved for 3 (15.8%), and subjective nonspecific improvements were observed in 4 patients (21.1%). Other symptoms improved for 2 patients who underwent surgery (10.5%), as described in Figure 3B.
Mean (SD) KPSS score at baseline was 79.4 (10.2), and the breakdown of these scores is described in Supplementary Table 4. For patients who underwent surgery with KPSS data available, KPSS improved for 2 patients (7.5%), remained stable for 15 (55.6%), and deteriorated for 10 (37.0%): for patients without surgical intervention, 12 (57.1%) remained stable and 9 (42.9%) deteriorated.
During the study, MPNST was suspected in 3 patients (not previously treated). The mean time interval from baseline FDG-PET/CT and MPNST diagnosis was 759 days (range: 349–1074). One patient (aged 29 at enrollment) had a thoracic lesion undetected by FDG-PET/CT at baseline. The other 2 patients with ANNUBP (aged 14 and 17 at enrollment) had lesions with suspicious uptake on baseline FDG-PET/CT, which were deemed nonmalignant upon biopsy; 1 had an abdominal lesion with an SUVmax of 7.9, and one had a retroperitoneal lesion with an SUVmax of 6.9. During the study, these 2 patients were deemed at high risk of malignant transformation due to increased SUVmax on FDG-PET/CT scans versus baseline, and an increase in tumor dimensions on follow-up MRI. The patient with the thoracic lesion developed a large symptomatic pleural effusion with dyspnea, and MRI revealed substantial progression of mediastinal disease. Neither patient had received treatment for ANNUBP ahead of study enrollment and both underwent biopsy or partial resection while receiving selumetinib.
During the study, 4 patients died, and 1 patient was lost to follow-up. One patient died due to respiratory infection associated with pretreatment tumor progression, and 3 due to MPNST.
Discussion
Real-world data on the use of MEK inhibitors, including selumetinib, for patients with NF1-PN are sparse but necessary to provide a nuanced perspective from real-world clinical practice.
This case series is a large cohort, representative of the heterogeneity of the real-world clinical population of neurofibromatosis. Although 52 cases were confirmed to have NF1, 1 patient had suspected confined mosaic NF1 and 1 had NF2-SWN. Loss-of-function variants of the NF2 gene cause the autosomal dominant disease NF2, which is less common than NF1, is typically adult-onset, and is treated with alternative agents to selumetinib.3,18 Mosaicism involving NF1 no longer meets the diagnostic criteria for NF1 as per the most recent international consensus recommendation, so the patient with suspected confined mosaic NF1 in this cohort was considered to have sphenoid wing dysplasia and PN.19
Common manifestations associated with NF1 were observed here as expected, including cutaneous lesions, OPG, scoliosis, neurological, and neurodevelopmental disorders.20 Additionally, it has been established that patients with NF1 are at an increased risk of developing cancers, primarily MPNST (2%–16% of patients with NF1), glioma, and breast cancer, which were observed in patients in this cohort.20–22
Approximately 15% of patients in this study transitioned from childhood to adulthood. Although selumetinib is currently indicated for use in pediatric patients with symptomatic, inoperable NF1-PN,9,11,12,23 the continuation of treatment observed in this real-world study reflects the need for long-term treatment options that can continue into adulthood or be initiated in adulthood in cases of late diagnosis. Although most patients are diagnosed in childhood,21 diagnostic delays can have serious repercussions, impacting QoL and disease intervention options.24
Diagnosis
Some symptoms of NF1 are age-dependent, and less than half of all sporadic cases (aged ≤1 year) meet the established criteria for NF1 diagnosis, which may lead to diagnostic delay.4,5 For these patients, genetic testing contributes to expediting diagnosis, detecting variants associated with severe disease, differentiating NF1 from Legius syndrome, and characterizing mosaicism.1,19 Microdeletions in the NF1 gene, which are associated with an increased risk of malignancies and MPSNT, were detected in 3 patients in this cohort. Each patient experienced stable disease and subjective improvement with selumetinib, with 1 patient also undergoing partial excision surgery.
The use of MRI at diagnosis can serve to determine the operability of superficial PN, provide a reference examination, or detect internal (unknown) PN lesions to inform monitoring or treatment, although for younger patients, MRI may not be a desirable assessment option.1,25 It is unclear whether the early diagnosis of PN modifies outcomes for all patients, but for symptomatic PN, diagnosis and treatment are more urgent. Our observations support that the route to diagnosis may differ on a case-by-case basis and that the use of genetic testing and/or MRI could improve and accelerate diagnosis regardless of patient age.
Alternatively, diagnosis of NF1 can be made based on medical history and clinical evaluation, without these tools.4,21 Most of this cohort (88.9%) had cutaneous manifestations (including cutaneous neurofibromas, café-au-lait spots, and intertriginous freckling) and 55.6% displayed ophthalmologic alterations. Additional features included musculoskeletal abnormalities, attention or learning difficulties (which may impact treatment adherence), and seizures. These clinical observations describe the heterogeneity of NF1 presentations that could improve disease awareness to facilitate diagnosis and monitoring via regular assessment of disease manifestations, symptoms, and ophthalmologic parameters. It is unclear whether the early diagnosis of PN modifies outcomes for all patients, but for symptomatic PN, diagnosis and treatment are more urgent.
Treatment
Until recently, NF1-PN has been exclusively treated surgically because conventional chemotherapy was associated with poor outcomes, although many agents have been trialed.26,27 Although the mainstay of treatment,28 surgery is not appropriate for approximately 50% of cases, so targeted treatments have been developed.9–12,23,26 This large cohort had a long follow-up period, offering real-world observations and valuable perspective on the effectiveness of selumetinib for patients with NF1-PN. The disease was well controlled for most of these patients, although the full extent of response could be more thoroughly assessed with more sensitive volumetric response criteria.
Surgery is a key treatment option for PN,26–28 and in this cohort, 8 patients underwent surgery before initiating pharmacological treatment, 5 while receiving selumetinib, and 6 both before and during treatment. Surgical intervention, particularly if needed before initiating pharmacological treatment, may indicate that the patient had more aggressive disease or a phase of rapid growth, which increases the risk of malignant transformation. Typically, clinicians in our center encounter patients who either progress rapidly or slowly on treatment. For those who experience slow disease progression, surgical intervention may complement pharmacological treatment to enhance treatment outcomes. In cases of rapid progression, surgery may help reduce the risk of malignant transformation or treat MPNST. In this study, surgical intervention may have been critical to achieving disease stability for some patients; stable disease was achieved by 10 (52.6%) patients who underwent surgical intervention and only 1 patient with MPNST experienced disease progression following surgery. Additionally, for patients who received selumetinib but did not undergo surgery, disease stability was achieved by 19 (54.3%) of patients, demonstrating that NF1-PN can be effectively managed with pharmacological intervention alone for some patients. In this cohort, only 11 patients underwent surgery during treatment, which should be taken into consideration when interpreting these results. Equally, in patients treated early, surgery may become unnecessary. Identifying those patients with the greatest clinical need for intervention is paramount; particularly for individuals with high risk of progression or who have already progressed to malignant disease.
For individuals for whom surgical intervention is not clearly beneficial, these data suggest that long-term selumetinib treatment effectively controls disease. Despite adverse events being recorded, patients in this study were effectively managed with dose interruption or reduction; only 2 discontinued treatment due to severe paronychia. The data presented here suggest that the likelihood of further dose reductions increases with every subsequent dose reduction, which has not been demonstrated in previous clinical or real-world studies but should be acknowledged for patients on long-term treatment.
Persistence was high in this cohort (96.3%, 52/54; median treatment duration 38.3 months), which demonstrates that with efficient patient management and a dedicated multidisciplinary team, treatment can be managed per individual case, and treatment persistence optimized.29
Patient Monitoring
Imaging techniques are not routinely used in all practice scenarios, largely due to limited availability or limited interpretability in less specialized centers. Imaging techniques, including FDG-PET/CT and MRI have, however, shown utility in the management of patients with NF1-PN. Practice guidelines are constantly evolving, but MRI has been shown to facilitate the detection of PN, and FDG-PET/CT has proved valuable for disease monitoring and directing biopsy when necessary, which could contribute to risk assessment for progression to MPNST and promote early intervention.21,27,30
Diagnosing MPNST can be challenging, but establishing the presence of PN can alert clinicians to the risk of MPNST, which is associated with poor outcomes and fatality.20,21 In this cohort, MRI was effectively used in disease staging to detect the risk of progression. Of the 3 cases of MPNST observed in this study, 2 progressed from ANNUBP and 1 from a suspicious mediastinal lesion that exhibited growth on treatment. These could be considered pertinent clinical markers for consideration as potential warning signs for MPNST. Although the incidence of MPNST in NF1 is low, pharmacological agents are ineffective and surgery is the only effective treatment option, so it is important for clinicians to be able to identify those with high-risk or stable disease.20,21,27 MRI can be used to assess the extent of lesions to identify an increased risk of MPNST.21,27,30 This study demonstrates that imaging techniques can effectively identify patients at risk of MPNST to support direct surgical removal.
In this cohort, suspicious lesions were removed before selumetinib treatment commenced and, as has proven valuable in previous studies, FDG-PET/CT is therefore essential to assess these lesions and to inform the intervention strategy for each patient.30
We also observe that MRI is not the only indicator of risk of progression in these patients. Superficial lesions can be assessed subjectively, requiring less costly and more widely available tools than MRI, and could speed up diagnosis and the recognition of high-risk patients. Our findings indicate that whole-body MRI is not required for every patient but should be considered over conventional localized MRI for patients at higher risk of malignant transformation or with more extensive or widespread PN burden. This is echoed by recent practice guidelines, which emphasize that the frequency of MRI should be adjusted based on the extent of surgical resection and the aggressiveness of the disease.28,31
Multidisciplinary care is of great importance when coordinating clinical and radiological assessments, pharmacological and surgical intervention, and effective patient follow-up.26 In this case series, we see a multidisciplinary approach being successfully implemented in a real-world clinical setting, which could provide guidance for other institutions.
Patient Characteristics
The cohort described here includes a lower proportion of pediatric cases that remained pediatric through to study completion (53.7%) than previously published case series.32 Many studies exclusively enroll pediatric patients and the average proportion of pediatric cases observed in a meta-analysis of 8 studies was 80.6%.32 There remains a need for data in older patient populations, where the risk of malignancy increases, and to refine long-term patient management. Additionally, this is the first report of real-world evidence for persistence with selumetinib in a partially adult population. As the management of NF1 evolves and the patient journey improves, it becomes more important to account for longer-term perspectives on clinical management.4
These insights may be particularly valuable for less specialized institutions, and to build a real-world picture of the effectiveness and safety of selumetinib for NF1-PN.
Study Limitations
Although the data presented here represent a large heterogeneous real-world NF1-PN cohort, case series are associated with some key limitations. These data are from a single center, so the generalizability of these findings may be limited. Clinical records were retrospectively analyzed, and therefore, cause and effect were not able to be established.33
Although imaging data are valuable, precise radiological data interpretation varies, and high-risk cases may remain undetected. In previously published reviews considering the role of FDG-PET/CT imaging techniques in detecting malignancy versus benign disease, the SUVmax values significantly overlapped, making clear differentiation of malignancy difficult.34 This study spanned up to 5 years of follow-up, but longer-term case studies are yet to be reported. This is particularly pertinent for pediatric patient populations, where disease stability is paramount, and longer follow-ups will provide valuable information about long-term disease management.
Notably, this study was conducted in an institute in Portugal where the national healthcare system ensures equal access to treatment, which may not be generalizable to other countries despite the necessity for treatment access, regardless of financial circumstances.
Conclusions
This case study series illustrates the heterogeneous nature of NF1 manifestations, disease course, and treatment outcomes. Sustained disease control is achieved for a subset of patients, and surgical intervention improves disease stability when implemented in certain patients. Although the diagnosis of NF1 does not require imaging per se, this case series highlights its importance for the detection and characterization of PN and MPNST, facilitating effective disease management as early as possible in the patient journey. Additionally, with patients of various ages, long-term challenges associated with patient management, such as resource and budget allocation for treatment, monitoring, and complication resolution, can be achieved. This case series supports the need for a multidisciplinary approach to disease management in an expert center. Even with large patient cohorts, efficient resource management and a dedicated team and institution can effectively manage and treat patients with a personalized approach to individual disease courses. Both imaging and clinical data suggest that selumetinib improved disease outcomes, and persistence remains high despite the incidence of adverse events, reflecting both treatment efficacy and a favorable treatment perception for patients and caregivers.
Supplementary Material
Acknowledgments
Clinical data were contributed by the authors of this study and a wider team, including Cláudia Faria, João Campagnolo, Emanuel Gouveia, Carmo Martins, Sofia Fernandes, Miguel Magalhães, Cecília Moura, Sofia Vinhais, João Conceição e Silva, Filipa Santos, Hugo Vasques, Cristina Amaro Rita Couceiro, Maria Jesus Moura, Filipe Silva, Filipa Teixeira, and Ana Mendes. The authors would like to thank Dr Hazel Shepherd, DPhil, of Helix, OPEN Health Communications, London, UK for providing medical writing assistance, funded by Alexion, AstraZeneca Rare Disease, in accordance with Good Publications Practice (GPP 2022) guidelines, which can be found at https://www.acpjournals.org/doi/epdf/10.7326/M22-1460.
Contributor Information
João Passos, Department of Neurology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Marta P Soares, Department of Genetic Medicine, Centro Hospitalar Universitário Lisboa Norte, E.P.E., Lisbon, Portugal.
Duarte Salgado, Department of Neurology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal; Unit of Pediatric Neuro-Oncology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Sofia Nunes, Unit of Pediatric Neuro-Oncology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Daniela Cavaco, Department of Endocrinology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Pedro M Garrido, Department of Dermatology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Mónica Coutinho, Department of Radiology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Inês Patrocínio Carvalho, Department of Nuclear Medicine, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Miguel Vilares, Department of Head and Neck Surgery, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Mafalda Ferreira, Department of Neurology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Cristina Lacerda, Department of Oncology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Conflict of interest statement
J.P. reports receiving consulting, advisory, travel, and speaker fees from Alexion and AstraZeneca; M.P.S. reports receiving travel fees from CTF Europe; D.S. reports receiving services from Alexion and serves as the President of the Portuguese Association for Neuro-Oncology; P.M.G. reports receiving honoraria from Leo Pharma; M.V. serves as the President of the College of Maxillofacial Surgery and the Maxillofacial Surgery Society. S.N., D.C., M.C., I.P.C., M.F., and C.L. have no conflicts of interest to declare.
Funding
Editorial assistance was funded by Alexion, AstraZeneca Rare Disease. Data were collected with no additional source of funding during the course of clinical practice at the Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Authorship statement
J.P. contributed to study conceptualization, provision of resources, data curation, acquisition of funding, study validation, investigation, development of methodology, project administration and supervision, and review and editing of the manuscript; M.P.S. and P.M.G. contributed to the study investigation, development of methodology, and review and editing of the manuscript; D.S. contributed to the study conceptualization including methodology, supervised and contributed to project administration, data visualization, and drafted, reviewed, and edited the manuscript; S.N. and M.C. reviewed and edited the manuscript; D.C. contributed to the study investigation; I.P.C. and M.F. contributed to the study investigation, and review and editing of the manuscript; C.L. contributed to project administration, study investigation, and review and editing of the manuscript; M.V. contributed to the study conceptualization including methodology, supervised and contributed to project administration, contributed resource, conducted investigations and data curation, validation and visualization, and drafted, reviewed, and edited the manuscript.
Content Contributions
Medical writing support was provided by Dr Hazel Shepherd, DPhil of Helix, OPEN Health Communications, funded by Alexion, AstraZeneca Rare Disease. Alexion provided courtesy review; however, authors retained control and final authority of publication content and decisions, including the choice of journal.
References
- 1.Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al. , eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 2.Hirbe AC, Gutmann DH.. Neurofibromatosis type 1: A multidisciplinary approach to care. Lancet Neurol. 2014;13(8):834–843. [DOI] [PubMed] [Google Scholar]
- 3.Blakeley JO, Plotkin SR.. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBella K, Szudek J, Friedman JM.. Use of the National Institutes of Health Criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3 Pt 1):608–614. [DOI] [PubMed] [Google Scholar]
- 5.Kehrer-Sawatzki H, Cooper DN.. Challenges in the diagnosis of neurofibromatosis type 1 (NF1) in young children facilitated by means of revised diagnostic criteria including genetic testing for pathogenic NF1 gene variants. Hum Genet. 2022;141(2):177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yoo HK, Amin S, et al. Clinical and humanistic burden among pediatric patients with neurofibromatosis type 1 and plexiform neurofibroma in the USA. Childs Nerv Syst. 2022;38(8):1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher MJ, Blakeley JO, Weiss BD, et al. Management of neurofibromatosis type 1-associated plexiform neurofibromas. Neuro Oncol. 2022;24(11):1827–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergoug M, Doudeau M, Godin F, et al. Neurofibromin structure, functions and regulation. Cells. 2020;9(11):2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Selumetinib (Koselugo) summary of product characteristics; 2023. https://www.ema.europa.eu/en/documents/product-information/koselugo-epar-product-information_en.pdf. Accessed January 23, 2024. [Google Scholar]
- 10.AstraZeneca. Koselugo approved in China for paediatric patients with neurofibromatosis type 1 and plexiform neurofibromas; 2023. https://www.astrazeneca.com/media-centre/press-releases/2023/koselugo-approved-in-china-for-paediatric-patients-with-neurofibromatosis-type-1-and-plexiform-neurofibromas.html. Accessed January 23, 2024. [Google Scholar]
- 11.AstraZeneca. Selumetinib granted orphan drug designation in Japan for neurofibromatosis type 1; 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/selumetinib-granted-orphan-drug-designation-in-japan-for-neurofibromatosis-type-1.html#. Accessed January 23, 2024. [Google Scholar]
- 12.U.S. Food and Drug Administration. FDA approves selumetinib for neurofibromatosis type 1 with symptomatic, inoperable plexiform neurofibromas; 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selumetinib-neurofibromatosis-type-1-symptomatic-inoperable-plexiform-neurofibromas. Accessed January 23, 2024. [Google Scholar]
- 13.Gross AM, Widemann BC.. Clinical trial design in neurofibromatosis type 1 as a model for other tumor predisposition syndromes. Neurooncol Adv. 2020;2(suppl 1):i134–i140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. ; European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. ; REiNS International Collaboration. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov. PASS of paediatric patients initiating selumetinib; 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05388370. Accessed January 23, 2024. [Google Scholar]
- 18.Evans DG. NF2-Related Schwannomatosis. In: Adam MP, Feldman J, Mirzaa GM, et al. , eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 19.Legius E, Messiaen L, Wolkenstein P, et al. ; International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC). Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet Med. 2021;23(8):1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huson SM, Compston DA, Harper PS.. A genetic study of von Recklinghausen neurofibromatosis in South East Wales. II. Guidelines for genetic counselling. J Med Genet. 1989;26(11):712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart DR, Korf BR, Nathanson KL, Stevenson DA, Yohay K.. Care of adults with neurofibromatosis type 1: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20(7):671–682. [DOI] [PubMed] [Google Scholar]
- 22.Uusitalo E, Rantanen M, Kallionpaa RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. [DOI] [PubMed] [Google Scholar]
- 23.European PMC. Selumetinib (Koselugo): CADTH Reimbursement Recommendation: Indication: For the treatment of pediatric patients aged 2 years and above, with neurofibromatosis type 1 who have symptomatic, inoperable plexiform neurofibromas; 2023. https://europepmc.org/article/NBK/NBK594382. Accessed January 23, 2024. [PubMed] [Google Scholar]
- 24.Cnossen MH, Smit FJ, de Goede-Bolder A, et al. Diagnostic delay in neurofibromatosis type 1. Eur J Pediatr. 1997;156(6):482–487. [DOI] [PubMed] [Google Scholar]
- 25.Miller DT, Freedenberg D, Schorry E, et al. ; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143(5):e20190660. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong AE, Belzberg AJ, Crawford JR, Hirbe AC, Wang ZJ.. Treatment decisions and the use of MEK inhibitors for children with neurofibromatosis type 1-related plexiform neurofibromas. BMC Cancer. 2023;23(1):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellerino A, Verdijk RM, Nichelli L, et al. Diagnosis and treatment of peripheral and cranial nerve tumors with expert recommendations: An EUropean Network for RAre CANcers (EURACAN) initiative. Cancers (Basel). 2023;15(7):1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross AM, Dombi E, Wolters PL, et al. Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro Oncol. 2023;25(10):1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meany H, Dombi E, Reynolds J, et al. 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) evaluation of nodular lesions in patients with Neurofibromatosis type 1 and plexiform neurofibromas (PN) or malignant peripheral nerve sheath tumors (MPNST). Pediatr Blood Cancer. 2013;60(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahlawat S, Blakeley JO, Langmead S, Belzberg AJ, Fayad LM.. Current status and recommendations for imaging in neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Skeletal Radiol. 2020;49(2):199–219. [DOI] [PubMed] [Google Scholar]
- 32.Sharawat IK, Panda PK, Sihag RK, Panda P, Dawman L.. Efficacy and safety profile of selumetinib in symptomatic inoperable plexiform neurofibromas. J Neurosurg Sci. 2022;66(6):501–510. [DOI] [PubMed] [Google Scholar]
- 33.Nissen T, Wynn R.. The clinical case report: A review of its merits and limitations. BMC Res Notes. 2014;7:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tovmassian D, Abdul Razak M, London K.. The role of [18F]FDG-PET/CT in predicting malignant transformation of plexiform neurofibromas in neurofibromatosis-1. Int J Surg Oncol. 2016;2016:6162182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.