Abstract
Intestinal webs are either congenital or acquired. There are few reported cases of either chemotherapy or nonsteroidal anti‐inflammatory medications leading to acquired intestinal webs in adults. There are limited descriptions of endoscopic interventions used for therapy of numerous duodenal webs in pediatrics. Here, we describe a 32‐month‐old patient undergoing chemotherapy who had multiple duodenal webs. The patient was diagnosed after failed gastrojejunostomy tube exchange via atypical contrast filling pattern and direct visualization with endoscopy. This patient likely has acquired duodenal webs from the combination of nonsteroidal anti‐inflammatory drug containing chemotherapy treatment and repeated tube trauma. Treatment involved serial esophagogastroduodenoscopy with a combination of endoscopic therapy including balloon dilation and incisional therapy with insulated‐tip knife and cautery scissors. The patient now tolerates G‐tube feedings.
Keywords: duodenal stenosis, intestinal atresia, membranectomy, NSAID‐induced stenosis
1. INTRODUCTION
Intestinal webs are a “mild form” of intestinal atresia. Webs are either congenital or acquired and can be located anywhere in the small intestine. Congenital webs are rare, 1 and arise due to failure of duodenal recanalization during weeks 8–10 in embryologic development. 2 Webs consist of mucosa and submucosa (lacks muscular layer) and can have a “wind‐sock” appearance due to distal ballooning of the web during peristalsis. 3 Presentation is often during transition from breastmilk/formula to solids. Partial duodenal obstruction can result in diagnostic delay. Acquired webs have been reported in adults taking nonsteroidal anti‐inflammatory medicines (NSAIDS) or chemotherapeutic agents. 4 , 5 Diagnosis is made by X‐ray, ultrasound, upper GI series, or endoscopy. 6 , 7 Plain films were diagnostic in only 58% of cases with “double‐bubble” sign. Contrast studies increased diagnostic rate to 71%. 8 Therapy for duodenal webs includes repeat dilations and/or excisional therapy (membranectomy). 9 , 10
2. CASE REPORT
A 32‐month‐old boy with disseminated atypical teratoid/rhabdoid tumor treated with antiangiogenic metronomic chemotherapy presented for gastrojejunostomy (GJ) tube replacement. Routine interventional radiology exchange of a 14 French GJ tube (Applied Medical Technology) was unsuccessful due to the inability to advance the tube over‐a‐wire beyond the second portion of the duodenum. Contrast injection during the procedure demonstrated an atypical contrast filling pattern in the duodenum raising suspicion for duodenal webs (Figure 1). Upper endoscopy confirmed seven duodenal webs and allowed for direct replacement of the 14 French GJ tube (Figure 2).
Figure 1.
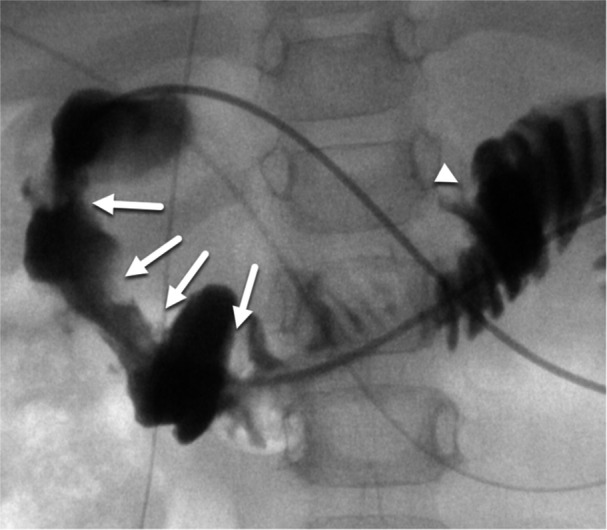
Contrast injection performed during fluoroscopically guided GJ‐tube exchange at time of web diagnosis. Multiple circumferential webs are present in the proximal duodenum (white arrows) resulting in luminal narrowing. Normal mucosal folds are present in the distal duodenum and jejunum (white arrowhead). GJ, gastrojejunostomy.
Figure 2.
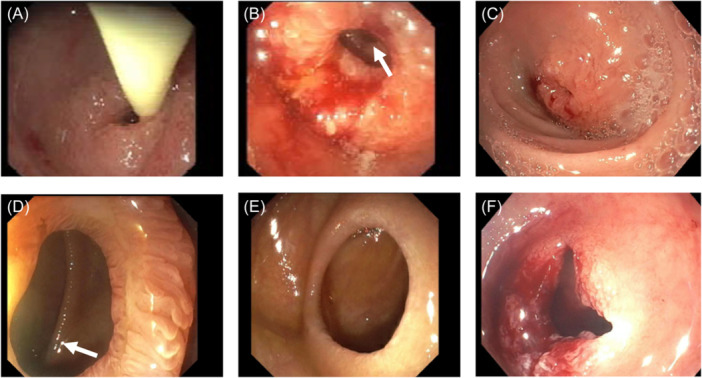
Diagnostic and therapeutic EGDs. (A) Avanos 6 French feeding tube passing through first web (EGD 1). (B) Visualization of web 2 and 3 (white arrow) on EGD 1. (C) Repeat EGD 2, revisualization of web 1, with recurrence after first dilation. (D) Visualization of web 5 with small ulcer and web 6 (white arrow) on EGD 2. (E) Web 6 with lateral wind‐sock appearance on EGD 2. (F) Web 1 after repeat dilation and incisional therapy on EGD 3. EGD, esophagogastroduodenoscopy.
This patient had a normal pyloric ultrasound at 3 months and normal upper GI series at 8 months old. These tests were obtained due to persistent vomiting leading to dehydration and weight loss. Persistent symptoms prompted brain imaging that revealed an obstructive brain mass. Initial GJ was placed at 9 months old after craniotomy for support through chemotherapy. This was followed by numerous uneventful GJ changes. Retrospective review of the GJ exchange images 4 months before web diagnosis revealed numerous nonobstructive duodenal webs. At the time of definitive diagnosis, webs had progressively narrowed. His medication regimen at that time included: topotecan, omeprazole, famotidine, diphenhydramine, and olanzapine. Chemotherapy before this included celecoxib, thalidomide, and fenofibrate. He was on celecoxib for 3 months. Duodenal biopsies revealed focal, mildly active inflammation with villous blunting and reactive epithelial changes thought to be secondary to the combination of chemotherapy and tube trauma during peristalsis.
Treatment involved serial esophagogastroduodenoscopy (EGD) with balloon dilation and web tissue cutting using insulated tip knife and cautery scissors via standard gastroscope (Olympus Inc, GIF‐H190, Olympus IT2 knife [KD‐611L], Olympus SB Knife JR cautery scissors [MD‐47703L]). Webs 1–5 required treatment, while webs 6–7 were patent to 10–14 mm, so they were not treated. For both insulated tip knife and cautery scissors, we used recommended manufacturer settings (detailed in Figure 3B).
Figure 3.
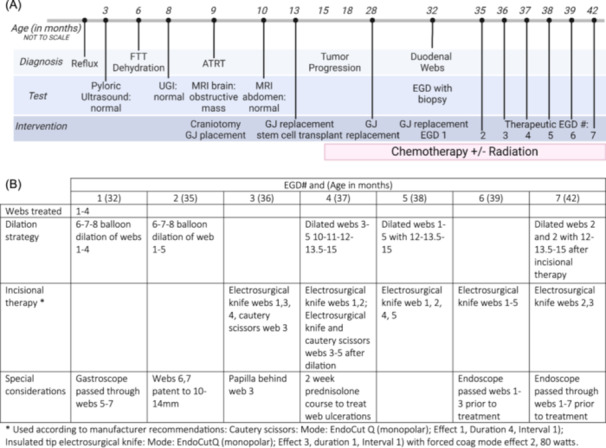
(A) Timeline of diagnoses, tests, and interventions from birth to 42 months old. (B) Therapeutic EGD settings and details. Figure made with Biorender.com. ATRT, atypical teratoid/rhabdoid tumor; EGD, esophagogastroduodenoscopy; FTT, failure to thrive; GJ, gastrojejunostomy; MRI, magnetic resonance imaging; UGI, upper gastrointestinal series.
He now tolerates G‐tube feedings and purees without emesis (>2 years since last endoscopic intervention). Timeline of GI and oncology diagnoses and interventions summarized in Figure 3.
3. DISCUSSION
Chronic NSAIDs or chemotherapy can lead to duodenal webs in adults. 4 , 5 There are no reports of celecoxib (NSAID) as a chemotherapeutic agent as the source of duodenal webs in pediatrics. The mechanism underlying small intestine mucosal pathology by NSAIDs is via enterocyte absorption of NSAIDs leading to the uncoupling of mitochondrial oxidative phosphorylation. This increases intestinal permeability, resulting in exposure to luminal contents, triggering neutrophil recruitment and activation, causing tissue inflammation. 11 If acute medication therapy or GJ‐tube presence alone initiated iatrogenic web formation in children, likely there would be numerous case reports of web formation. Therefore, we propose that the combination of NSAIDs, chemotherapy, and peristalsis around a GJ tube led to the development of seven consecutive duodenal webs in this patient. He was unlikely born with congenital webs, as he had a normal upper GI before GJ placement, prior GJ tube replacements with fluoroscopy were uneventful, and he had previously tolerated gastric feeds and transition from formula to solids.
The major duodenal papilla is frequently within or near intestinal webs because most webs are in the second portion of the duodenum. If there is bilious vomiting, or bile visualized in the proximal duodenum on endoscopy, the papilla is likely proximal to the web. During excisional therapy, it is necessary to assess papilla location and cut away from it to avoid injury. Typically, cutting toward the lateral duodenal wall minimizes risk of papillary trauma or iatrogenic pancreatitis. In a prior case report, endoscopists treating a congenital duodenal web with a poorly visualized papilla made a small incision in the center of the web, then balloon dilated the opening. Even with a 5.9 mm gastroscope, directly visualization of the papilla was not possible, so they removed the web piecemeal to avoid blindly damaging the papilla. 12
There are few reports of insulated‐tip electrosurgical knife endoscopic incision alone in pediatrics, 13 , 14 and limited literature describing combined balloon and endoscopic excisional therapy. 12 Electrosurgical knife therapy is used for endoscopic submucosal dissections, particularly in the setting of dysplastic polyp resections. The electrosurgical knife has a ceramic‐coated tip to minimize transfer of energy to distally surrounding tissues. In the case of a duodenal web that is comprised of only submucosa and mucosa, we chose the electrosurgical insulated tip knife with the intention of minimizing thermal transfer to the major duodenal papilla and duodenal wall distal to knife tip.
Due to the need for numerous web resections in a thrombocytopenic and leukopenic patient, we performed seven therapeutic EGDs. At the completion of each EGD, the GJ tube was replaced under fluoroscopic guidance by IR. Luminal contrast visualized using fluoroscopy was utilized to confirm that there was no perforation. Based on web appearance and nearly complete recurrence after balloon dilation alone after the first EGD (Figure 2C), we combined excisional therapy (using electrosurgical insulated tip knife and cautery scissors) with balloon dilation for a more durable result for subsequent EGDs. Electrocautery scissors allow for tissue grasping and creation of deeper, straighter incisions. However, web and patient orientation limited its sole use, which is why therapy in this case relied on careful electrosurgical knife treatment. This cautious approach helped limit thermal delivery and allowed for better bleeding control during web excisions. Argon plasma coagulation was used in each EGD for bleeding control. In this patient, the papilla was located distal to the 3rd web along the medial wall of the duodenum, so care was taken to cut laterally when treating the 3rd web. Papilla location was determined based on bile outflow and use of patient repositioning. Timing of repeat endoscopies was dependent on recurrence of G‐tube feeding intolerance, which was a clinical symptom of web recurrence (often 4–6 weeks after prior EGD, see Figure 3). After the final therapeutic intervention, the duodenal lumen was patent to 15 mm and the patient is tolerating full G‐tube and oral feeds.
To our knowledge, there are no reports using combined balloon dilation, endoscopic electrosurgical knife, and cautery scissors to treat multiple or dilation‐refractory duodenal webs in children. We recommend consideration of combined approach, repeat endoscopic therapy in future cases of pediatric duodenal webs before surgical bowel resection. Finally, it is uncommon to be unable to replace a GJ tube unless there is a mechanical complication. 15 Therefore, failed GJ exchanges should be evaluated for underlying structural anomalies.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Verbal informed patient consent has been obtained; they are aware of and agree with the intent for this article to be published.
ACKNOWLEDGMENTS
The authors have no funding to report.
Tjaden NEB, Acord M, Minturn J, Allukian M, Mamula P. Thirty‐two‐month‐old with multiple duodenal webs diagnosed after failed gastrojejunostomy exchange successfully treated with combination endoscopic therapy. JPGN Rep. 2024;5:483‐487. 10.1002/jpr3.12107
REFERENCES
- 1. Ward K, Harbie KA, Islam S. Case report of duodenal obstruction from multiple webs. J Pediat Surg Case Rep. 2016;8:30‐33. [Google Scholar]
- 2. Gershon MD. Endodermal Derivatives, Formation of the Gut and Its Subsequent Rotation. Columbia University; 2004. https://www.columbia.edu/itc/hs/medical/humandev/2004/Chapt18-Endoderm.pdf
- 3. Sugandhi N, Agarwala S. E27 Duodenal atresia. In: Carachi R, Agarwala S, Bradnock TJ, Lim Tan H, Cascio S Basic Techniques in Pediatric Surgery: An Operative Manual. Springer Berlin Heidelberg; 2013:322‐324. [Google Scholar]
- 4. Lim E. Gastrointestinal: ibuprofen‐induced duodenal stricture resulting in malnutrition. J Gastroenterol Hepatol. 2010;25:1330. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Li JZ, Yang YH, Huang XL, Wang Y, Wu B. Intestinal atresia following chemotherapy, presenting as superior mesenteric artery syndrome: a case report. Mol Clin Oncol. 2017;7:543‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pittschieler K, Gentili L, Pittschieler K, Gentili L. Endoscopic diagnosis of duodenal stenosis. J Pediatr Gastroenterol Nutr. 1997;24:359‐360. [DOI] [PubMed] [Google Scholar]
- 7. Nicholson MR, Acra SA, Chung DH, Rosen MJ. Endoscopic diagnosis of duodenal stenosis in a 5‐month‐old male infant. Clin Endosc. 2014;47:568‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailey PV, Tracy, Jr. TF , Connors RH, Mooney DP, Lewis JE, Weber TR. Congenital duodenal obstruction: a 32‐year review. J Pediatr Surg. 1993;28:92‐95. [DOI] [PubMed] [Google Scholar]
- 9. Poddar U, Jain V, Yachha S, Srivastava A. Congenital duodenal web: successful management with endoscopic dilatation. Endosc Int Open. 2016;04:E238‐E241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibarguen‐Secchia E. P0764 endoscopic resection of duodenal web. J Pediatr Gastroenterol Nutr. 2004;39:S347. [Google Scholar]
- 11. Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non‐steroidal anti‐inflammatory drug (NSAID)‐induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48:107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beeks A, Gosche J, Giles H, Nowicki M. Endoscopic dilation and partial resection of a duodenal web in an infant. J Pediatr Gastroenterol Nutr. 2009;48:378‐381. [DOI] [PubMed] [Google Scholar]
- 13. Lee SS, Hwang ST, Jang NG, et al. A case of congenital duodenal web causing duodenal stenosis in a down syndrome child: endoscopic resection with an insulated‐tip knife. Gut Liver. 2011;5:105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okamatsu T, Arai K, Yatsuzuka M, et al. Endoscopic membranectomy for congenital duodenal stenosis in an infant. J Pediatr Surg. 1989;24:367‐368. [DOI] [PubMed] [Google Scholar]
- 15. Koo KSH, Reis J, Manchester J, Chaudry G, Dillon B. Effects of mechanical complications on radiation exposure during fluoroscopically guided gastrojejunostomy exchange in the pediatric population. Dysphagia. 2018;33:251‐257. [DOI] [PubMed] [Google Scholar]


