Abstract
Peptide antibiotics are widespread in nature and, by providing a rapid first line of defense, may be key players in the innate immune system. Although epithelia are the main barriers shielding the internal environment from microorganisms, the role for peptide antibiotics in epithelial protection is unclear. We recently reported that the human cationic antimicrobial protein hCAP18, the precursor of the antimicrobial peptide called LL-37, is not expressed by normal human keratinocytes but is induced in various inflammatory skin disorders. In the present study we demonstrate that hCAP18 is consistently expressed at both mRNA and protein levels in squamous epithelia of the mouth, tongue, esophagus, cervix, and vagina in humans. The gene for hCAP18 contains promoter elements that are potentially regulated by interleukin-6, and our data further show a colocalization between interleukin-6 and hCAP18 expression in these tissues. Our finding that hCAP18 is widely produced in squamous epithelia suggests a role for this peptide in epithelial antimicrobial defense. Furthermore, colocalization with interleukin-6 indicates a potential local mechanism for the upregulation of hCAP18 at the epithelial surfaces.
Peptide antibiotics may serve a key protective role in the host defense. They are participants in the innate immune system (nonadaptive immune system), acting as effector molecules with the capacity to kill a broad spectrum of microorganisms (6). In contrast to highly specific adaptive immunity, the innate immune system provides a rapid and nonspecific response and thereby contributes to the first line of defense. The first peptide antibiotics were discovered in the 1980s when cecropins were isolated from insects (25) and defensins were isolated from rabbit macrophages (24). Numerous antimicrobial peptide antibiotics occur in nature, and over a dozen have been identified in humans, including several salivary histatins, lactoferricin, six α-defensins, two β-defensins, and the human cationic antimicrobial protein hCAP18.
hCAP18, the C-terminal domain of which is called LL-37, has been isolated from specific granules of human neutrophil granulocytes and is structurally distinct from the defensins (9, 15). hCAP18 belongs to the cathelicidin family of proteins characterized by a conserved cathelin proregion and a variable antibacterial peptide in the C-terminal domain. The gene for hCAP18, named CAMP, is the only member of the cathelicidin family so far identified in humans. CAMP appears to have a restricted spatial expression pattern and has thus far been reported to be produced constitutively only in bone marrow, in testis, and in airway epithelium and to be upregulated in skin epithelium in association with inflammation (1, 5, 12). The promoter region of the CAMP gene contains potential binding sites for the transcription factors, the acute-phase response factor, and the nuclear factor for interleukin-6 (IL-6) (15). IL-6 regulates the activation of these two transcription factors, indicating that this cytokine may play an important role in modulating CAMP gene expression (2, 3). IL-6 is a multifunctional cytokine involved in various inflammatory responses and is consistently found in association with infectious processes (22).
Epithelia serve a critical role in separating the organism from the environment. In addition to providing fundamental physical and mechanical shields, epithelia function as active immunological organs by presenting antigens and producing cytokines (7), and epithelia are, in fact, able to produce antibacterial peptides (5, 16, 23). Thus, different members of the defensin family are encountered in several human epithelia and are induced by human keratinocytes in culture (16, 19, 30); a recent study by Bals et al. described the expression of hCAP18 in airway epithelium (5). We have previously shown that hCAP18 is produced in human epidermis in association with a variety of inflammatory disorders (12). These findings led us to suggest that hCAP18, although not constitutively expressed in the epidermis, is induced nonspecifically in response to inflammation. The present study further delineates the expression of hCAP18 in nonkeratinizing human squamous epithelia, and we report here that hCAP18 was consistently expressed at both mRNA and peptide levels in squamous epithelia of the buccal mucosa, tongue, esophagus, cervix, and vagina. In all tissues the expression of hCAP18 colocalized with the immunoreactivity for IL-6, a finding which is noteworthy in view of the potential role for this cytokine in modulating hCAP18 gene expression. Our findings suggest the presence of a powerful antimicrobial defense system at the epithelial interfaces and a putative local mechanism for its regulation.
MATERIALS AND METHODS
Tissues.
Formalin-fixed and paraffin-embedded archival human tissues, including samples from the buccal mucosa (n = 3), tongue (n = 2), esophagus (n = 2), cervix (n = 5), and vagina (n = 3), were obtained from the Department of Pathology, Karolinska Hospital, Stockholm, Sweden. All material was obtained at surgery (Table 1).
TABLE 1.
Clinical patient data
| Tissuea | Histopathological evaluation | Associated condition | Sexb | Age (yr) |
|---|---|---|---|---|
| Tongue | Normal epithelia | Sjögren’s syndrome | M | 74 |
| Tonguea | Normal epithelia | Control, 4 mo after nonspecific ulcer | M | 75 |
| Buccal mucosa | Normal epithelia | Oral cavity tumor | F | 60 |
| Buccal mucosa | Normal epithelia | None | F | 70 |
| Buccal mucosa | Normal epithelia | None | F | 55 |
| Esophagusa | Mild esophagitis | Helicobacter gastritis | F | 20 |
| Esophagus | Normal epithelia | Gastric carcinoma | M | 60 |
| Cervix | Mild dysplasia | None | F | 30 |
| Cervixa | Severe dysplasia | Inflammation | F | 35 |
| Cervixa | Normal epithelia | Uterus myoma | F | 47 |
| Cervix | Normal epithelia | None | F | 43 |
| Cervix | Mild dysplasia | Inflammation | F | 35 |
| Vagina | Normal epithelia | None | F | 43 |
| Vagina | Hyperplastic epithelia | Inflammation | F | 42 |
| Vagina | Normal epithelia | None | F | 31 |
Tissues visualized in figures.
M, mule; F, female.
Preparation of RNA probes.
A 435-bp hCAP18 full-length cDNA (9) was subcloned in Bluescript KS 11 and, after linearization with BamHI and EcoRI, was used as a template for in vitro transcription to generate 35S-labeled antisense and sense probes. After transcription, the RNA probes were ultrafiltered (Micron 100; Amicon, Inc., Beverly, Mass.) before hybridization.
Northern analysis.
To ascertain the specificity of the probe derived from full-length hCAP18 cDNA, we performed a Northern analysis by using a multiple-tissue Northern blot containing poly(A)+ RNA from samples from the stomach, thyroid, spinal cord, lymph node, trachea, adrenal gland, and bone marrow (Clontech Laboratories). The quality of the blot was confirmed through hybridization with cDNA probes detecting ubiquitously expressed transcripts. Prehybridization and hybridization with a randomly 32P-labeled cDNA probe for hCAP18 were performed according to the manufacturer’s instructions. The blots were washed at 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at 65% and, after overnight autoradiography, were evaluated with a phosphorimager system (Fuji Bas 1000).
In situ hybridization.
In situ hybridization was performed essentially as described earlier (26). Briefly, 5-μm sections were hybridized overnight with 2.5 × 106 to 5.0 × 106 cpm of 35S-labeled RNA probes at 55°C. After hybridization, the slides were washed under stringent conditions that included incubation with 50 μg of RNase A (Sigma) per ml for 30 min at 37°C and were processed for autoradiography. Autoradiographic exposure was for 3 to 4 weeks.
Immunohistochemistry. (i) hCAP18.
Protein A-purified antibodies raised in rabbits against recombinant hCAP18 were used at a 1:500 dilution (27). Deparaffinized, rehydrated sections were treated with 1% H2O2 in methanol for 30 min at room temperature to quench endogenous peroxidase activity. After being rinsed in phosphate-buffered saline, the sections were microwave treated twice for 5 min. All sections were stained according to the indirect peroxidase method (17) by using a Vectastain ABC Elite Kit (Vector Laboratories) and following the manufacturer’s instructions. Control sections from the same tissues were similarly processed except that no hCAP18 antibody was added. As an additional control, we performed immunohistochemistry as described above with rabbit serum diluted 1:500 (Dako, Copenhagen, Denmark) instead of primary hCAP18 antibody.
(ii) Immunoadsorption of hCAP18.
To ascertain the specificity of the immunostaining, we performed immunoadsorption, adding 10 μl of the hCAP18 recombinant protein (27) to 150 μl of a 1:500 dilution of anti-hCAP18 antibody. The final concentration of the hCAP18 recombinant peptide in the reaction mixture was 33 μg/ml. The mixture was preincubated at 4°C overnight with gentle shaking prior to immunohistochemical analyses according to the protocol described above. Control sections were processed according to the same protocol except that no peptide was added to adsorb the antibody.
(iii) IL-6.
A monoclonal IL-6 antibody diluted 1:50 (Janssen, Beerse, Belgium) (8) was used for the immunostaining on serial sections from the same tissues. The sections were stained according to the indirect peroxidase method described above.
RESULTS
Probe specificity.
Northern blot analysis confirmed the specificity of the hCAP18 probe. A single prominent message of less than 1 kb was detected specifically in bone marrow (Fig. 1).
FIG. 1.
Autoradiogram of a multiple tissue Northern blot hybridized with a 0.4-kb CAP18 cDNA fragment. The sizes of the RNA markers are indicated on the left. A single transcript of less than 1 kb was detected specifically in bone marrow, in agreement with published data.
hCAP18 mRNA and peptide are expressed in human squamous epithelia.
All tissues were reviewed by an experienced pathologist and were evaluated as listed in Table 1. In all tissues there was a positive signal upon in situ hybridization for hCAP18 mRNA in epithelial cells (Fig. 2B and 4B and D). In most tissues the signal for hCAP18 mRNA was more prominent in the basal layers of the epithelium and decreased toward the surface (Fig. 2B and 4B), but in cervical tissues found to be dysplastic and inflammatory the strongest expression of hCAP18 mRNA was seen in the upper part of the epithelium toward the surface (Fig. 4D). Sections hybridized with sense RNA probe showed a only background autoradiographic signal (Fig. 2D).
FIG. 2.
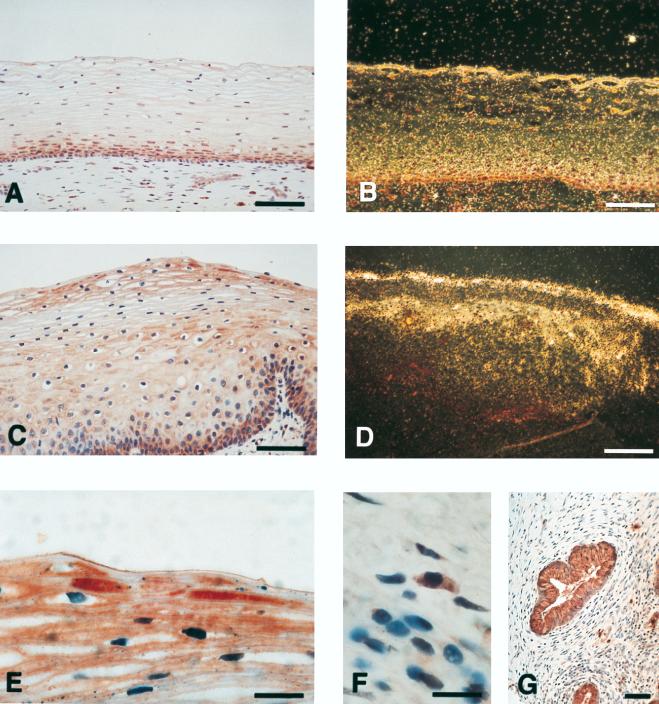
hCAP18 is expressed in the squamous epithelium of the tongue and colocalizes with immunoreactivity for IL-6. (A) Section of tongue demonstrating positive immunostaining for hCAP18 in the epithelium. Immunoreactivity (red precipitate) is most pronounced in the basal cell layers, but scattered immunopositive cells can also be seen in the suprabasal layers. (B) Section from the same tissue hybridized with antisense 35S-labeled cRNA probe for hCAP18 mRNA. Intense autoradiographic signal for hCAP18 mRNA (appears as white grains under dark-field illumination) is seen in the lower portion of tongue epithelium. (C) Immunoreactivity for IL-6 is seen in tongue epithelium with the same pattern as for hCAP18 and in submucosal blood vessels. (D) Hybridization with the control sense probe for hCAP18 lacks the autoradiographic signal for hCAP18 mRNA. The shiny appearance is due to the autofluorescence of the tissue. Bars, 100 μm.
FIG. 4.
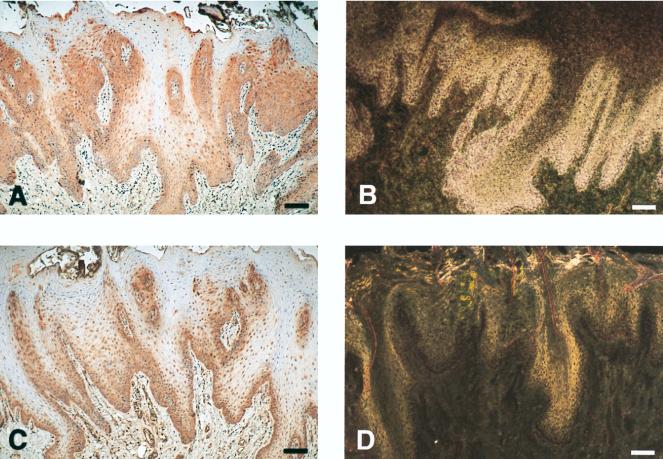
In the dysplastic and inflammatory cervix, hCAP18 is expressed in a bandlike pattern in the superficial epithelial layers. (A) Noninflammatory cervix demonstrates positive immunoreactivity for hCAP18 predominantly found in the basal epithelium. (B) In situ hybridization shows matching signal for hCAP18 mRNA in the same tissue. The shiny appearance of the surface layer is due to the autofluorescence of the tissue and does not represent an autoradiographic signal. (C) Sample of a dysplastic and inflammatory cervix demonstrates a striking band of hCAP18 immunoreactivity in the superficial epithelium. (D) In situ hybridization shows that hCAP18 is actively expressed in the outermost layers of the epithelium. (E) High-power view of superficial hCAP18-immunoreactive epithelial cells. (F) hCAP18-immunoreactive fibroblast-like cell in the cervix stroma (same tissue sample as in panel A). (G) hCAP18-immunoreactive mucous glands in the cervix (same tissue sample as in panel C). Bars: A to D and G, 100 μm; E and F, 25 μm.
In all tissues there was immunoreactivity for hCAP18 in areas of squamous epithelium expressing hCAP18 mRNA, indicating the ability of these cells to produce hCAP18 peptide (Fig. 2A, 3A, and 4A, C, and E). As mentioned above, in noninflammatory tissues the signal for hCAP18 mRNA was primarily detected in the basal epithelial layers, although hCAP18 immunoreactivity was also seen in occasional suprabasal cells, suggesting that under normal conditions hCAP18 is mainly transcribed in the basal epithelial layers and is then translated and transported toward the surface in the process of normal cell turnover. However, in the two dysplastic and inflammatory cervix samples, hCAP18 was strongly expressed in the outermost cell layers at both mRNA and protein levels, indicating a spatially distinct induction of hCAP18 under these conditions. In addition, in all cervix tissues there was positive signal and immunoreactivity for hCAP18 in scattered stromal cells and in the mucous glands (Fig. 4F and G). The stromal cells had a fibroblastic appearance but were not further characterized. As an internal positive control, strong immunostaining for hCAP18 was consistently detected in neutrophil granulocytes (not shown). Control slides without hCAP18 antibody were devoid of immunoreactivity, as were slides processed with only rabbit serum (Fig. 3B). Immunoadsorption at 33 μg of hCAP18 peptide per ml almost completely abolished immunoreactivity for hCAP18 (Fig. 3C).
FIG. 3.
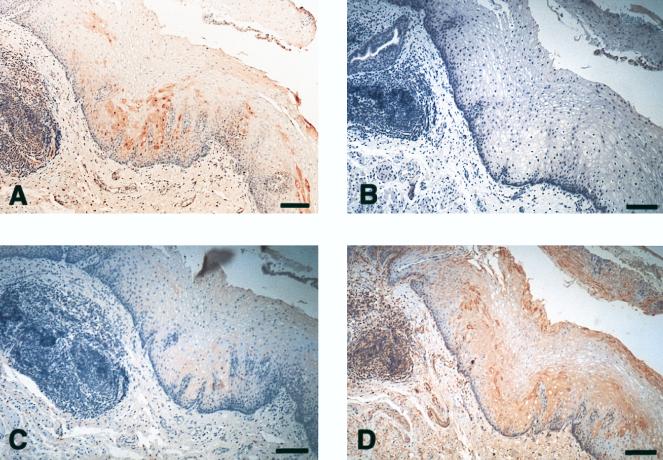
hCAP18 and IL-6 colocalize in esophagus epithelia. (A) Section of esophagus demonstrates hCAP18-immunoreactive epithelial cells. (B) No immunoreactivity is detected in the epithelium when rabbit serum is used instead of hCAP18 antibody. (C) Immunoadsorption with hCAP18 recombinant peptide almost completely abolished hCAP18 immunoreactivity in esophagus epithelium. (D) Immunostaining for IL-6 demonstrates immunoreactive cells in the same areas as for hCAP18. Bars, 100 μm.
Expression of hCAP18 colocalizes with immunoreactivity for IL-6 in squamous epithelia.
Since the gene for hCAP18 contains promoter elements regulated by IL-6, we performed immunohistochemistry with antibodies directed against this cytokine. In all investigated tissues, immunoreactivity for IL-6 colocalized with that for hCAP18 (Fig. 2C and 3D).
DISCUSSION
In the present investigation we demonstrate that the antimicrobial peptide hCAP18 is expressed at both RNA and protein levels in nonkeratinized squamous epithelia in humans. Most samples showed only minor histopathological signs of inflammation, indicating that there may be a basal constitutive expression of hCAP18 in these tissues. This contrasts with what we previously reported in human skin, where there was no detectable expression of hCAP18 in normal quiescent epidermis although there was a pronounced upregulation in association with a variety of inflammatory conditions (12). One might argue that a continuous expression of antimicrobial activity may be more critical in epithelia lacking an outer keratinized cover. Still, hCAP18 is likely to be a regulated gene, and in the present investigation there was a spatially distinct pattern of hCAP18 production in the cervical samples exhibiting dysplasia and inflammation compared to normal noninflamed tissues (Fig. 4). In the former case, the expression of hCAP18 was clearly upregulated in the superficial layers, creating a striking shield-like band (Fig. 4C to E). Interestingly, in all of the cervical samples, hCAP18 was also detected in scattered stromal cells and in the mucous glands (Fig. 4F and G), indicating that hCAP18 may serve a key role in the defense of this organ.
The complete functional repertoire of hCAP18 remains to be fully elucidated. The C-terminal domain of hCAP18 exhibits potent antibacterial activity in vitro against both gram-positive and gram-negative bacteria, a trait which most likely reflects a major biological role for this protein (18, 20, 28). In addition, several studies have demonstrated that hCAP18 binds to the endotoxin bacterial lipopolysaccharide, neutralizing many of its biological effects (11, 21). Thus, hCAP18 may play a role in the protection against septic shock as was demonstrated in a pig model of endotoxemia (29). Interestingly, the induction of defensins in the trachea is mediated by CD14 on the epithelial cell surface, further supporting a role for epithelial cells in the local host defense (10). Besides direct effects against microbial invasion, it seems conceivable that antibacterial peptides, including hCAP18, serve additional biological roles. Thus, defensins modulate the inflammatory response through the enhancement of chemotaxis, and another peptide, NK lysin, possesses antitumor activity (4). Interestingly, a porcine homologue of hCAP18, PR-39, can induce syndecans in association with wound healing (14). Syndecans are cell surface proteoglycans required for cellular response to growth factors and thereby important in tissue repair. It is plausible that hCAP18 plays a similar role in tissue repair processes in humans.
In all of the tissues there was a striking colocalization between hCAP18 expression and immunoreactivity for IL-6 (Fig. 2 and 3). IL-6 is a proinflammatory cytokine produced by a variety of cell types, including keratinocytes. Typically, the production of IL-6 is induced in response to bacterial products and tissue damage. The regulation of the hCAP18 gene appears to be complex and remains to be fully clarified. However, the identification of several elements responsive to IL-6 in the gene promoter strongly suggests that this cytokine plays a prominent role in modulating the transcription of the hCAP18 gene. Thus, the colocalization of IL-6 and hCAP18 reported here is suggestive of a mechanism through which hCAP18 may be rapidly upregulated in epithelial cells in response to microbial or other challenges.
The existence of immediate and efficient antimicrobial defense systems in epithelial tissues seems both natural and necessary. Epithelia form the borders between the organism and the frequently hostile environment and are continuously exposed to a large variety of potential intruders. Relying solely on the highly evolved but slow adaptive immune system would not be quick enough to stop the invasion, growth, and subsequent takeover by pathogens capable of rapid division. Indeed, there is accumulating evidence that peptide antibiotics are prominent players in the protection of epithelial surfaces. Thus, the expression of hCAP18 was recently demonstrated in airway epithelium, and the peptides human beta defensin 1 and human beta defensin 2 were reported in human skin; the latter was also found to be upregulated by contact with different microorganisms (5, 13, 16). Our findings demonstrate that hCAP18 is widely expressed in squamous epithelia and support the notion of an innate antimicrobial shield at epithelial interfaces. The coexpression of hCAP18 with IL-6 might provide a local mechanism for modulating hCAP18 gene transcription in these tissues.
ACKNOWLEDGMENTS
We thank Kerstin Bruce, Anna Hulthén, and Anna-Lena Kastman for excellent technical assistance.
This work was supported by grants from the Karolinska Institute, the Welander-Finsen Foundation, the SSAC Foundation for Research in Antimicrobial Therapy, and the Swedish Psoriasis Association.
REFERENCES
- 1.Agerberth B A, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Nishio Y, Inoue M, Wang X-J, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 4.Andersson M, Gunne H, Agerberth B A, Bergman T, Sillard R, Jörnvall H, Mutt V, Olsson B, Wigzell H, Dagerlind Å, Boman H G, Gudmundsson G H. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14:1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 7.Bos J D, Kapsenberg L. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 8.Brakenhoff J P, de Hon F D, Fontaine V, ten Boekel E, Schooltink H, Rose-John S, Heinrich P C, Content J, Aarden L A. Development of a human interleukin-6 receptor antagonist. J Biol Chem. 1994;269:86–93. [PubMed] [Google Scholar]
- 9.Cowland J B, Johnson A H, Borregaard N. hCAP-18, a cathelin/bactenecin like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 10.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher M A, Kloczewiak M A, Loiselle P M, Ogata M, Vermeulen M W, Zanzot E M, Warren H S. A novel peptide-IgG conjugate, CAP18 106-138-IgG that binds and neutralizes endotoxin and kills gram-negative bacteria. J Infect Dis. 1997;175:621–632. doi: 10.1093/infdis/175.3.621. [DOI] [PubMed] [Google Scholar]
- 12.Frohm M, Agerberth B A, Ahangari G, Ståhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 13.Fulton C, Anderson M G, Zasloff M, Bull R, Quinn A. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350:1750–1751. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 14.Gallo R L, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci USA. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudmundsson G H, Agerberth B A, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 16.Harder J, Bartels J, Christophers E, Schröder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 17.Hsu S M, Raine L, Fanger H. Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PA) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 18.Johansson J, Gudmundsson G H, Rottenberg M E, Berndt K D, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 19.Krisanaprakornkit S, Weinberg A, Perez C N, Dale B A. Expression of the peptide antibiotic human β-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larrick J W, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright S C. Antimicrobial activity of rabbit CAP 18-derived peptides. Antimicrob Agents Chemother. 1993;37:2534–2539. doi: 10.1128/aac.37.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larrick J W, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J-M, Warren S H, Wright S C. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–240. [PubMed] [Google Scholar]
- 22.Le M, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588–602. [PubMed] [Google Scholar]
- 23.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 24.Selsted M E, Brown D M, DeLange R J, Lehrer R I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983;258:14485–14489. [PubMed] [Google Scholar]
- 25.Steiner H, Hultmark D, Engström Å, Bennich H, Boman H G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 26.Ståhle-Bäckdahl M, Sudbeck B D, Eisen A Z, Welgus H G, Parks W C. Expression of 92 kDa type IV collagenase in eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992;99:497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen O, Cowland J B, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 28.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderMeer T J, Menconi M J, Zhuang J, Wang H, Murtaugh R, Bouza C, Stevens P, Fink M P. Protective effects of a novel 32-amino acid C-terminal fragment of CAP18 in endotoxemic pigs. Surgery. 1995;117:656–662. doi: 10.1016/s0039-6060(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]