Abstract
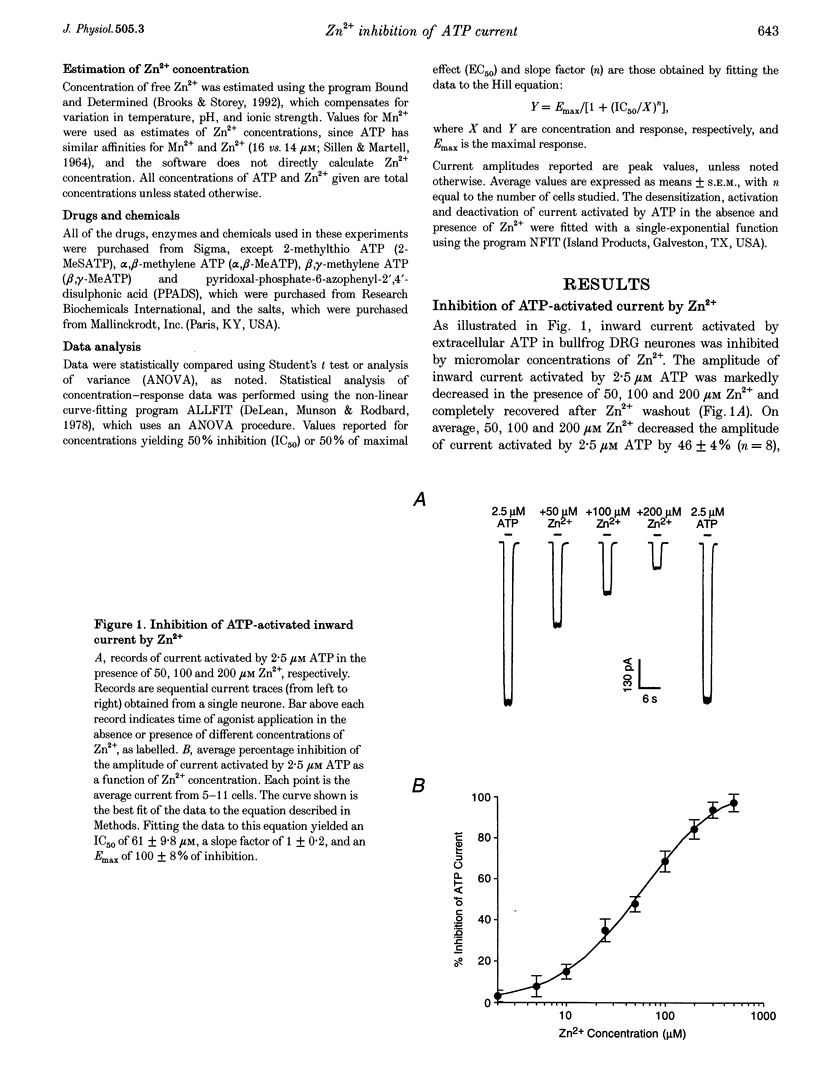
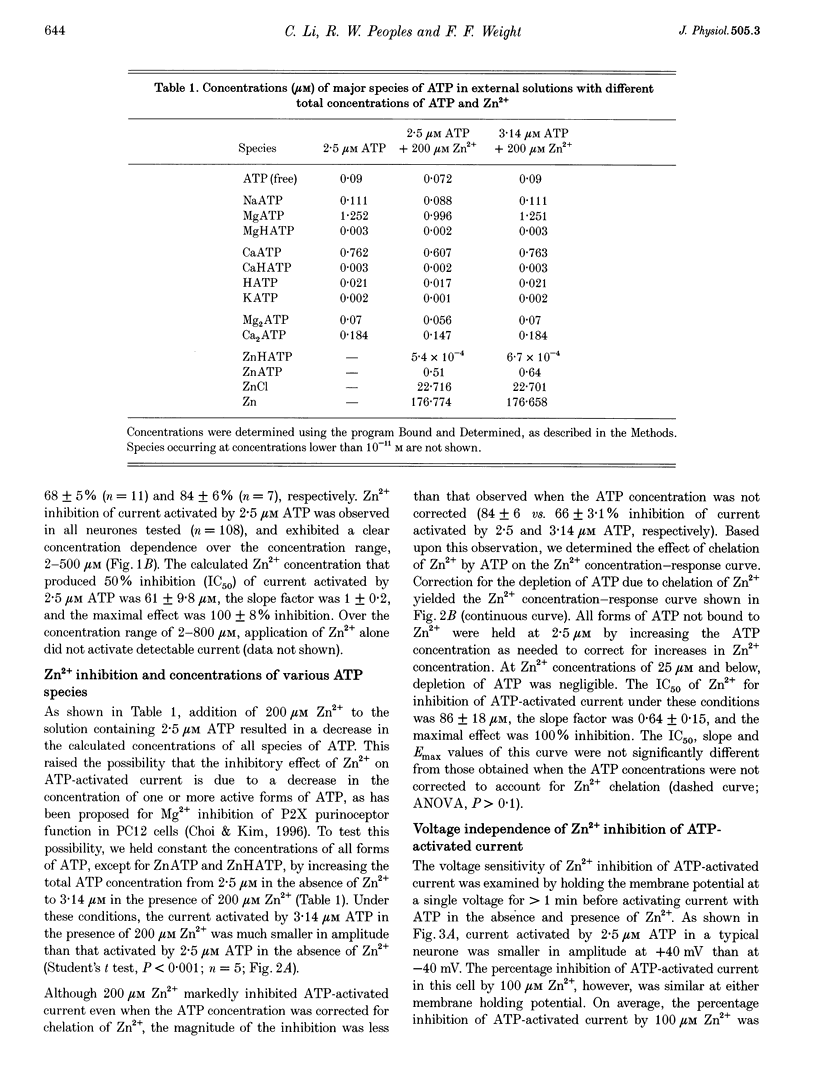
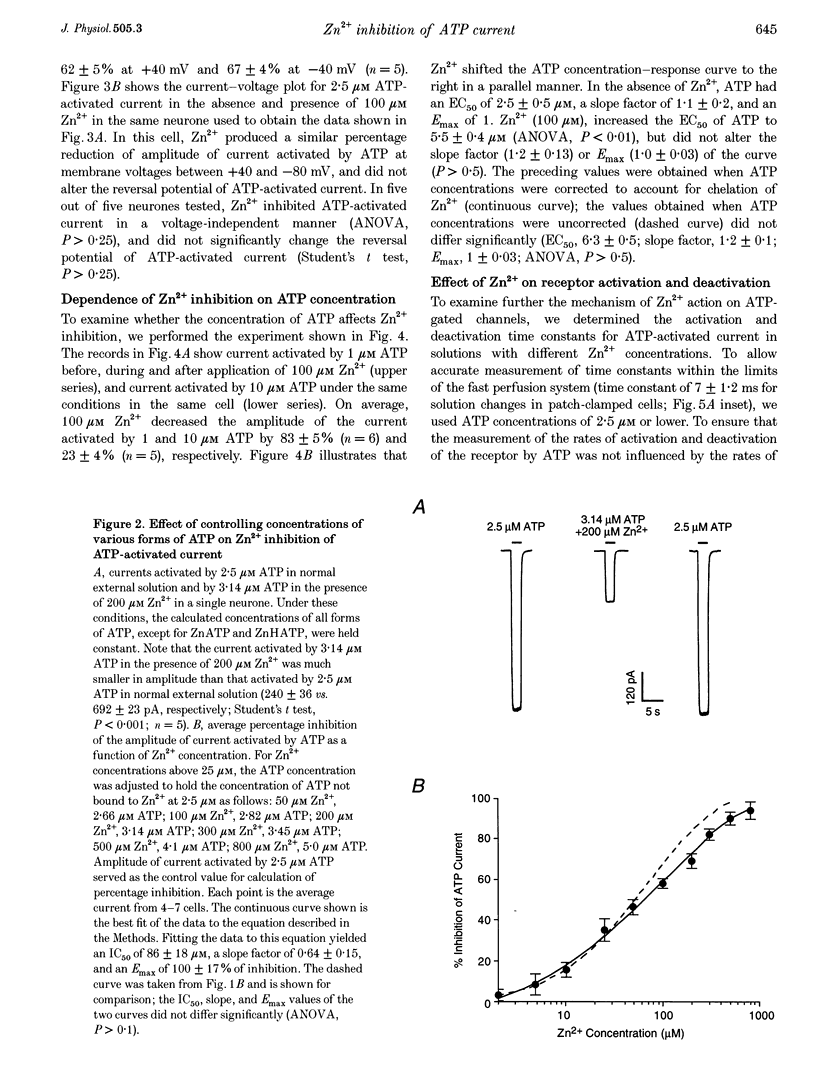
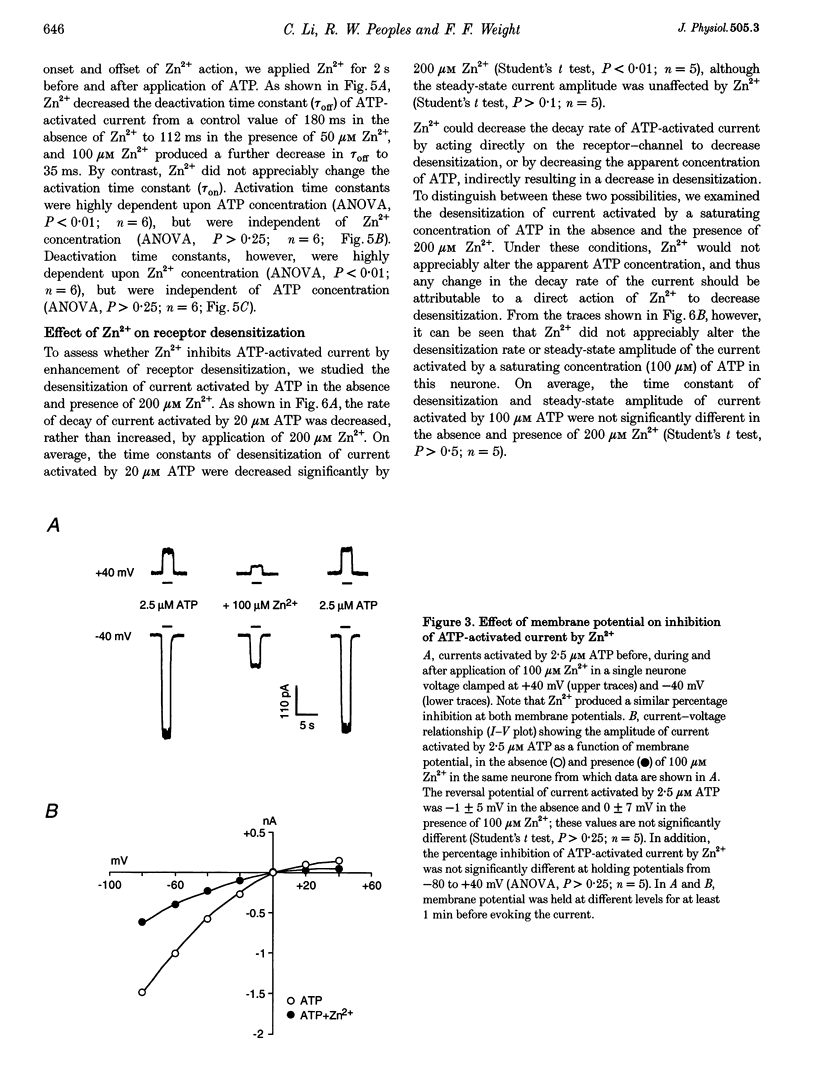
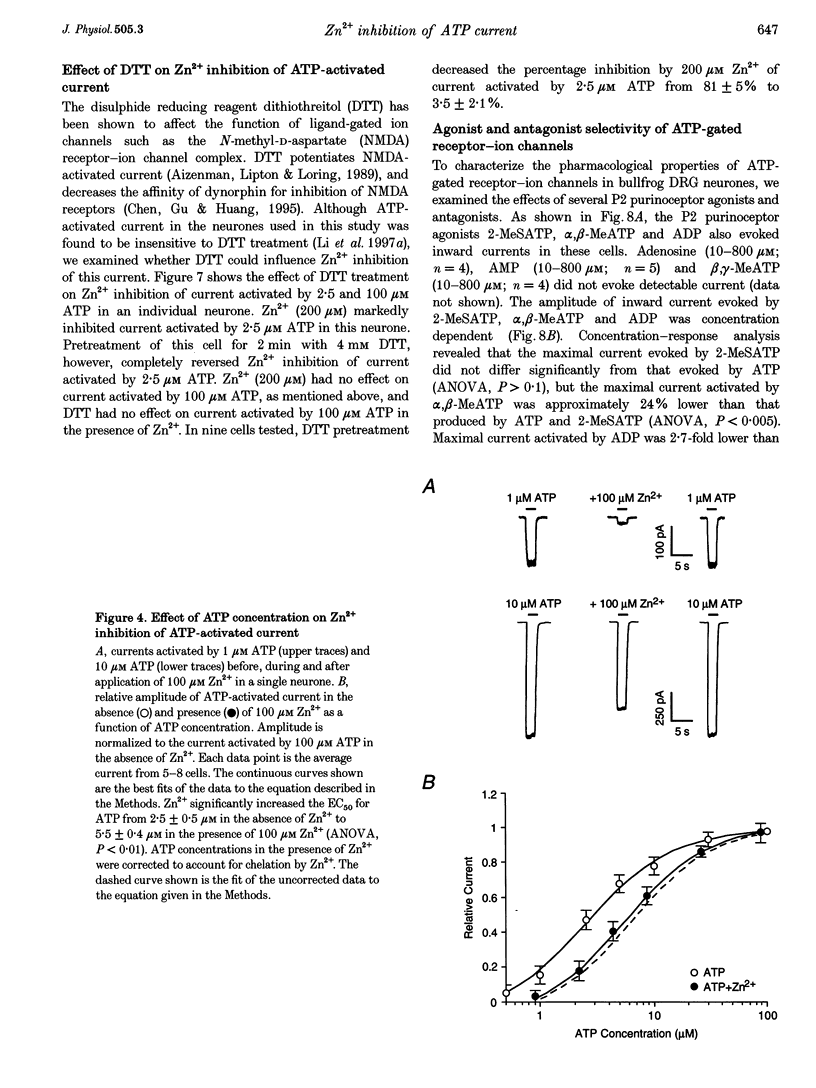
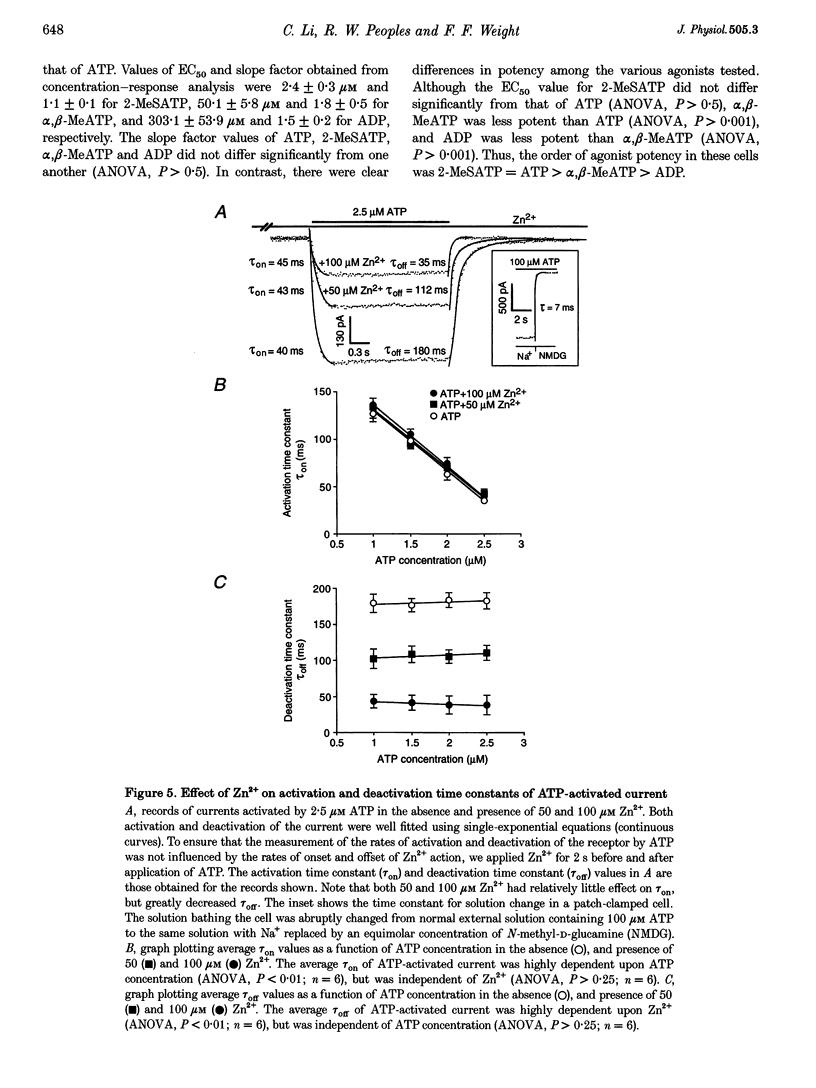
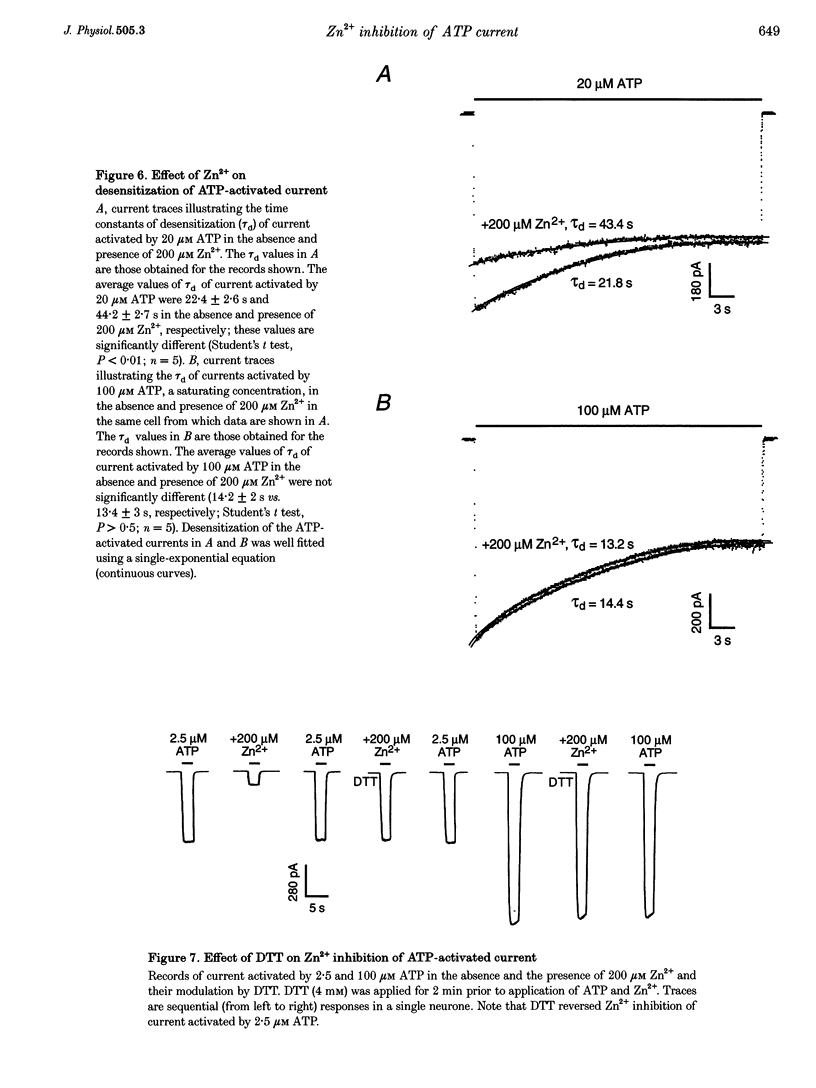
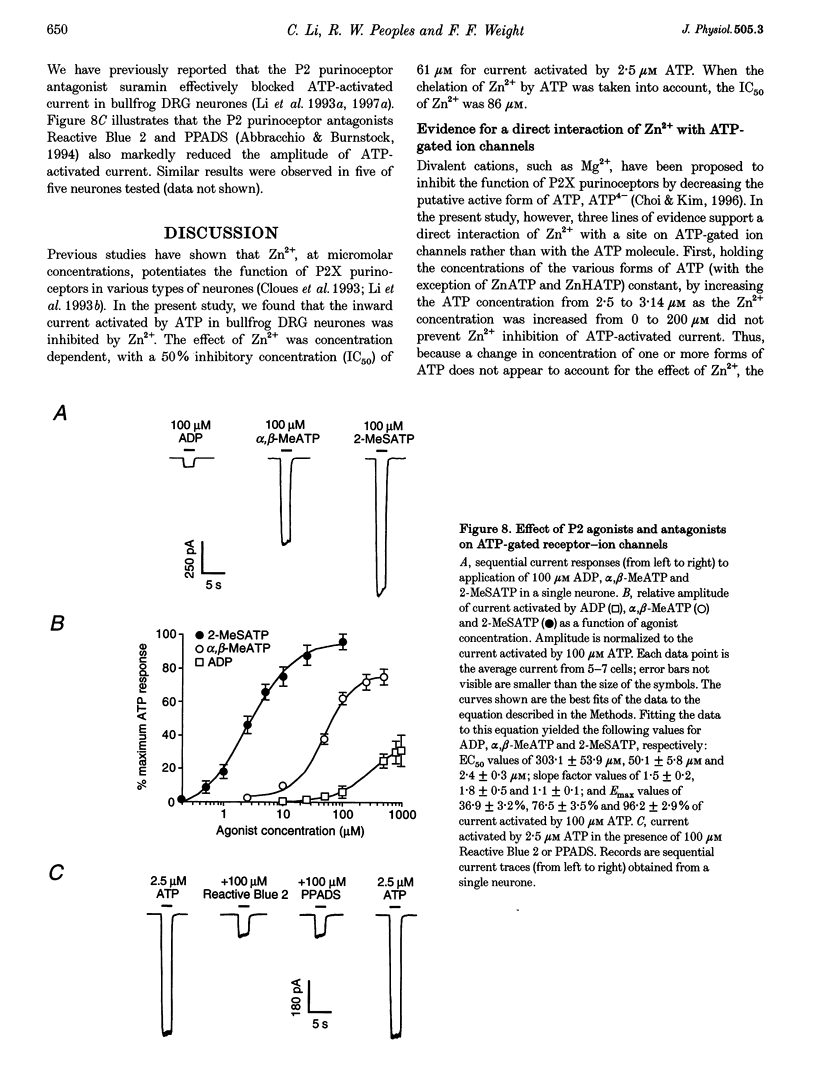
1. The effect of Zn2+ on ATP-activated current was studied in bullfrog dorsal root ganglion (DRG) neurones using the whole-cell patch-clamp technique. 2. Zn2+ (2-800 microM) inhibited current activated by submaximal concentrations of ATP. The Zn2+ concentration that produced 50% inhibition (IC50) of current activated by 2.5 microM ATP was 61 +/- 9.8 microM. When ATP concentrations were adjusted to account for chelation of Zn2+, the IC50 of Zn2+ was 86 +/- 18 microM. 3. The inhibitory action of Zn2+ on ATP-gated channels did not appear to be due to a decrease in the concentration of one or more species of ATP. 4. Zn2+ inhibition of ATP-activated current was independent of membrane potential between -80 and +40 mV, and did not involve a shift in the reversal potential of the current. 5. Zn2+ (100 microM) shifted the ATP concentration-response curve to the right in a parallel manner, increasing the EC50 for ATP from 2.5 +/- 0.5 microM to 5.5 +/- 0.4 microM. 6. Zn2+ decreased the time constant of deactivation of ATP-gated ion channels without affecting the time constant of activation or desensitization. 7. Dithiothreitol (DTT) reversed Zn2+ inhibition of ATP-activated current. 8. 2-Methylthio ATP, alpha,beta-methylene ATP and ADP activated current with EC50 values of 2.4 +/- 0.3. 50.1 +/- 5.8 and 303.1 +/- 53.9 microM, respectively. Adenosine, AMP or beta,gamma-methylene ATP did not evoke detectable current. 9. Reactive Blue 2 and pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid inhibited ATP-activated current. 10. The results suggest that Zn2+ can inhibit P2X purinoceptor function by decreasing the affinity of the binding site for ATP. These observations provide the first evidence for this action of Zn2+ on a neurotransmitter-gated ion channel. Furthermore, the receptor-channel in these neurones appears to be a novel member of the P2X purinoceptor class.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Aizenman E., Lipton S. A., Loring R. H. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989 Mar;2(3):1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. P., Storey K. B. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992 Feb 14;201(1):119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Brown C., Tanna B., Boarder M. R. PPADS: an antagonist at endothelial P2Y-purinoceptors but not P2U-purinoceptors. Br J Pharmacol. 1995 Nov;116(5):2413–2416. doi: 10.1111/j.1476-5381.1995.tb15088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Gu Y., Huang L. Y. The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. J Neurosci. 1995 Jun;15(6):4602–4611. doi: 10.1523/JNEUROSCI.15-06-04602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. Y., Kim K. T. Characterization of Na+ influx mediated by ATP(4-)-activated P2 purinoceptors in PC12 cells. Br J Pharmacol. 1996 Jun;118(4):935–940. doi: 10.1111/j.1476-5381.1996.tb15489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson D. W. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Westbrook G. L. Kinetics of AP5 dissociation from NMDA receptors: evidence for two identical cooperative binding sites. J Neurophysiol. 1994 Jun;71(6):2566–2569. doi: 10.1152/jn.1994.71.6.2566. [DOI] [PubMed] [Google Scholar]
- Cloues R., Jones S., Brown D. A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflugers Arch. 1993 Jul;424(2):152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Marshall G. R., Wong E. H., Woodruff G. N. The affinities, potencies and efficacies of some benzodiazepine-receptor agonists, antagonists and inverse-agonists at rat hippocampal GABAA-receptors. Br J Pharmacol. 1987 Jul;91(3):601–608. doi: 10.1111/j.1476-5381.1987.tb11253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Leff P. How should P2X purinoceptors be classified pharmacologically? Trends Pharmacol Sci. 1995 May;16(5):168–174. doi: 10.1016/s0165-6147(00)89010-0. [DOI] [PubMed] [Google Scholar]
- Khakh B. S., Humphrey P. P., Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J Physiol. 1995 Apr 15;484(Pt 2):385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Li C., Aguayo L., Peoples R. W., Weight F. F. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993 Oct;44(4):871–875. [PubMed] [Google Scholar]
- Li C., Peoples R. W., Li Z., Weight F. F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8264–8267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Weight F. F. Cu2+ potently enhances ATP-activated current in rat nodose ganglion neurons. Neurosci Lett. 1996 Nov 15;219(1):45–48. doi: 10.1016/s0304-3940(96)13186-4. [DOI] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Weight F. F. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflugers Arch. 1997 Feb;433(4):446–454. doi: 10.1007/s004240050299. [DOI] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Weight F. F. Mg2+ inhibition of ATP-activated current in rat nodose ganglion neurons: evidence that Mg2+ decreases the agonist affinity of the receptor. J Neurophysiol. 1997 Jun;77(6):3391–3395. doi: 10.1152/jn.1997.77.6.3391. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M. Inhibition of 5-HT3 receptor-mediated ion current by divalent metal cations in NCB-20 neuroblastoma cells. J Neurophysiol. 1991 Oct;66(4):1329–1337. doi: 10.1152/jn.1991.66.4.1329. [DOI] [PubMed] [Google Scholar]
- Robertson S. J., Rae M. G., Rowan E. G., Kennedy C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. Br J Pharmacol. 1996 Jun;118(4):951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. Z., North R. A. Excitation of rat locus coeruleus neurons by adenosine 5'-triphosphate: ionic mechanism and receptor characterization. J Neurosci. 1993 Mar;13(3):894–899. doi: 10.1523/JNEUROSCI.13-03-00894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sullivan J. M., Traynelis S. F., Chen H. S., Escobar W., Heinemann S. F., Lipton S. A. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994 Oct;13(4):929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Buell G., North R. A. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995 May;18(5):224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Ueno S., Harata N., Inoue K., Akaike N. ATP-gated current in dissociated rat nucleus solitarii neurons. J Neurophysiol. 1992 Sep;68(3):778–785. doi: 10.1152/jn.1992.68.3.778. [DOI] [PubMed] [Google Scholar]
- Wright J. M., Li C. Zn2+ potentiates steady-state ATP activated currents in rat nodose ganglion neurons by increasing the burst duration of a 35 pS channel. Neurosci Lett. 1995 Jul 7;193(3):177–180. doi: 10.1016/0304-3940(95)11694-r. [DOI] [PubMed] [Google Scholar]
- Yakushiji T., Tokutomi N., Akaike N., Carpenter D. O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987 Sep;22(3):1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


