Abstract
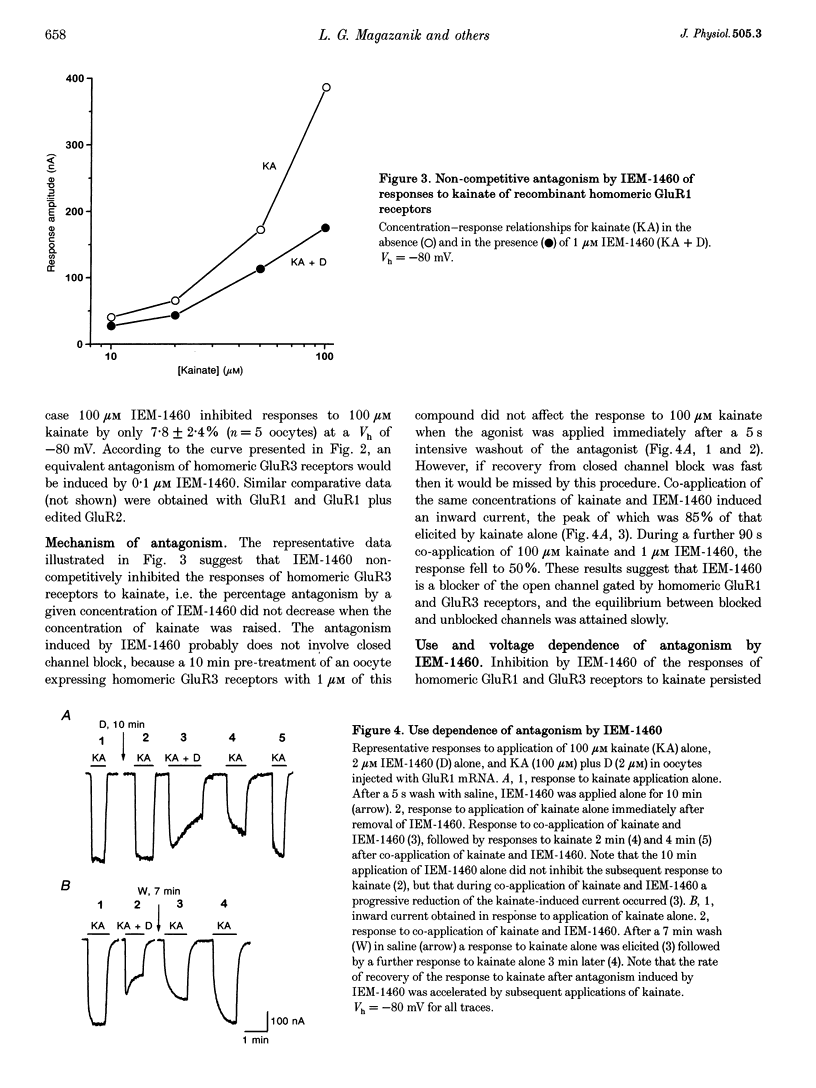
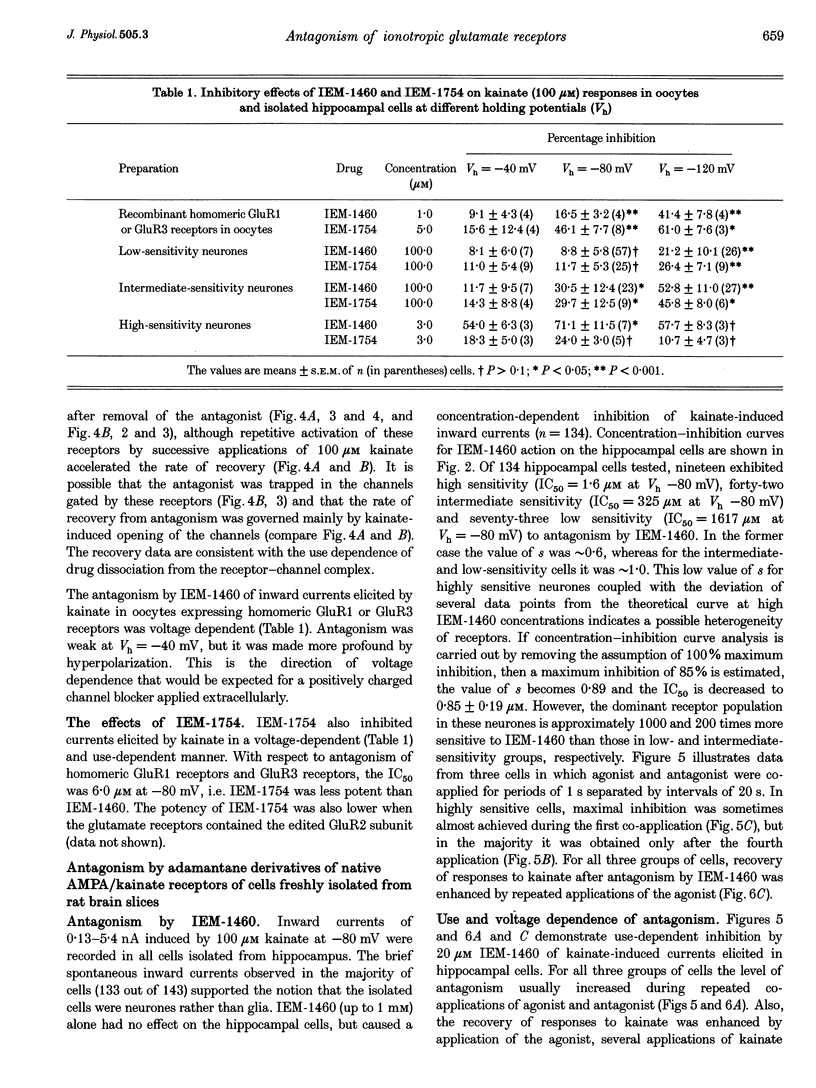
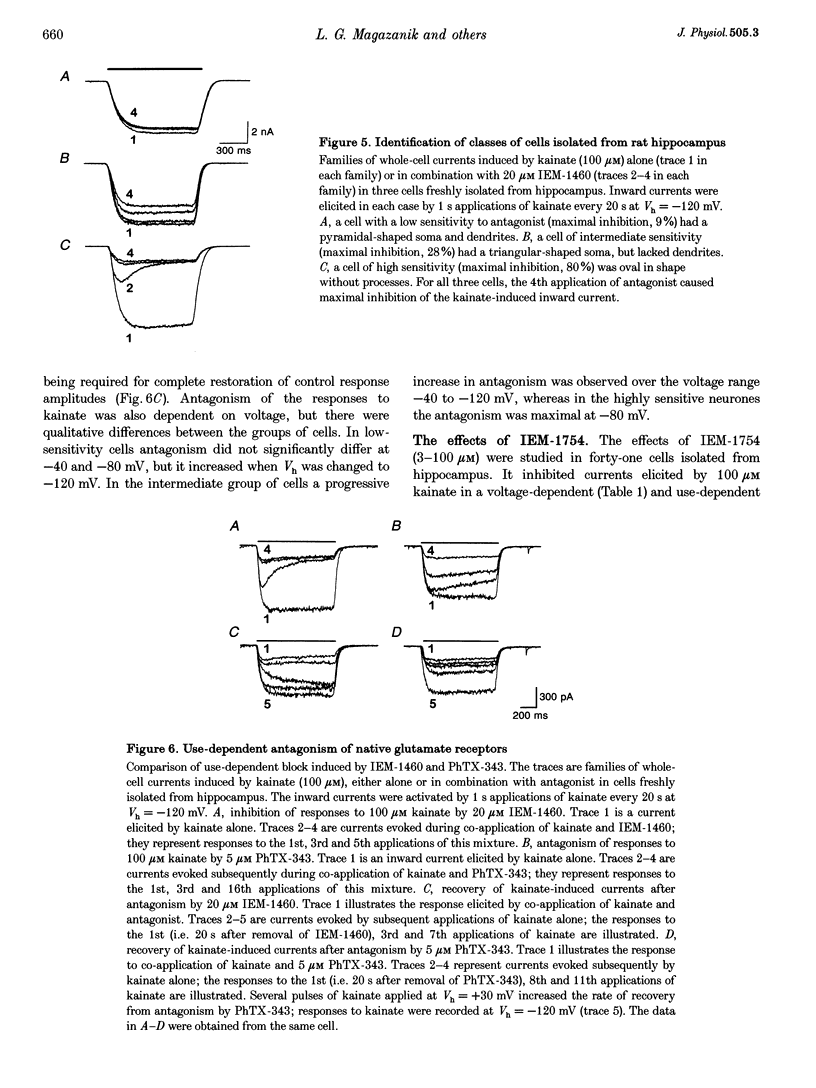
1. The effects of two adamantane derivatives, 1-trimethylammonio-5-(1-adamantane-methyl-ammoniopentane dibromide) (IEM-1460) and 1-ammonio-5-(1-adamantane-methylammoniopentane dibromide) (IEM-1754) on kainate-induced currents were studied in Xenopus oocytes expressing recombinant ionotropic glutamate receptors and in freshly isolated neurones from rat hippocampal slices. 2. The adamantane derivatives caused use- and voltage-dependent block of open channels of recombinant AMPA receptors. This antagonism was dependent on receptor subunit composition; channels gated by recombinant, homomeric GluR1 and GluR3 receptors exhibited a higher sensitivity to block than those gated by receptors containing edited GluR2 subunits. In the former cases, IEM-1460 had an IC50 of 1.6 microM at a holding potential (Vh) of -80 mV and IEM-1754 was 3.8 times less potent than IEM-1460. In contrast, 100 microM IEM-1460 inhibited responses to 100 microM kainate of receptors containing edited GluR2 subunits by only 7.8 +/- 2.4% (n = 5 oocytes at a Vh of -80 mV. 3. Native AMPA/kainate receptors in isolated hippocampal cells were inhibited by adamantane derivatives in a use- and voltage-dependent manner. This antagonism was dependent on cell type: pyramidal neurones were less sensitive to IEM-1460 (IC50 = 1617 microM at Vh = -80 mV) than interneurones (IC50 = 1.6 microM at Vh = -80 mV). IEM-1460 and IEM-1754 were equipotent when applied to pyramidal neurones, but IEM-1754 was less potent (approximately 3 times) than IEM-1460 when applied to interneurones. 4. It is concluded that the presence of the edited GluR2 subunit in recombinant AMPA receptors and native AMPA/kainate receptors inhibits channel block by organic cations and that adamantane derivatives are potentially valuable tools for identifying classes of AMPA/kainate receptors and their roles in synaptic transmission.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonov S. M., Dudel J., Franke C., Hatt H. Argiopine blocks glutamate-activated single-channel currents on crayfish muscle by two mechanisms. J Physiol. 1989 Dec;419:569–587. doi: 10.1113/jphysiol.1989.sp017887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov S. M., Johnson J. W., Lukomskaya N. Y., Potapyeva N. N., Gmiro V. E., Magazanik L. G. Novel adamantane derivatives act as blockers of open ligand-gated channels and as anticonvulsants. Mol Pharmacol. 1995 Mar;47(3):558–567. [PubMed] [Google Scholar]
- Blaschke M., Keller B. U., Rivosecchi R., Hollmann M., Heinemann S., Konnerth A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet P., Audinat E., Lambolez B., Crépel F., Rossier J., Iino M., Tsuzuki K., Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994 Feb;12(2):383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Brackley P. T., Bell D. R., Choi S. K., Nakanishi K., Usherwood P. N. Selective antagonism of native and cloned kainate and NMDA receptors by polyamine-containing toxins. J Pharmacol Exp Ther. 1993 Sep;266(3):1573–1580. [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Donaldson P. L., Gration K. A., Lambert J. J., Piek T., Ramsey R., Spanjer W., Usherwood P. N. Block of locust muscle glutamate receptors by delta-philanthotoxin occurs after receptor activations. Brain Res. 1982 Jun 3;241(1):105–114. doi: 10.1016/0006-8993(82)91233-1. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hume R. I., Heinemann S. F. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992 Oct;12(10):4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Green A. C., Nakanishi K., Usherwood P. N. Polyamine amides are neuroprotective in cerebellar granule cell cultures challenged with excitatory amino acids. Brain Res. 1996 Apr 22;717(1-2):135–146. doi: 10.1016/0006-8993(96)00042-x. [DOI] [PubMed] [Google Scholar]
- Götz T., Kraushaar U., Geiger J., Lübke J., Berger T., Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997 Jan 1;17(1):204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Iino M., Koike M., Isa T., Ozawa S. Voltage-dependent blockage of Ca(2+)-permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones. J Physiol. 1996 Oct 15;496(Pt 2):431–437. doi: 10.1113/jphysiol.1996.sp021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T., Iino M., Itazawa S., Ozawa S. Spermine mediates inward rectification of Ca(2+)-permeable AMPA receptor channels. Neuroreport. 1995 Oct 23;6(15):2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- Isa T., Itazawa S., Iino M., Tsuzuki K., Ozawa S. Distribution of neurones expressing inwardly rectifying and Ca(2+)-permeable AMPA receptors in rat hippocampal slices. J Physiol. 1996 Mar 15;491(Pt 3):719–733. doi: 10.1113/jphysiol.1996.sp021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995 Nov;15(5):987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994 Jun;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Single channel studies of non-competitive antagonism of a quisqualate-sensitive glutamate receptor by argiotoxin636--a fraction isolated from orb-web spider venom. Brain Res. 1988 Sep 6;459(2):312–327. doi: 10.1016/0006-8993(88)90647-6. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Burnashev N., Jonas P. Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995 Jul 15;486(Pt 2):305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Burnashev N., Sakmann B., Seeburg P. H. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993 Mar;10(3):491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Leranth C., Szeidemann Z., Hsu M., Buzsáki G. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR2/3 subunits in most interneurons. Neuroscience. 1996 Feb;70(3):631–652. doi: 10.1016/s0306-4522(96)83003-x. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol. 1993 Mar;462:373–392. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophys J. 1986 Nov;50(5):981–986. doi: 10.1016/S0006-3495(86)83538-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Randle J. C. Tetrabutylammonium induces a voltage-dependent block of kainate current in Xenopus oocytes injected with rat brain mRNA. Can J Physiol Pharmacol. 1990 Aug;68(8):1069–1078. doi: 10.1139/y90-161. [DOI] [PubMed] [Google Scholar]
- Sudan H. L., Kerry C. J., Mellor I. R., Choi S. K., Huang D., Nakanishi K., Usherwood P. N. The action of philanthotoxin-343 and photolabile analogues on locust (Schistocerca gregaria) muscle. Invert Neurosci. 1995;1(2):159–172. doi: 10.1007/BF02331913. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H., Oguro K., Masuzawa T., Kawai N. Ca(2+)-dependent non-NMDA receptor-mediated synaptic currents in ischemic CA1 hippocampal neurons. J Neurophysiol. 1994 Mar;71(3):1190–1196. doi: 10.1152/jn.1994.71.3.1190. [DOI] [PubMed] [Google Scholar]
- Vorobjev V. S., Sharonova I. N., Haas H. L. A simple perfusion system for patch-clamp studies. J Neurosci Methods. 1996 Oct;68(2):303–307. doi: 10.1016/0165-0270(96)00097-0. [DOI] [PubMed] [Google Scholar]
- Vorobjev V. S. Vibrodissociation of sliced mammalian nervous tissue. J Neurosci Methods. 1991 Jul;38(2-3):145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Petralia R. S., Blahos J I. I., Niedzielski A. S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996 Mar 15;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993 Jun;3(3):291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]


