Summary
Psychological interventions for sleep-wake disorders have medium-to-large effect sizes, however whether behavioral randomized controlled trials (RCTs) targeted underserved populations or addressed contextual and cultural factors is unknown. We conducted a systematic review to: (a) examine sociodemographic characteristics of behavioral RCTs for prevalent sleep-wake disorders and sleep disturbances that targeted undeserved adults, (b) identify types of cultural adaptations (surface-level, deep-level), and (c) describe intervention effectiveness on primary sleep outcomes. Overall, 6.97% of RCTs (56 studies) targeted underserved groups (veterans, women, racial/ethnic minorities, low socioeconomic status, disability status); 64.29% made surface-level and/or deep-level cultural adaptations. There was a lack of racial/ethnic, socioeconomic, sexual orientation, and linguistic diversity. Most cultural adaptations were made to behavioral therapies, and cognitive behavioral therapy for insomnia (CBT-I). Surface-level cultural adaptations to the delivery modality and setting were most common. Deep-level cultural adaptations of the content and core intervention components were also typical. Intervention effectiveness varied by type of adapted intervention and participant population. RCTs of adapted CBT-I interventions among participants with a definite sleep disorder or sleep disturbance showed consistent significant reductions in adverse sleep outcomes versus control. These findings have important implications for the use of cultural adaptations to address behavioral sleep medicine disparities.
Keywords: cultural tailoring, sleep, psychological treatments, effectiveness, underserved, mental health care disparities
Sleep-wake disorders such as insomnia, obstructive sleep apnea, and nightmare disorder, have a population prevalence ranging from six to 38% in the United States, and are associated with substantial public health burden, including increased risk of cardiovascular disease, obesity, depression, motor vehicular or workplace accidents, and death (1–11). Sleep-wake disorders as well as sleep disturbances such as short sleep duration and poor sleep quality are also associated with substantial economic costs and account for roughly $100 billion per year in direct and indirect costs (12–15). It is no surprise then that there is concerted national attention to improving both pernicious sleep disorders and sleep disturbances in the United States (16).
Meta-analyses and systematic reviews of randomized controlled trials (RCTs) testing psychological interventions for sleep-wake disorders indicate that psychological interventions such as Cognitive Behavioral Therapy for Insomnia or Behavioral Therapies (e.g., stimulus control, sleep restriction therapies) are both efficacious and effective for the resolution or amelioration of sleep disorders or sleep disturbances in the short- and long-term, with medium-to-large effect sizes among adults (17–19). However, none of these meta-analyses or systematic reviews examined the effectiveness of these interventions among underserved groups or populations at higher risk of one of these disorders or disturbances (e.g., insomnia, short sleep duration) or at greater risk of experiencing disproportionate burden from these conditions, including racial/ethnic minorities, women, sexual orientation or gender minorities, immigrants, linguistic minorities, veterans, those with a disability, and those from lower socioeconomic groups. While one systematic review addressed behavioral treatments for sleep disturbances in children and adults with intellectual disabilities, none of the included studies were of RCTs targeting adults (20). As such, RCTs of psychological interventions for sleep disorders that target underserved adult populations for whom modifications or changes to the intervention may be warranted in order to maximize its effectiveness are sparse.
A cultural adaptation of a psychological intervention refers to the systematic process of changing an evidence-based treatment to include culturally sensitive components (e.g., language, culture, and context) that are consonant with a patient’s worldview (21, 22). These changes can include surface-level cultural adaptations such as translating the intervention to a patient’s language of preference when working with linguistic minorities and/or deep-level cultural adaptations such as incorporating sociocultural values such as familismo in the intervention by incorporating family members into the treatment paradigm when working with Latina/os (21, 22). Deep-level cultural adaptations can also include integration of concepts and metaphors into the intervention content that are culturally consonant with the target population (e.g., use of proverbs) (21, 22). Systematic reviews of cultural adaptations of psychological interventions for depression, and behavioral health issues indicate adapted interventions yield more favorable improvements and higher retention rates than control conditions, particularly for racial/ethnic minorities (23–27). In fact, results from meta-analyses of cultural adaptations of psychological interventions corroborate these findings and indicate a large overall effect size (g = 0.67, p<.001) for adapted interventions compared to no intervention or other interventions, and a medium effect size (g = 0.52) for the adapted versus un-adapted version of the same intervention (23, 25). Of note, most of these systematic reviews and meta-analyses focused on RCTs of psychological interventions with cultural adaptations for racial/ethnic minorities, which represent but one of many underserved or marginalized groups in the United States. Further, to our knowledge, none of these reviews of cultural adaptations included psychological interventions that targeted a prevalent sleep disorder or sleep disturbance as a primary outcome. As such, significant gaps remain in our understanding of the types of cultural adaptations made to psychological interventions for common sleep-wake disorders and sleep disturbances, and in turn, their effect on primary sleep outcomes among underserved groups in the United States.
The primary aims of this systematic review were to (a) examine the sociodemographic characteristics of RCTs of psychological interventions for prevalent sleep-wake disorders or sleep disturbances that targeted underserved groups including racial/ethnic minorities, those with low socioeconomic status, immigrants, sexual orientation minorities, women, veterans, and those with a disability, (b) identify the types of surface-level or deep-level cultural adaptations made to psychological interventions for prevalent sleep-wake disorders or sleep disturbances, and (c) describe the effectiveness of the culturally adapted psychological interventions for prevalent sleep-wake disorders or sleep disturbances on primary sleep outcomes. Herein, we focused on sleep-wake disorders with a national population prevalence of about 5% or greater, these included insomnia, nightmare disorder, obstructive sleep apnea, restless legs syndrome, and circadian rhythm sleep-wake disorder shift-work type (11), and sleep disturbances that may or may not be concomitant with these conditions.
METHODS
Protocol and Registration
Our systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting and reporting the article search (28, 29). This systematic review is registered as: PROSPERO2016:CRD42016039070 (www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016039070)
Eligibility Criteria, Information Sources, and Search
Eligible articles had to: (a) be conducted in the US mainland (b) written in English; (c) be a randomized controlled trial or comparative effectiveness trial that uses random assignment to compare groups; (d) target an underserved group, specifically non-White racial/ethnic group, women, low socioeconomic status backgrounds, immigrants, sexual orientation minorities, veterans, or individuals from a disability status group; (e) focus on adults; (f) compare a psychological or behavioral intervention to minimal or no intervention, another psychological intervention, or a pharmacological intervention; (g) include one of the following psychological interventions or its derivatives as a primary intervention: Behavioral Therapy, Cognitive Behavioral Therapy, Relaxation Therapy, Sleep Hygiene, Motivational Enhancement Therapy, Stimulus Control Therapy, Mindfulness Based Stress Reduction, self-help, psycho-education; (h) include one of the following primary sleep outcomes: insomnia, obstructive sleep apnea, nightmare disorder, restless legs syndrome, circadian rhythm sleep-wake disorder shift-work type, sleep quality, sleepiness symptoms, sleep duration, circadian processes.
We searched the biomedical electronic databases Ovid MEDLINE, EMBASE, The Cochrane Library, CINAHL, PsycINFO, AMED, Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform for research articles, and conference proceedings on randomized controlled trials of psychological interventions for prevalent sleep-wake disorders published in peer-reviewed journals from inception to January 16, 2020. All relevant subject headings and free-text terms were used to represent underserved groups, and the sleep-wake disorders and psychological interventions under investigation. Terms were applied to limit results to RCTs and to adult populations sampled in the United States. Additional records were identified by scanning the reference lists of relevant studies and reviews, using the Similar Articles feature in PubMed and the Cited Reference Search in ISI Web of Science. Relevant study and clinical trials registry websites such as Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform were also searched. The specific search terms used within each electronic database are detailed in the Supplemental Files.
Study Selection, Data Collection Process, and Data Items
We used a two-stage process to determine article eligibility. First, two of four review authors (CA, EPM, IPI, LGC, TV) independently scanned the abstract and title of every record retrieved for initial screen. Second, all potentially relevant articles were retrieved as full-text for further assessment. During both stages, discrepancies were resolved through consensus.
For studies that met the inclusion criteria, two of four review authors (CA, IPI, LGC, TV) independently extracted key study and adaptation characteristics after a period of calibration. All discrepancies were resolved through consensus. Study characteristics included Intervention characteristics including type of intervention, delivery modality, duration, intensity and comparator; trial characteristics including trial identifier, design, and duration; cultural adaptation including presence/absence of adaptation, type of adaptation, description of adaptation; outcomes including primary and secondary outcomes, summary of primary sleep outcomes results; participant sociodemographic characteristics including sample size, population group, percentage of women, race/ethnicity, age, education, nativity or immigrant status, veterans status, sexual orientation minority, disability, household income, and unemployment status. We also extracted participant inclusion and exclusion criteria to determine whether participants had a sleep disorder or sleep disturbance diagnosis at the time of enrollment. A determination of definite sleep disorder or sleep disturbance was made when studies directly assessed for and confirmed the presence a sleep disorder or sleep disturbance diagnosis, and included this information in the inclusion criteria. A determination of probable sleep disturbance was made when studies directly assessed for any sleep disturbance but did not include endorsement of a sleep disturbance as part of the inclusion criteria. A determination of potential sleep disturbance was made when studies did not directly assess for the presence of a sleep disturbance but based on the scientific literature the participant population would likely have a sleep disturbance concomitant with the co-morbid health or mental health condition. Information was also extracted on the following secondary outcomes: quality of life and psychological factors (e.g., depressive symptoms, anxiety symptoms), fatigue, mood, pain, PTSD, stress, substance use, and suicidality (Supplemental Table).
Following Barrera, Castro, Strycker & Toobert (21), who summarized existing cultural adaptation frameworks (30–33), we distinguished between surface-level and deep-level cultural adaptations, and extracted these data. Surface-level cultural adaptations modify intervention materials and messages to include observable, “superficial” characteristics of the target population (32). Surface-level cultural adaptations included linguistic strategies (e.g. bilingual and bicultural materials and staff, translation of materials), peripheral strategies (i.e. inclusion of culturally familiar activities and lifestyle elements), constituent involving strategies (e.g. use of community health workers, same race/ethnicity or status role models), delivery modality (e.g. delivery of the intervention in group settings), and setting (e.g. safe locations familiar to participants). Deep-level cultural adaptations refer to the integration of cultural elements into intervention activities and messages (as described in (21). Herein, deep-level cultural adaptations included the incorporation of sociocultural strategies (e.g. incorporation of cultural values in intervention design or implementation, involvement of family or important social members), linguistic strategies (e.g. adjustment of materials to literacy level), use of social support and networks, changes to the content of the intervention (e.g., inclusion of trauma-related sleep disturbances content), and changes to the core components of the intervention in order to address symptoms unique to that target population (e.g., combining imagery rehearsal therapy and behavioral therapy for veterans to address nightmares and other sleep disturbances).
We categorized articles accordingly to the type of psychological intervention. Primary psychological interventions of the selected studies included Acceptance and Commitment Therapy (ACT), Behavioral Therapy (BT), Cognitive Behavioral Therapy (CBT), Cognitive Behavioral Therapy for Insomnia (CBT-I), Cognitive Processing Therapy (CPT), Complementary and Alternative Medicine (CAM) therapies, Imagery Rehearsal Therapy (IRT), Mindfulness-based Stress Reduction (MBSR), and Problem-solving Therapy (PST). Also, Clinical Emotional Freedom Techniques, and Mission Reconnect program were grouped as Eclectic Therapy. CAM interventions that focused broadly on mind-body practices such as meditation, yoga, Tai-Chi, and relaxation practices were considered.
Risk of Bias in Individual Studies
Three of the authors (CA, LGC, IPI) independently assessed the risk of bias in all selected articles following the Cochrane Risk of Bias assessment tool (34). We assessed selection bias due to inadequate generation of a randomized sequence or allocation concealment, performance bias due to knowledge of the allocated interventions by participants and personnel during the study, detection bias due to knowledge of allocated interventions by outcome assessors, attrition bias due to the amount, nature, or handling of incomplete outcome data, and reporting bias due to selective outcome reporting. We rated whether included articles exhibited low risk, high risk or unclear risk across each bias domain. To calibrate our evaluation process for assessing risk of bias, we completed and discussed ratings on five randomly selected articles. We achieved adequate inter-rater reliability once we reached consensus on rating across the five bias domains. Next, each paper was independently assessed by two reviewers and all discrepancies were resolved through consensus among three of the authors (CA, LGC, IPI).
RESULTS
Study Selection
Overall, 8,182 unique records were identified and screened (Figure 1). The original search yielded 8,721 records, and 1 additional record was identified through other sources. A total of 7,378 records were excluded after the initial screen. A total of 803 full-text articles were then assessed for eligibility; of these 747 full-text articles were excluded. Reasons for exclusion included wrong setting (n=329), wrong study design (n=215), wrong participant population (n=184), wrong outcomes (n=9), wrong comparator (n=4), wrong language (n=4), wrong intervention (n=1), and full-text not retrieved (n=1). This resulted in a total of 56 studies that met inclusion criteria and were included in the qualitative synthesis. As such, only 6.97% of full-text articles assessed for eligibility focused on psychological interventions for sleep-wake disorders for underserved populations.
Figure 1.
PRISMA Flowchart of Review of Cultural Adaptations of Psychological Interventions for Prevalent Sleep Disorders and Sleep Disturbances. Final n includes two articles from one study (Berger et al, 2009a, Berger et al., 2009b).
Study and Trial Characteristics
Table 1 displays the study characteristics of the 56 RCT studies that met inclusion criteria. The average sample size was 85.23 participants (SD=54.49, Range=11–219) across arms, 40.91 (SD=26.18, Range=6–106) in the intervention arm, and 38.40 (SD=24.09, Range=5–91) in the control arm. The average percentage of women participants was 55.35% (SD =43.22). The average percentage of White participants was 64.86% (SD=21.67), followed by 28.16% (SD=23.38) who were Black participants, 9.23% (SD=10.35) Other Ethnicity participants, 9.45% (SD=10.23) Latina/o participants, and 4.06% (SD=4.26) Asian participants. The average age was 51.75 (SD=11.76, Range=29–77.1), and average education was 14.42 years (SD=1.20, Range=12–15.75). Of the included studies reporting employment (n=15) and disability status (n=6), the average percentage of participants who were unemployed was 40.78% (SD=26.13), and the average percentage of those with a disability was 36.46% (SD=31.81). By and large, studies did not include immigrants, linguistic minorities, or participants who identified as a sexual orientation minority (i.e., LGBTQ+), with two exceptions. One study reported that 24.02% of participants received treatment in Spanish, and another study reported that 4% of the sample was a gender minority (transgender). Twenty-three studies (41.07%) focused on women, one study (1.79%) focused on men in prison, five studies (8.93%) focused on older adults, and 25 studies (44.64%) focused on veteran population. One study focused on unemployed adults diagnosed with HIV/AIDS (1.79%).
Table 1.
Study characteristics of included studies of Cultural Adaptations of Psychological Interventions for Prevalent Sleep-Wake Disorders (N =56)
| Article | Underserved population | N | Women (%) | White (%) | Latino (%) | Black (%) | Asian (%) | Other (%) | Age M (SD) | Education M (SD); %)a | Income (%) | Disability (%) | Unemployment (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptance and Commitment Therapy | |||||||||||||
| 75. Herbert et al., 2017 | Veterans with chronic, nonterminal pain condition | 129 | 7.8 | 47.0 | 14 | 28 | 5 | 6 | 52 (13.3) | - | < $20,000 : 36 | - | - |
| 76. Lang et al., 2017 | Veterans | 160 | 20.0 | 75.0 | 13.1 | 11.3 | 3.8 | 10 | 34.2 (8) | 14.2 (2.1); HS or less 20.2 | < $10,000 : 13.1 $10,000 −24,999: 25.6 | - | 26.9 |
| 77. Mosher et al., 2018 | Women with Stage IV breast cancer | 47 | 100 | 89.4 | - | - | - | - | Tx: 59.3 (12.0); Ctrl: 53.3 (10.9) | Tx: 14.3 (2.1); Ctrl: 14.5 (2.6) | ≤ $50,999 : 31.9 | - | 4.3 |
| Behavioral Therapy | |||||||||||||
| 63, 64. Berger et al., 2009a,bc | Women with breast cancer | 219 | 100 | 96.5 | 3.5 | - | - | - | Tx:51.6; Ctrl: 52.9 | ≤ HS 19.7 | < $20,000 : 5.8 | - | - |
| 69. Gebara et al., 2019 | Older veterans | 11 | 27.3 | 81.8 | - | 18.2 | - | - | Tx: 64 (2.5); Ctrl: 66.2 (5.4) | <HS 18.2 | - | 27.3 | - |
| 65. Germain et al., 2012 | Veterans with sleep complains | 50 | 10.0 | 82.0 | - | - | - | - | Tx: 40 (14.1); Ctrl1: 39.4 (11.9); Ctrl2: 43.6 (14.) | - | - | - | - |
| 66. Germain et al., 2014 | Combatexposed Military Veterans | 40 | 15.0 | 78.0 | - | - | - | - | Tx: 40.9 (12.0); Ctrl: 35.9 (11.2) | - | - | - | - |
| 67. Johnson et al., 2016 | Veteran men with nocturia | 72 | 0 | 47.2 | 1.4 | 45.8 | - | 4.2 | Tx: 66.2 (12.8); Ctrl.1: 63.7 (11.7); Ctrl.2: 67.8 (10.8) | - | < $30,000 : 61.1 | - | - |
| 70. Lee et al., 2020d | Adults diagnosed with | 51 | 26.0 | 49.0 | - | 38. | - | - | 57 (6.8) | ≤ HS 28 | - | - | 100e |
| HIV/AIDS with fatigue | |||||||||||||
| 68. Martin et al., 2017 | Veterans 60 yrs or older | 42 | 7.1 | 71.4 | - | - | - | - | 77.1 (9.9) | 14.5 (2.5) | - | - | 100 |
| Cognitive Behavioral Therapy | |||||||||||||
| 71. Brenes et al., 2016 | Rural older adults | 141 | 81.6 | 90.8 | - | 5.7 | - | 3.5 | 66.8 (6.2) | < HS: 5 | ≤ $24,999 : 23.4 | - | - |
| 72. Heapy et al., 2017 | Veterans with chronic back pain | 125 | 22.4 | 64.5 | 7.3 | 25.8 | - | 2.4 | 57.9 (11.6) | 13.9 (2.1) | - | 18.4 | 15.2 |
| 73. Stanley et al., 2016 | Older adults from lowincome, or minority communities with anxiety worry | 40 | 9.0 | 15.0 | 2.5 | 82.5 | - | 2.5 | 62.9 (8) | 13.6 (1.8) | < $10,000 : 38.5 $10,000 - $20,000 : 28.2 | 26.3 | - |
| 74. Stanley et al., 2018 | Older adults from lowincome, or minority communities with anxiety or worry | 134 | 81.3 | 17.1 | 4.5 | 76.8 | - | 1.5 | 66.9 (9.2) | 13.6 (2.8) | < $10,000 : 26.9 | - | - |
| Cognitive Behavioral Therapy for Insomnia | |||||||||||||
| 35. Alessi et al., 2016 | Older veterans with insomnia | 159 | 3.1 | 78.6 | 6.3 | 4.4 | - | 7.6 | 72.2 (7.7) | < HS: 3.8, HS: 15.7 | - | - | 76.1 |
| 50. Cain et al., 2019 | Pregnant women | 53 | 100 | 41.5 | 26.4 | 56.6 | - | 3.8 | 29.1 (5.6) | - | - | - | - |
| 51. Chakravorty et al., 2019 | Alcoholdependent veterans | 22 | 0 | - | - | 73 | - | - | 54.5 (6.9) | 12 (0.8) | - | - | 64 |
| 36. Drake et al., 2019 | Postmenopaus al women with insomnia | 150 | 100 | 52.0 | 0.7 | 39.3 | - | 2 | 56.4 (5.6) | - | - | - | - |
| 38. Edinger et al., 2009 | Veterans with insomnia | 81 | 13.6 | 58.0 | - | - | - | - | 54.2 (13.7) | - | - | - | - |
| 37. Edinger & Sampson, 2003 | Veterans | 20 | 14.0 | 75.0 | 0 | 25 | 0 | 0 | 51 (13.7) | - | - | - | - |
| 39. Epstein & Dirksen, 2007 | Women with breast cancer | 72 | 100 | 95.8 | 0 | 2.8 | 0 | 1.4 | Tx: 57.1 (9.8); Ctrl: 59.1 (10.6) | Tx: 15.7 (3); Ctrl: 15.2 (2.5) | - | - | - |
| 40. Fiorentino et al., 2010 | Breast cancer survivors | 14 | 100 | 85.7 | 7.1 | - | 7.1 | 7.1 | 61 (11.6) | - | - | - | - |
| 41. Fung et al., 2016 | Older veterans | 134 | 3.0 | 78.4 | - | - | - | - | 72.2 (7.7) | <HS 4.5; HS 14.2 | - | - | - |
| 52. Kalmbach et al., 2019 | Postmenopaus al women | 150 | 100 | 52 | 0.7 | 39.3 | - | 2 | 56.4 (5.6) | - | - | - | 28.7 |
| 42. Laurel Franklin et al., 2018 | Rural veterans with PTSD | 18 | 0 | 28 | - | 66 | - | 6 | 53.8 (12) | HS 22.3 | - | - | 28 |
| 54. Manber et al., 2019f | Pregnant women | 179 | 100 | 48 | 38 | 3.4 | 14.5 | 28.5 | 33 | - | - | - | - |
| 43. Margolies et al., 2013 | Veterans with PTSD | 40 | 10.0 | 40 | - | 60 | - | - | 37.7 (9.1) | - | - | - | - |
| 44. Matthews et al., 2014 | Women breast cancer survivors with chronic insomnia | 56 | 100 | - | - | - | - | - | Tx: 52.2 (6.9); Ctrl: 52.9 (7.8) | C: 66.1 | Tx: $60,001 $80,000 ; Ctrl: $40,001 $60,000 b | - | - |
| 45. McCurry et al., 2016 | Postmenopaus al women with insomnia symptoms and hot flashes | 106 | 100 | 91.5 | - | 0.9 | - | 7.6 | 54.8 (4.2) | < HS or GED: 4.7 | - | - | - |
| 46. Palesh et al., 2018 | Women with Stage I-III breast cancer undergoing chemotherapy | 71 | 100 | 9 | 0 | 3 | 1 | 0 | 52.5 (9.8) | - | - | - | - |
| 47. Pigeon et al., 2017 | Veterans with a diagnosis of MDD and sleep problems | 27 | 11.1 | 81.5 | - | 18.5 | - | - | - | < HS 7.4; HS 37 | - | - | 29.6 |
| 53. Pigeon et al., 2019 | Veterans | 50 | 20.0 | 70 | 4 | - | - | - | 54.8 | ≤ HS or GED 38 | - | - | - |
| 48. Scogin et al., 2018 | Rural middleaged and older adults | 40 | 90 | 57.5 | - | - | - | - | 58.1 (5.6) | Tx: 13.5 5); Ctrl: 12.7 (1.9) | - | 32.5 | 17.5 |
| 49. Ulmer et al., 2011 | Veterans with PTSD | 22 | 38.8 | 33.3 | - | 33.3 | - | 33.3 | 46 (11.1) | - | - | - | - |
| Cognitive Processing Therapy | |||||||||||||
| 78. Galovski et al., 2016 | Women interpersonal assault survivors | 92 | 100 | 50 | 3 | 50 | - | 20 | 36.9 (11.8) | 14.2 (2.9); > HS: 50% | ≤ $20,000 : 74 | - | - |
| 79. Galovski et al., 2009 | Women sexual assault survivors suffering from PTSD | 108 | 100 | 84.3 | - | - | - | - | 33 (10.) | 15 (2) | ≤ $20,000 : 29.6 | - | - |
| 80. Gutner et al., 2013 | Women rape victims with PTSD | 171 | 100 | 71.6 | - | 25.4 | - | 3 | ITT sample 32 (10) | 14.4 (2.3) | - | - | - |
| Complementary and Alternative Therapy | |||||||||||||
| 55. Innes & Selfe, 2012 | Older women | 20 | 100 | 75 | - | 25 | - | 20 | Tx: 58.4 (2); Ctrl: 58.9 (2.9) | <4 years C: 35% | - | - | 35 |
| 56. Irwin et al., 2017 | Breast cancer Survivors with insomnia | 90 | 100 | 85.6 | - | - | - | - | Tx: 59.6 (7.9); Ctrl: 60 (9.3) | Tx: 15.8 (1.2); Ctrl: 15.7 (1.4) | - | - | 38.9 |
| 61. Jamison et al., 2019 | Veterans with current or recent PTSD | 80 | 12.5 | 43.8 | - | 22.5 | 7.5 | 26.3 | 53.1 (10.8) | - | - | - | - |
| 57. Nakamura et al., 2017 | Veterans with Gulf War Illness Symptoms | 60 | 10 | 85 | 8.3 | 6.7 | 0 | 5 | 50.7 (7.3) | - | - | - | - |
| 58. Nakamura et al., 2011 | Veterans with self-reported sleep disturbance | 63 | 4.8 | - | - | - | - | - | Tx: 49.9 (10.3); Ctrl: 53.8 (10.4) | - | - | - | - |
| 59. Nidich et al., 2016 | Men in prison | 181 | 0 | 52.5 | 6.6 | 16 | - | 24.9 | 29 | - | - | - | - |
| 62. Porter et al., 2019 | Women with metastatic breast cancer | 63 | 100 | 74 | - | - | - | - | 57.3(11.5) | 17(6.9); HS/SC: 28.6 | ≤ $50,000 : 39.7 | - | 71.4 |
| 60. Stoerkel et al., 2018 | Women with breast cancer for whom surgery would be their initial treatment | 100 | 100 | 54 | 14 | 20 | 4 | - | 31–35: 1%; 36–45: 11%; >45: 81% | <HS:0; HS: 10% | - | - | - |
| Eclectic Therapy | |||||||||||||
| 85. Alschuler et al., 2018 | Adults with multiple sclerosis that causes disability | 28 | 92.9 | 82.1 | - | 7.1 | 3.6 | 3.6 | Tx: 59.8 (7.7); Ctrl: 59.8 (6.5) | HS or GED: 7.1% | - | 100 | - |
| 86. Church et al., 2016 | Veterans with PTSD | 21 | 33.0 | - | - | - | - | - | 56 | - | - | - | - |
| 87. Kahn et al., 2016 | Veterans | 160 | 18.8 | 52.5 | 25.4 | 11.6 | 5 | 7.7 | 33.4 (6.6) | < HS: 0; HS or GED: 11 | - | - | - |
| Imagery Rehearsal Therapy | |||||||||||||
| 89. Cook et al., 2010 | Male veterans with chronic, severe PTSD | 124 | 0 | 41.9 | - | 51.6 | - | 6.4 | 59.4 (3.6) | <HS: 15.3; HS: 39.5 | - | - | 30.3 |
| 90. Harb et al., 2019 | Veterans | 108 | 13.9 | 58.3 | - | 37 | - | 4.6 | 37.1 | ≤ HS 34.3 | - | - | 56.5e |
| 88. Krakow et al., 2001 | Women sexual assault survivors with PTSD | 168 | 100 | 62.5 | - | - | - | 37.5 | Tx: Completer 40 (11.2), Noncomple ter 37 (12.7); Ctrl: Completer 36 (9.3), Noncomple | < C: 62 | ≤ $10,000 : 45 ter 31 (10.5) | - | - |
| Mindfulness-based Stress Reduction | |||||||||||||
| 81. Cash et al., 2015 | Women with fibromyalgia | 91 | 100 | - | - | - | - | - | - | - | - | - | - |
| 82. Lengacher et al., 2015 | Women diagnosed with breast cancer | 79 | 100 | 73.4 | 11.4 | 10.1 | - | 5.1 | 57 (9.7) | <HS: 20 | < $10,000 : 11.7 $10,000 - $20,000 : 20.8 | - | - |
| 83. Shapiro et al., 2003 | Women with Stage II breast cancer who were cancerfree at the time of the study | 63 | 100 | 85.7 | 7.9 | 3.2 | - | - | 57 (9.7) | HS: 49.2 | - | 14.3 | - |
| 84. Witek Janusek et al., 2019 | Women with breast cancer | 164 | 100 | 76.8 | 4.3 | 14 | 1.2 | 1.8 | 55.1 | - | $10,000 - $29,000 : 17.7 | - | - |
| Problem-solving Therapy | |||||||||||||
| 91. Bedford et al., 2018 | College student veterans with at least mild depression | 24 | 20.8 | 70.8 | 29.2 | - | - | - | 32.7 (7.5) | - | - | - | 45.8 |
Note: Separate values for treatment and control condition were reported if an overall value was not reported; Reference number precedes author name in Article column. PTSD= Post-Traumatic Stress Disorder; MDD=Major Depressive Disorder
HS: High School, SC: Some College, C: College graduate
Income refers to median annual income
Values reported are from Berger et al., 2009b
Lee et al., 2020 was the only article to report percentage gender minority characteristics: Transgender 4%
Lee et al., 2020: unemployed included people who were unemployed, retired, and/or had a disability; Hard et al., 2019: unemployed included people who were unemployed and/or retired
Manber et al., 2019 reported 24.02% of participants received treatment in Spanish.
There was variation in the primary psychological interventions tested. Twenty (35.71%) studies tested CBT-I as the primary intervention (35–54). Eight (14.29%) tested Complementary and Alternative Therapies (CAM) (55–62). Seven (12.50%) tested Behavioral Therapy (BT) (63–70). Four (7.14%) tested Cognitive Behavioral Therapy (CBT) (71–74). Three (5.36%) tested Acceptance and Commitment Therapy (ACT) (75–77). Three (6.67%) tested Cognitive Processing Therapy (CPT) (78–80). Four (7.14%) tested Mindfulness Based Stress Reduction (MBSR) (81–84). Three (5.36%) tested some form of Eclectic Therapy (ET) (85–87). Three (5.36%) tested Imagery Rehearsal Therapy (IRT) (88–90). One (1.79%) tested Problem-Solving Therapy (PST) (91).
Of included studies, 57.14% (n=32) included participants with a definite diagnosis of a sleep disorder or endorsement of a sleep disturbance, 16.07% (n=9) included participants with a probable sleep disturbance, and 26.79% (n=15) included participants with a potential sleep disturbance (Table 2). Of studies with a definite sleep disorder or sleep disturbance diagnosis, 75% targeted insomnia, 9.38% targeted nightmares and poor sleep quality, 6.25% targeted poor sleep quality, 3.12% targeted nightmares and insomnia, 3.12% targeted nocturia, and 3.12% targeted restless legs syndrome. All of the intervention studies testing CBT-I or IRT had a definite diagnosis or endorsement of a sleep disorder or sleep disturbance. Roughly half of the studies testing BT or CAM interventions had a definite diagnosis or endorsement of a sleep disorder or sleep disturbance. Studies on ACT, CBT, CPT, ET, MBSR, and PST were largely if not exclusively conducted with participants who had a probable or potential sleep disorder or sleep disturbance. There was also variation in the delivery modality for the primary intervention. The most common comparator was sleep hygiene or a sleep education program. Three RCTs (5.3%) tested the effectiveness of a surface-level culturally adapted behavioral intervention against the same un-adapted intervention. The overwhelming majority of studies (91.07%) utilized a 2-arm RCT trial design. The average trial duration was 7.69 weeks (SD=4.51), and the range for intervention follow-up was two weeks to up to 10 years. Importantly, eleven of studies (19.64%) tested telehealth delivery (telephone, teleconference, internet, mobile, audio files).
Table 2.
Trial Characteristics of Psychological Interventions for Prevalent Sleep-Wake Disorders (N =56)
| Article | Primary Intervention | Sleep disorder or disturbance | Determination | Delivery Modality | Comparator | N Tx/Ctrl | Trial Design | Duration (Intervention/FU) |
|---|---|---|---|---|---|---|---|---|
| Acceptance and Commitment Therapy | ||||||||
| 75. Herbert et al., 2017 | VTC-ACT | - | Potential | Video teleconferencing | In person ACT | 64/65 | 2 arm RCT | 8w/6m |
| 76. Lang et al., 2017 | ACT | - | Probable | In Person (1:1) | Present-Centered Therapy | 80/80 | 2 arm RCT | 12w/3,6,9,12m |
| 77. Mosher et al., 2018 | ACT | - | Probable | Telephone | Education/Support | 23/24 | 2 arm RCT | 6w/8,12w post-baseline |
| Behavioral Therapy | ||||||||
| 63,64. Berger et al., 2009a,b1 | BT | - | Potential | In Person (1:1) | Healthy Eating | 88/85 | 2 arm RCT | 1m/90,365d |
| 69. Gebara et al., 2019 | BBTI | Insomnia | Definite | In Person (1:1) and telephone sessions | Wait-list | 6/5 | 2 arm RCT | 4w/4w |
| 65. Germain et al., 2012 | BT,IRT | Nightmares + Poor Sleep Quality | Definite | In Person (1:1) and telephone sessions | Medication Arms (Placebo or Prazosin) | 17/ Ctrl.1 18, Ctrl.2 15 | 3 arm RCT | 8w/4m |
| 66. Germain et al., 2014 | BT | Insomnia | Definite | In Person (1:1) and telephone sessions | Sleep Education | 20/20 | 2 arm RCT | 4w/6m |
| 67. Johnson et al., 2016 | M-BET | Nocturia | Definite | In person or telephone (1:1) | Standard drug therapy for nocturia (α- blocker) and combined M-BET + α-blocker | 23 / Ctrl.1 25, Ctrl.2 24 | 3 arm RCT | 10w/12w post-baseline |
| 70. Lee et al., 2020 | BT | - | Potential | In Person (1) and telephone sessions | Attention control group (dietary strategies) | 25/26 | 2 arm RCT | 4w/1,2,3m |
| 68. Martin et al., 2017 | BT | - | Potential | - | Sleep Education | 21/21 | 2 arm RCT | 4w/4m |
| Cognitive Behavioral Therapy | ||||||||
| 71. Brenes et al., 2016 | CBT | - | Probable | Telephone and workbook | Nondirective Supportive TherapyTelephone | 70/71 | 2 arm RCT | 11w/4,9,15m post-randomization |
| 72. Heapy et al., 2017 | IVR-CBT | - | Potential | Telephone | In-person CBT | 62/63 | 2 arm RCT | 10w/3,6,9m |
| 73. Stanley et al., 2016 | CBT | - | Probable | In person (1:1 first session) and patient preference (telephone or in person) | Community Resources and Emotional Support | 20/20 | 2 arm RCT | 3m/- |
| 74. Stanley et al., 2018 | CBT | - | Probable | Patient preference (telephone or in person) and telephone booster sessions | Enhanced Community Care with Resource Counseling | 70/64 | 2 arm RCT | 6m/9m |
| Cognitive Behavioral Therapy for Insomnia | ||||||||
| 35. Alessi et al., 2016 | CBT-I | Insomnia | Definite | In person (smallgroups of 3–5 ppl or 1:1) | Sleep Education | 106/53 | 2 arm RCT | 6w/6,12m |
| 50. Cain et al., 2019 | CBT-I | Insomnia | Definite | In Person (Group) and online | Group prenatal visits | 27/26 | 2 arm RCT | 7w/- |
| 51. Chakravorty et al., 2019 | CBT-I | Insomnia | Definite | In Person (1:1) | Usual Care | 11/11 | 2 arm RCT | 8w/3,6m |
| 36. Drake et al., 2019 | CBT-I | Insomnia | Definite | In person | Sleep Hygiene, Sleep Restriction | 50/50,50 | 3 arm RCT | 6w/6m |
| 37. Edinger & Sampson, 2003 | CBT-I | Insomnia | Definite | In Person (1:1) and take-home audiocassettes and educational materials | Sleep Hygiene | 10/9 | 2 arm RCT | 2w/3m after treatment |
| 38. Edinger et al., 2009 | CBT-I | Insomnia | Definite | In Person (1:1) | Sleep Hygiene | 41/40 | 2 arm RCT | 8w/6m |
| 39. Epstein & Dirksen, 2007 | CBT-I | Insomnia | Definite | In Person (Group) and telephone sessions | Sleep Education and Hygiene | 34/38 | 2 arm RCT | 6w/2w posttreatment |
| 40. Fiorentino et al., 2010 | CBT-I | Insomnia | Definite | In Person (1:1) | Delayed Treatment | 6/8 | 2 arm RCT | 6w/6w posttreatment |
| 41. Fung et al., 2016 | CBT-I | Insomnia | Definite | In Person (1:1 and Group) | Sleep Education | - | 2 arm RCT | 6w/6m and 12m post-baseline |
| 52. Kalmbach et al., 2019 | CBT-I | Insomnia | Definite | In Person (1:1) | Sleep Hygiene, Sleep Restriction | 50/50 | 3 arm RCT | 6w/2w,6m |
| 42. Laurel Franklin et al., 2018 | Telephone based CBT-I | Insomnia | Definite | Telephone | In person CBT-I | 11/7 | 2 arm RCT | 8w/1,3m |
| 54. Manber et al., 2019 | CBT-I | Insomnia | Definite | In Person (1:1) | Modified Pseudo Desensitization Therapy for Insomnia | 89/90 | 2 arm RCT | 5w/- |
| 43. Margolies et al., 2013 | CBT-I, IRT | Insomnia | Definite | In Person (1:1) | Wait-list | 20/20 | 2 arm RCT | 6w/- |
| 44. Matthews et al., 2014 | CBT-I | Insomnia | Definite | In Person (1:1) | Behavioral Placebo Treatment | 30/26 | 2 arm RCT | 6w/3,6m |
| 45. McCurry et al., 2016 | Telephonebased CBT-I | Insomnia | Definite | Telephone (first session in person or telephone) | Menopause Education | 53/53 | 2 arm RCT | 8w/8,24w posttreatment |
| 46. Palesh et al., 2018 | BBT-CI | Insomnia | Definite | In person and telephone | Health Eating Education Learning | 37/37 | 2 arm RCT | 3–4w/1m |
| 47. Pigeon et al., 2017 | Brief CBT-I | Insomnia | Definite | In person and telephone | Sleep Hygiene | 13/14 | 2 arm RCT | 4w/3m posttreatment |
| 53. Pigeon et al., 2019 | Brief CBT-I | Insomnia | Definite | In Person (1:1) | Usual Care | 24/26 | 2 arm RCT | 6w/- |
| 48. Scogin et al., 2018 | Integrated CBTD+CBT-I | Insomnia | Definite | Video teleconferencing | Usual C are | 22/18 | 2 arm RCT | 10w/3m |
| 49. Ulmer et al., 2011 | CBT-I, IRT | Insomnia | Definite | In Person (1:1) | Usual Care | 12/9 | 2 arm RCT | 12w/10y |
| Cognitive Processing Therapy | ||||||||
| 78. Galovski et al., 2016 | CPT,H | Insomnia | Definite | In Person (1:1) | Symptom monitoring control condition before beginning standard CPT | 44/48 | 2 arm RCT | 15w/3m posttreatment |
| 79. Galovski et al., 2009 | CPT | - | Probable | In Person (1:1) | Prolonged Exposure | 54/54 | 2 arm RCT | 6w/2w, 9m posttreatment |
| 80. Gutner et al., 2013 | CPT | - | Probable | In Person (1:1) and home practice assignments | Prolonged Exposure | 81/90 | 2 arm RCT | 6w/3,9m, longterm: 5 to 19y |
| Complementary and Alternative Therapy | ||||||||
| 55. Innes & Selfe, 2012 | Y | RLS | Definite | In Person (Group) and home practice assignments | Educational film | 10/10 | 2 arm RCT | 8w/- |
| 56. Irwin et al., 2017 | TCC | Insomnia | Definite | In Person (Group) | CBT-I | 45/45 | 2 arm RCT | 3m/6,15m |
| 61. Jamison et al., 2019 | CART | - | Probable | In Person (1:1) | Wait-list | 47/33 | 2 arm RCT | 4w/1,6m |
| 58. Nakamura et al., 2011 | MBB | Poor sleep quality | Definite | - | Sleep Hygiene | 35/28 | 2 arm RCT | 2w/- |
| 57. Nakamura et al., 2017 | MBB | Poor sleep quality | Definite | In Person (Group) | Sleep Education | 33/27 | 2 arm RCT | 3w/3m |
| 59. Nidich et al., 2016 | TM | - | Potential | In Person (Group) | No-treatment | 90/91 | 2 arm RCT | 4m/4m |
| 62. Porter et al., 2019 | Y | - | Potential | In Person (1:1) | Social Support Group | 43/20 | 2 arm RCT | 8w/6m |
| 60. Stoerkel et al., 2018 | SCT | - | Potential | Audio-files | Usual Care | 51/49 | 2 arm RCT | 2w/2w |
| Elective Therapy | ||||||||
| 85. Alschuler et al., 2018 | RI | - | Potential | Teleconference for groups and take-home materials | Wait-list | 12/16 | 2 arm RCT | 6w |
| 86. Church et al., 2016 | TAU,EFT | - | Probable | In Person (1:1) | Treatment as usual wait-list | 12/9 | 2 arm RCT | 6m/3,6m |
| 87. Kahn et al., 2016 | MR | - | Potential | Internet and mobile | MR + PREP for Strong Bonds program, PREP only, waitlist | 40/MR+PREP 40; PREP 40; Waitlist Control 40 | 4 arm RCT | 16w/8,16 |
| Imagery Rehearsal Therapy | ||||||||
| 89. Cook et al., 2010 | IRT | Nightmares+ Poor sleep quality | Definite | In Person (Group) | Sleep and Nightmare Management Treatment | 61/63 | 2 arm RCT | 6w/1,3,6m |
| 90. Harb et al., 2019 | IRT, CBT-I | Nightmares + Poor sleep quality | Definite | In Person (1:1) | CBT-I | 53/55 | 2 arm RCT | 6w3,6m |
| 88. Krakow et al., 2001 | IRT | Nightmares + Insomnia | Definite | In Person (Group) | Wait-list | 88/80 | 2 arm RCT | 6w/3,6m |
| Mindfulness-based Stress Reduction | ||||||||
| 81. Cash et al., 2015 | MBSR | - | Potential | In Person (Group) and home practice assignments | Wait-list | 51/40 | 2 arm RCT | 8w/2m |
| 82. Lengacher et al., 2015 | MBSR | - | Potential | - | Usual Care | 38/41 | 2 arm RCT | 6w/12w |
| 83. Shapiro et al., 2003 | MBSR | - | Potential | In Person (Group) | Stress management | 31/32 | 2 arm RCT | 6w/3,9m |
| 84. Witek Janusek et al., 2019 | MBSR | - | Potential | In Person (Group) | Active Control | 84/80 | 2 arm RCT | 8w/1,6m |
| Problem-solving Therapy | ||||||||
| 91. Bedford et al., 2018 | ePST | - | Potential | Online | Minimal contact | 12/12 | 2 arm RCT | 6w/12 following the initial treatment session |
Note: Determination refers to the likelihood of the sleep disorder or disturbance based on review of the participant inclusion and exclusion criteria. Reference number precedes author name in Article column. ACT=Acceptance and Commitment Therapy; BBT-CI=Brief Behavioral Therapy for Cancer-Related Insomnia; BT=Behavioral Therapy; BTI-MV=Brief Behavioral Treatment of Insomnia; CBT=Cognitive Behavioral Therapy; CBT-I=Cognitive Behavioral Therapy for Insomnia; CPT=Cognitive Processing Therapy; EFT=Clinical Emotional Freedom Techniques; ePST=computer guided Problem-Solving Treatment; H=Hypnosis; IRT=Imagery Rehearsal Therapy; Integrated CBT-D+CBT-I= Integrated CBT (CBT for depression and CBT for insomnia); IVR-CBT=Interactive voice response-based cognitive behavioral therapy; MBB=Mind-body Bridging; M-BET=Multicomponent behavioral combined with exercise therapy; MBSR=Mindfulness-based stress reduction; MR=Mission Reconnect program; RI=Resilience intervention; SCT=Self-care toolkit; TAU=Treatment as usual ; TCC=Tai Chi Chih; TM=Transcendental Meditation; VTC-ACT=Video teleconferencing Acceptance and Commitment Therapy; Y=Yoga.
Definite= directly assessed for and confirmed the existence of a sleep disorder or sleep disturbance and included this in the inclusion criteria; Probable=directly assessed for any sleep disturbance but did not include endorsement of a sleep disturbance as part of the inclusion criteria. Potential= sleep did not assess for a sleep disturbance but based on the scientific literature would likely have a sleep disturbance concomitant with the co-morbid health or mental health condition.
Values reported are from Berger et al., 2009b.
Risk of Bias Within Studies
Table 3 reports the risk of bias assessment for all included studies. While 42 (75.0%) of the included studies had low risk of selection bias due to the use of random sequence generation in the randomized controlled trial design, 14 studies or 25% did not describe the random sequence generation method used and were assessed as unclear risk of bias. Similarly, ratings of selection bias due to allocation concealment were much more mixed. Twenty-seven (48.21%) were rated as low risk of bias for allocation concealment, two (3.57%) were rated high risk of bias for allocation concealment, and 27 (48.21%) were rated unclear risk of bias for allocation concealment because there was insufficient information to permit judgment of risk. Twenty-four studies (42.86%) were rated as high risk for performance bias due to lack of blinding of participants and personnel, whereas of the remaining studies, 18 (32.14%) did not describe any measures used to blind study participants and personnel from knowledge of which intervention the participant received and as such were rated as unclear risk of performance bias, and 14 (25.00%) were rated low risk of performance bias. Twenty-four studies (42.86%) were rated as low risk for detection bias due to blinding of outcome assessors, whereas nine (16.07%) were rated high risk for detection bias because the outcome assessors had knowledge of the intervention assignment, and 23 (41.07%) were rated as unclear risk of detection bias because there was insufficient information to permit judgment of risk. The overwhelming number of studies (n=41, 73.21%) were rated low risk for attrition bias due to the amount, nature, or handling of incomplete data, whereas 11 (19.64%) were rated high risk of attrition bias, and four (7.14%) were rated as unclear risk for attrition bias. Twenty-six studies (46.43%)were rated unclear risk for reporting bias because insufficient information was provided to evaluate low or high risk for reporting bias due to selective outcome, 28 (50%) were rated low risk for reporting bias, and two (3.57%) were rated high risk of reporting bias. Of note, only five studies (8.93%) were rated as low risk of bias across the six domains, thereby representing the highest quality studies (35, 36, 56, 68, 90). Of these, three evaluated CBT-I or BT as the primary intervention against sleep education (35, 36, 68), one evaluated Tai Chi Chih against CBT-I (56), and another evaluated IRT and CBT-I against CBT-I alone in veterans (90).
Table 3.
Risk of Bias Assessment of Included Studies of Psychological Interventions for Sleep-Wake Disorders
| Article | Selection Bias: Random Sequence Generation | Selection Bias: Allocation Concealment | Performance Bias | Detection Bias | Attrition Bias | Reportin’ Bias |
|---|---|---|---|---|---|---|
|
| ||||||
| 75. Herbert et al., 2017 | Low | Low | High | Low | Low | Low |
| 76. Lang et al., 2017 | Low | Low | Unclear | Unclear | Low | Unclear |
| 77. Mosher et al., 2018 | Low | Unclear | Unclear | Low | Low | Unclear |
| 63,64. Berger et al., 2009a,b | Low | Low | High | Unclear | Low | Unclear |
| 69. Gebara et al., 2019 | Unclear | Unclear | High | Unclear | Low | Unclear |
| 65. Germain et al., 2012 | Low | Unclear | Unclear | High | Low | Low |
| 66. Germain et al., 2014 | Low | Unclear | High | Unclear | Low | Low |
| 67. Johnson et al., 2016 | Low | Low | Unclear | Low | High | Low |
| 70. Lee et al., 2020 | Low | Unclear | Unclear | Unclear | Low | Low |
| 68. Martin et al., 2017 | Low | Low | Low | Low | Low | Low |
| 71. Brenes et al., 2016 | Unclear | Unclear | Unclear | Low | High | Low |
| 72. Heapy et al., 2017 | Low | Low | High | High | Low | Low |
| 73. Stanley et al., 2016 | Low | Unclear | Unclear | Low | Low | Low |
| 74. Stanley et al., 2018 | Low | Low | Unclear | Unclear | Low | Low |
| 35. Alessi et al., 2016 | Low | Low | Low | Low | Low | Low |
| 50. Cain et al., 2019 | Low | Low | High | High | Low | High |
| 51. Chakravorty et al., 2019 | Low | Low | High | Unclear | Low | Low |
| 36. Drake et al., 2019 | Low | Low | Low | Low | Low | Low |
| 37. Edinger & Sampson, 2003 | Unclear | Unclear | High | Unclear | Low | Unclear |
| 38. Edinger et al., 2009 | Low | Unclear | High | Unclear | Unclear | Low |
| 39. Epstein & Dirksen, 2007 | Low | Unclear | High | Unclear | High | Unclear |
| 40. Fiorentino et al., 2010 | Low | Unclear | High | Unclear | High | Unclear |
| 41. Fung et al., 2016 | Low | Low | Low | Low | High | Unclear |
| 52. Kalmbach et al., 2019 | Unclear | Unclear | Low | Low | Unclear | High |
| 42. Laurel Franklin et al., 2018 | Low | Unclear | Low | Low | High | Low |
| 54. Manber et al., 2019 | Low | Low | Low | Low | High | Low |
| 43. Margolies et al., 2013 | Unclear | Unclear | High | High | Low | Unclear |
| 44. Matthews et al., 2014 | Low | Low | Low | Unclear | Low | Unclear |
| 45. McCurry et al., 2016 | Unclear | Unclear | Low | Low | Low | Low |
| 46. Palesh et al., 2018 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| 47. Pigeon et al., 2017 | Low | Unclear | Unclear | Low | High | Unclear |
| 53. Pigeon et al., 2019 | Low | Low | High | Low | Low | Low |
| 48. Scogin et al., 2018 | Unclear | Unclear | Unclear | Low | High | Unclear |
| 49. Ulmer et al., 2011 | Unclear | Unclear | Unclear | High | Low | Unclear |
| 78. Galovski et al., 2016 | Low | Low | Unclear | Unclear | Low | Unclear |
| 79. Galovski et al., 2009 | Unclear | Unclear | Unclear | Low | Low | Unclear |
| 80. Gutner et al., 2013 | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| 55. Innes & Selfe, 2012 | Low | Low | High | Low | Unclear | Unclear |
| 56. Irwin et al., 2017 | Low | Low | Low | Low | Low | Low |
| 61. Jamison et al., 2019 | Unclear | Unclear | High | High | Low | Low |
| 58. Nakamura et al., 2011 | Low | Low | Unclear | Unclear | Low | Unclear |
| 57. Nakamura et al., 2017 | Low | Unclear | High | Low | Low | Low |
| 59. Nidich et al., 2016 | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| 62. Porter et al., 2019 | Low | Low | Low | Low | High | Unclear |
| 60. Stoerkel et al., 2018 | Low | Unclear | High | Unclear | Low | Low |
| 85. Alschuler et al., 2018 | Low | Unclear | High | Unclear | Low | Low |
| 86. Church et al., 2016 | Low | Low | High | High | Low | Unclear |
| 87. Kahn et al., 2016 | Low | Low | High | High | Low | Low |
| 89. Cook et al., 2010 | Low | Low | Unclear | Low | Low | Unclear |
| 90. Harb et al., 2019 | Low | Low | Low | Low | Low | Low |
| 88. Krakow et al., 2001 | Low | Unclear | High | Low | Low | Unclear |
| 81. Cash et al., 2015 | Low | High | High | Unclear | Low | Low |
| 82. Lengacher et al., 2015 | Low | Low | Low | Unclear | Low | Low |
| 83. Shapiro et al., 2003 | Unclear | High | High | Unclear | Unclear | Unclear |
| 84. Witek Janusek et al., 2019 | Low | Low | Low | Unclear | Low | Low |
| 91. Bedford et al., 2018 | Low | Low | High | High | Low | Unclear |
Note: Reference number precedes author name in Article column.
Results of Individual Studies
Types of Cultural Adaptations.
Thirty-six studies (64.29%) conducted either a surface -and/or deep-level cultural adaptation of a psychological intervention for one of the specified underserved groups, whereas the remaining 20 (35.71%) did not adapt the intervention to the underserved target population group (Table 4). In particular, of the adapted studies, 17 (30.36%) conducted both a surface-level and a deep-level cultural adaptation, 16 studies (28.57%) conducted only a surface-level adaptation, and three studies (5.36%) conducted only a deep-level cultural adaptation. Of studies having conducted a cultural adaptation, CBT-I (n=14; 38.89%) BT (n=5; 13.89%), CBT (n=4; 11.11%), and CAT (n=4, 11.11%) were the most commonly adapted treatments. Cognitive Processing Therapy and Problem-Solving Therapy were not adapted for any of the specified underserved target populations identified in this review. There was wide variation in the types of surface- or deep-level cultural adaptations made across psychological interventions.
Table 4.
Types of Cultural Adaptations of Psychological Interventions for Prevalent Sleep-Wake Disorders (N=56)
| Article | Underserved Population | Primary Intervention | Cultural adaptationa | Surface adaptation | Deep adaptation |
|---|---|---|---|---|---|
| Acceptance and Commitment Therapy | |||||
| 75. Herbert et al., 2017 | Veterans with chronic, nonterminal pain condition | VTC-ACT | Surface | Delivery modality (video conferencing) | - |
| 76. Lang et al., 2017 | Veterans | ACT | None | - | - |
| 77. Mosher et al., 2018 | Women with Stage IV breast cancer | ACT | Both | Delivery modality (telephone) | Content (inclusion of cancer related experiences) |
| Behavioral Therapy | |||||
| 63,64. Berger et al., 2009a,b | Women with breast cancer | BT | Both | Setting (safe location: Treatment took place in a location of patient’s preference or at home) | Core component (relaxed sleep hygiene guidelines, usual sleep time plus one hour in response to chemotherapy); Content (inclusion of patient reported symptoms after chemotherapy) |
| 69. Gebara et al., 2019 | Older veterans | BBTI | None | - | - |
| 65. Germain et al., 2012 | Veterans with sleep complains | BT,IRT | Deep | - | Core component (combined BT elements and IRT) |
| 66. Germain et al., 2014 | Combat-exposed Military Veterans | BTI-MV | Deep | - | Content (included information about military specific factors relating to sleep) |
| 67. Johnson et al., 2016 | Veteran men with nocturia | M-BET | None | - | - |
| 70. Lee et al., 2020 | Adults diagnosed with HIV/AIDS with fatigue and unemployed, retired or on disability | BT | Surface | Other (provision of hands on activities and materials) | - |
| 68. Martin et al., 2017 | Veterans 60 yrs or older | BT | Both | Treatment dose (duration; length of sessions) | Core component (substitution of sleep compression in place of sleep restriction therapy and modifications of standard stimulus control instructions) |
| Cognitive Behavioral Therapy | |||||
| 71. Brenes et al., 2016 | Rural older adults | CBT | Surface | Delivery modality (telephone) | - |
| 72. Heapy et al., 2017 | Veterans with chronic back pain | IVR-CBT | Surface | Delivery modality (interactive voice response) | - |
| 73. Stanley et al., 2016 | Older adults from lowincome, or minority communities with anxiety or worry | CBT | Both | Delivery modality (telephone or in person); Setting (at home or a community partner site); Treatment dose (duration; number of sessions) | Sociocultural strategies and cultural values (incorporated religion and/or spirituality coping skills) |
| 74. Stanley et al., 2018 | Older adults from low income, or minority communities with anxiety or worry | CBT | Both | Constituent-involving strategies (delivery modality by nonclinicians); Delivery modality (telephone or in person); Setting (at home or a community partner site); Treatment dose (duration; inclusion of monthly booster sessions for up to 3months) | Linguistic (adjustment of materials to literacy level); Sociocultural strategies and cultural values (incorporated religion and/or spirituality coping skills) |
| Cognitive Behavioral Therapy for Insomnia | |||||
| 35. Alessi et al., 2016 | Older veterans with insomnia disorder | CBT-I | Surface | Constituent-involving strategies (delivery modality by nonclinicians) | - |
| 50. Cain et al., 2019 | Pregnant women | CBT-I | Both | Setting (delivered at gynecological clinics ); Delivery modality (online); Constituent-involving strategies (intervention delivered by specialists in obstetrics and gynecology) | Core component (sessions addressed sleep hygiene during pregnancy and the postpartum period; sleep restriction was excluded from weekly discussions but remained in the online information and participants were advised not to utilize this method during pregnancy); Content (newborn care ) |
| 51. Chakravorty et al., 2019 | Alcohol-dependent veterans | CBT-I | Surface | Setting (safe location: Veterans hospital) | - |
| 36. Drake et al., 2019 | Postmenopausal women with insomnia | CBT-I | None | - | - |
| 37. Edinger & Sampson, 2003 | Veterans | CBT-I | None | - | - |
| 38. Edinger et al., 2009 | Veterans with primary/secondary insomnia | CBT-I | None | - | - |
| 39. Epstein & Dirksen, 2007 | Women with breast cancer | CBT-I | Surface | Delivery modality (group setting) | - |
| 40. Fiorentino et al., 2010 | Breast cancer survivors | CBT-I | Deep | - | Content (Inclusion of thoughts and fears of occurrence of cancer) |
| 41. Fung et al., 2016 | Older veterans | CBT-I | None | - | - |
| 52. Kalmbach et al., 2019 | Postmenopausal women | CBT-I | None | - | - |
| 42. Laurel Franklin et al., 2018 | Rural veterans with PTSD | Telephonebased CBT-I | Surface | Delivery modality (telephone) | - |
| 54. Manber et al., 2019 | Pregnant women | CBT-I | Both | Linguistic (intervention delivered in Spanish) | Core component (sleep restriction therapy modified for pregnancy with initial time in bed recommendations equal to average total sleep time plus 30min and never less than 5.5h, and tips to improve postpartum sleep); Content (education about infant sleep development) |
| 43. Margolies et al., 2013 | Veterans with PTSD | CBT-I, IRT | Both | Setting (safe location: Veterans hospital) | Core component (combined CBT-I elements and IRT) |
| 44. Matthews et al., 2014 | Women breast cancer survivors with chronic insomnia | CBT-I | None | - | - |
| 45. McCurry et al., 2016 | Postmenopausal women with insomnia symptoms and hot flashes | Telephonebased CBT-I | Both | Constituent-involving strategies (use of role models: sleep coaches were Women); Delivery modality (telephone) | Content (sleep changes during menopause) |
| 46. Palesh et al., 2018 | Female with Stage I-III breast cancer undergoing chemotherapy | BBT-CI | Both | Delivery modality (telephone); Treatment dose (duration; shortened session duration); Treatment dose (timing; intervention sessions scheduled based on the participant’s chemotherapy regime); Setting (delivered in the clinic while patients are undergoing chemotherapy infusion) | Content (education about cancerassociated circadian disruption) |
| 47. Pigeon et al., 2017 | Veterans with a diagnosis of MDD and sleep problems | Brief CBT-I | Surface | Delivery modality (telephone); Setting (primary care) | - |
| 53. Pigeon et al., 2019 | Veterans | Brief CBT-I | Surface | Setting (safe location: Veterans hospital) | - |
| 48. Scogin et al., 2018 | Rural middle-aged and older adults | Integrated CBTD+CBT-I | Both | Delivery modality (video conferencing) | Linguistic (adjustment of materials to literacy level); Sociocultural strategies and cultural values (research psychotherapists also participated in a cultural sensitivity workshop) |
| 49. Ulmer et al., 2011 | Veterans with PTSD | CBT-I, IRT | Both | Setting (safe location: veterans hospital) | Content (trauma-related sleep disturbances) |
| Cognitive Processing Therapy | |||||
| 78. Galovski et al., 2016 | Women interpersonal assault survivors | CPT,H | None | - | - |
| 79. Galovski et al., 2009 | Women sexual assault survivors suffering from PTSD | CPT | None | - | - |
| 80. Gutner et al., 2013 | Women rape victims with PTSD | CPT | None | - | - |
| Complementary and Alternative Therapy | |||||
| 55. Innes & Selfe, 2012 | Older women | Y | Both | Constituent-involving strategy (use of role models: designed and taught by a senior Iyengar yoga instructor with over 30 years of experience) | Core component (designed for older, sedentary adults) |
| 56. Irwin et al., 2017 | Breast cancer survivors with insomnia | TCC | None | - | - |
| 61. Jamison et al., 2019 | Veterans with current or recent PTSD | CART | Surface | Treatment dose (intensity; respiration rate of nine breaths per minute) | - |
| 58. Nakamura et al., 2011 | Veterans with selfreported sleep disturbance | MBB | Surface | Setting (safe location: Veterans hospital) | - |
| 57. Nakamura et al., 2017 | Veterans with Gulf War Illness Symptoms | MBB | None | - | - |
| 59. Nidich et al., 2016 | Men in prison | TM | None | - | - |
| 62. Porter et al., 2019 | Women with metastatic breast cancer | Y | Deep | - | Core component (designed mindful yoga program to meet the needs of women with MBC) |
| 60. Stoerkel et al., 2018 | Women with breast cancer for whom surgery would be there initial treatment | SCT | None | - | - |
| Eclectic Therapy | |||||
| 85. Alschuler et al., 2018 | Adults with multiple sclerosis that causes disability | RI | Surface | Delivery modality (telephone, group setting) | - |
| 86. Church et al., 2016 | Veterans with PTSD | TAU,EFT | None | - | - |
| 87. Kahn et al., 2016 | Veterans | MR | Both | Delivery modality (online) | Involvement of family (veteranpartner dyads) |
| Imagery Rehearsal Therapy | |||||
| 89. Cook et al., 2010 | Male veterans with chronic, severe PTSD | IRT | Both | Delivery modality (group setting); Setting (safe location: Veterans hospital) | Content (addressed combat-related nightmares) |
| 90. Harb et al., 2019 | Veterans | IR,CBT-I | Both | Setting (safe location: Veterans hospital) | Core component (no sleep restriction; combined CBT-I elements and IRT) |
| 88. Krakow et al., 2001 | Women sexual assault survivors with PTSD | IRT | None | - | - |
| Mindfulness-based Stress Reduction | |||||
| 81. Cash et al., 2015 | Women with fibromyalgia | MBSR | None | - | - |
| 82. Lengacher et al., 2015 | Women diagnosed with breast cancer | MBSR | Both | Delivery modality (group settings); Treatment duration (shortened number of sessions) | Content (fear of recurrence, physical emotional symptoms) |
| 83. Shapiro et al., 2003 | Women with Stage II breast cancer who were cancer-free at the time of the study | MBSR | Surface | Treatment dose (timing; intervention delivered after completion of cancer treatment due to peak in stress) | - |
| 84. Witek Janusek et al., 2019 | Women with breast cancer | MBSR | Surface | Setting (safe location: Cancer wellness center) | - |
| Problem-solving Therapy | |||||
| 91. Bedford et al., 2018 | College student veterans with at least mild depression | ePST | None | - | - |
Note: Reference number precedes author name in Article column. ACT=Acceptance and Commitment Therapy; BBT-CI=Brief Behavioral Therapy for Cancer-Related Insomnia; BT=Behavioral Therapy; BTI-MV=Brief Behavioral Treatment of Insomnia; CBT=Cognitive Behavioral Therapy; CBT-I=Cognitive Behavioral Therapy for Insomnia; CPT=Cognitive Processing Therapy; EFT=Clinical Emotional Freedom Techniques; ePST=computer guided Problem-Solving Treatment; H=Hypnosis; IRT=Imagery Rehearsal Therapy; Integrated CBT-D+CBT-I= Integrated CBT (CBT for depression and CBT for insomnia); IVR-CBT=Interactive voice response-based cognitive behavioral therapy; MBB=Mind-body Bridging; M-BET=Multicomponent behavioral combined with exercise therapy; MBSR=Mindfulness-based stress reduction; MDD=Major Depressive Disorder; MR=Mission Reconnect program; PTSD=Post-traumatic stress disorder; RI=Resilience intervention; SCT=Self-care toolkit; TAU=Treatment as usual ; TCC=Tai Chi Chih; TM=Transcendental Meditation; VTC-ACT=Video teleconferencing Acceptance and Commitment Therapy; Y=Yoga.
Both refers to the conduct of surface-level and deep-level cultural adaptations.
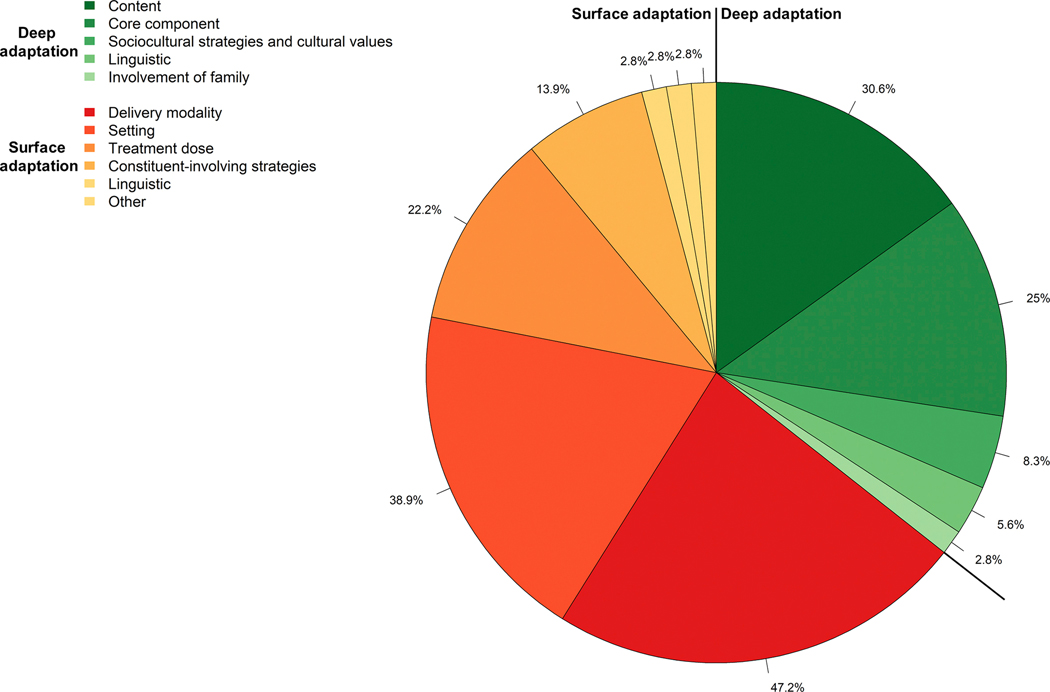
There were several types of surface-level cultural adaptations identified, including changes to the delivery modality (n=17; 47.22%), setting (n=14; 38.89%), treatment dose (i.e., duration, intensity, or timing) (n=8; 22.22%), use of constituent –involving strategies (n=5; 13.89%), linguistic changes (n=1; 2.78%), and other type of surface-level adaptation (i.e., provision of materials) (n=1, 2.78%). Surface-level cultural adaptations of delivery modality were mostly focused on adaptations of in-person one-on-one interventions (ACT, CBT, CBT-I, eclectic treatments, IRT, MBSR) for telephone delivery for women with breast cancer, rural older adults, post-menopausal women, veterans including veterans living in rural settings, older adults with low-incomes, and adults with multiple sclerosis (42, 45–47, 71, 73, 74, 77, 85), for virtual/digital/online delivery for veteran populations, rural middle-aged and older adults, or pregnant women (48, 50, 72, 75, 77) or for group delivery among women with breast cancer, veterans, and adults with multiple sclerosis (39, 82, 85, 89). Surface-level cultural adaptations to the setting were made to accommodate delivery in a safe and known location such as a patient’s home (63, 64), at home or a community partner site (73, 74), Veterans hospital (43, 49, 51, 53, 58, 89, 90), or at a clinic or cancer wellness center for breast cancer patients (46, 84) or gynecological clinic for pregnant women (50). Eight studies altered the treatment dose, that is the treatment duration, intensity, or timing. Four of these targeted older adults and made adaptions to the treatment duration by either reducing the duration of individual sessions of BT or MBSR intervention for older veterans or women diagnosed with breast cancer (68, 82), reducing the number of total sessions of CBT for older adults with low-incomes (73) or adding additional monthly booster sessions of CBT for up to 3 months post treatment based on patient preference for older adults from low-income communities (74). An additional study that focused on adapting CBT-I for women with breast cancer undergoing chemotherapy also shortened the session duration (46). One study adapted the treatment intensity by increasing the number of respirations per minute to nine breaths instead of six for the use of biofeedback among veterans with PTSD (61). Additionally, two studies made alterations to the timing of intervention. In particular, the MBSR intervention was delivered after completion of cancer treatment due to peak in stress concurrent to cancer treatment (83). Similarly, Palesh, Scheiber (46) scheduled the CBT-I sessions for in-clinic delivery based on participant’s chemotherapy regimen. Five studies incorporated constituent-involving strategies. Of these, two focused on women’s sleep health used role models that mirrored the target population; women sleep coaches in a study with menopausal women (45), and a senior woman yoga instructor in a study with older women (55). an additional two studies included non-clinicians in the delivery of CBT-I for older veterans with insomnia, or the delivery of CBT for older adults from low-income backgrounds (35, 74), and one study had CBT-I delivered by specialists in obstetrics and gynecology (50). Of note, Lee, Jong (70) made Other type of surface adaptation by providing materials (i.e., noise machine or fan to reduce noise in bedroom environment) to facilitate the implementation of BT for adults with HIV/AIDS who were unemployed or disabled.
Deep-level cultural adaptations included content-level adaptations (n=11; 30.56%), core component adaptations (n=9; 25.00%), incorporation of sociocultural strategies and cultural values (n=3; 8.33%), linguistic adaptations (n=2; 5.56%), and involvement of family (n=1; 2.78%). Deep-level cultural adaptations of intervention content mostly included the a priori inclusion of cancer related experiences to ACT, thoughts and fears of recurrence of cancer to CBT-I and MBSR, and patient reported symptoms following chemotherapy to BT among women breast cancer survivors (40, 64, 77, 82), education about cancer-related circadian changes (46), psycho-education about sleep changes during menopause in CBT-I among postmenopausal women (45), inclusion of trauma-related sleep disturbances to CBT-I or combat-related nightmares for veterans with PTSD in IRT (49, 89), or education on newborn care or infant sleep development (50, 54). Only one study that targeted veterans included general information about military specific factors relating to sleep in BT for combat-exposed military veterans (66). Deep-level cultural adaptations of BT core components included a loosening of sleep hygiene guidelines such as adding one hour to sleep recommendation to reflect the need for more rest in response to chemotherapy for BTs that targeted women with breast cancer (63, 64), substituting sleep compression for sleep restriction therapy, and modifying standard stimulus control instructions in older adult veteran population (68). Three studies with veteran populations changed core intervention components by combining either BT or CBT-I, and IRT to address nightmares in context of insomnia and other sleep disturbances (43, 65, 90) or eliminating sleep restriction altogether (90). An additional two studies targeting pregnant women, either excluded the discussion of sleep restriction from the weekly sessions (50), or modified sleep restriction instructions such that the sleep window was never less than 5.5 hours (54). Of note, Kahn et al. (87) conducted a deep-level cultural adaptation to a core component of an MBSR, support, and massage-based eclectic therapy through the inclusion of family, or romantic partner (veteran-partner dyads) in the treatment, such that the dyad and not an individual person was the patient. In another study targeting older, sedentary women, the yoga instruction was adapted to better suit the needs of the older women (55). Similarly, a study targeting women with metastatic breast cancer tailored a mindful yoga intervention through the integration of yoga poses that minimized the risk of falling or vertebrae fractures, and chairs to offset balance (62). Three studies incorporated sociocultural strategies and cultural values into the psychological intervention. Specifically, Stanley et al. (73, 74) integrated religion and spirituality in CBT for low-income older adults in both of their studies. In addition, in Scogin, Lichstein (48) psychotherapists delivering an integrated CBT-I and CBT for depression treatment to rural middle-aged and older adults participated in a cultural sensitivity workshop. Two studies made linguistic deep-level cultural adaptations. Specifically, Stanley, Wilson (74) and Scogin, Lichstein (48) used linguistic strategies to adjust the intervention materials to the literacy level of the older adult low-income and rural target population.
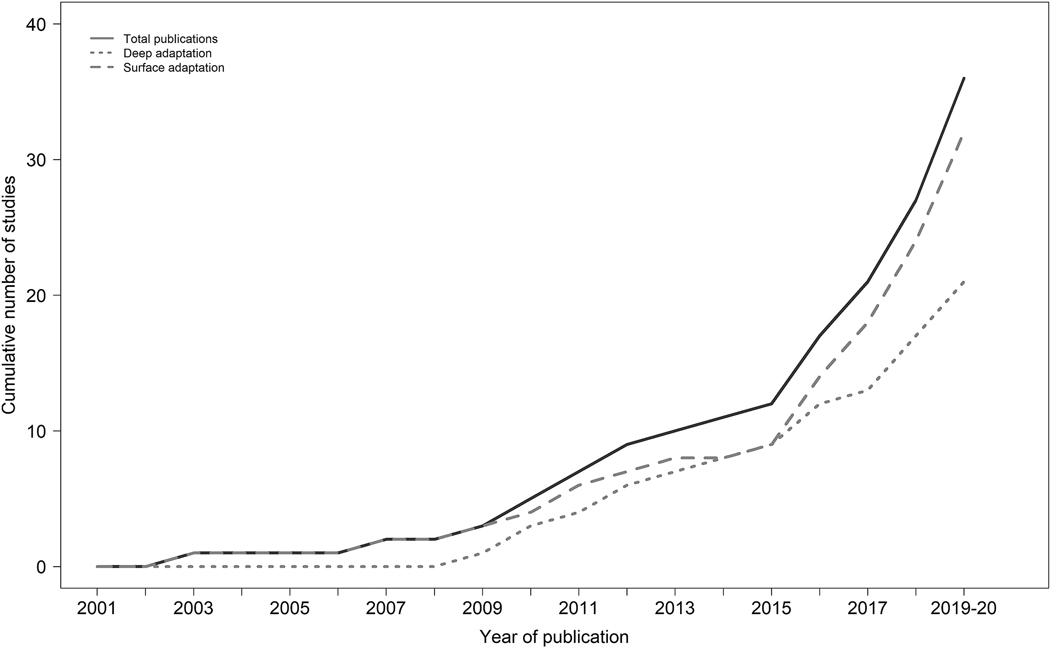
Publication Trends of Cultural Adaptations of Psychological Interventions Targeting Sleep-Wake Disorders or Sleep Disturbances.
Figure 3 illustrates the publication trends of surface-level and deep-level cultural adaptations of the peer-reviewed empirical literature published up until January 16, 2020. Of note, 2001 was the first year that a peer-reviewed article that utilized an RCT design and targeted one of the seven underserved groups and met inclusion criteria for this review was published. Overall, 2008 to 2015 saw a marked increase in the number of publications conducting surface-level cultural adaptations and deep-level cultural adaptations to psychological interventions, and the rate of publication of adapted interventions has only increased since 2015 . The publication rate for studies conducting a surface-level cultural adaptation markedly increased from 2015 to January 2020, which mirrors the overall trend for total publications of psychological interventions with underserved groups. Similarly, the overall rate of increase of deep-level cultural adaptations from 2008 to January 2020 appears to have remained the same.
Figure 3.
Legend. Publication Trends of Cultural Adaptations of Psychological Interventions for Prevalent Sleep Disorders and Sleep Disturbances.
Effectiveness of Cultural Adaptations of Psychological Interventions on Primary Sleep Outcomes by Presence of Sleep Disorder or Sleep Disturbance
Table 5 summarizes the RCT results on effectiveness of included studies on primary sleep outcomes classified according to the likelihood of a sleep disorder or sleep disturbance diagnosis in participant population and by type of primary intervention. Sleep quality (n=40; 71.43%) was the most common primary sleep outcome of included RCTs, followed by insomnia (n=30; 53.57%), sleep efficiency (n=21; 37.5%), sleep duration (n=21; 37.5%), nightmare frequency (n=8; 14.29%), and sleepiness (n=4; 7.14%).
Table 5.
Summary of Results of Effectiveness of Cultural Adaptations and Psychological Interventions on Primary Sleep Outcomes Organized by Participant Population (N=56)
| Article | Primary Intervention | Comparator | Cultural adaptation | Sleep Disorder or Disturbance | Analysis | Insomnia | Nightmare Frequency | Sleep duration | Sleep efficiency | Sleepiness | Sleep quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant Population with Definite Sleep Disorder or Disturbance Diagnosis | |||||||||||
| Behavioral Therapy | |||||||||||
| 69. Gebara et al., 2019 | BBTI | Wait-list | None | Insomnia | Pre-Tx vs 4w | n.s. | |||||
| 65. Germain et al., 2012 | BT,IRT | Medication Arms (Placebo or Prazosin) | Deep | Nightmares + Poor Sleep Quality | Pre-Tx vs 8w; PostTx vs 12w FU | n.s.;** (Ctrl 2) | n.s. | n.s.b | n.s.b | n.s. | |
| 66. Germain et al., 2014 | BT | Sleep Education | Deep | Insomnia | Pre-Tx vs 4w | * | n.s. | * | |||
| 67. Johnson et al., 2016 | M-BET | Standard drug therapy for nocturia (α-blocker) and combined M-BET + αblocker | None | Nocturia | Pre-Tx vs 12w FU | n.s.a | *a | n.s. | n.s. | ||
| Cognitive Therapy for Insomnia | |||||||||||
| 35. Alessi et al., 2016 | CBT-I | Sleep Education | Surface | Insomnia | Pre-Tx vs 6w; PreTx vs 6,12m FU | ***; ***; *** | n.s.a,*b ;n.s.a,*b; n.s.a,*b | ***; ***; *** | |||
| 50. Cain et al., 2019 | CBT-I | Group prenatal visits | Both | Insomnia | Pre-tx vs. 2nd tri, 3rd tri, postpartum | n.s.; *; ** | n.s.b; n.s.b; n.s.b | ||||
| 51. Chakravorty et al., 2019 | CBT-I | Usual Care | Surface | Insomnia | Pre-Tx vs 8w; PreTx vs 3,6m FU | *;*;* | n.s.b;n.s.b ; n,s,b | n.s.;n.s.;n,s, | |||
| 36. Drake et al., 2019 | CBT-I | Sleep Hygiene, Sleep Restriction | None | Insomnia | Pre-Tx vs PostTx,6m FU | ***;*** | n.s. b; n.s. b | * b; n.s. b | ***; *** | ||
| 37. Edinger & Sampson, 2003 | CBT-I | Sleep Hygiene | None | Insomnia | 2w vs 3m FU | *** | n.s.b | *b | * | ||
| 38. Edinger et al., 2009 | CBT-I | Sleep Hygiene | None | Insomnia | Pre-Tx vs 8w; Post- Tx vs 6m FU | *; * | *b | ||||
| 39. Epstein & Dirksen, 2007 | CBT-I | Sleep Education and Hygiene | Surface | Insomnia | Pre-Tx vs 2w Post- Tx | **b | n.s.b | *** | |||
| 40. Fiorentino et al., 2010 | CBT-I | Delayed Treatment | Deep | Insomnia | Pre-Tx vs 6w; Post- Tx vs 6w FU | *; * | *an.s.b | **b | **; * | ||
| 41. Fung et al., 2016 | CBT-I | Sleep Education | None | Insomnia | No SDB: Pre-Tx vs 6,12m FU; SDB: Pre-Tx vs 6,12m FU | n.s.a,b, n.s.a,b ; n.s.a,*b, n.s.a, *b | *,*; **,** | ||||
| 52. Kalmbach et al., 2019 | CBT-I | Sleep Hygiene, Sleep Restriction | None | Insomnia | Pre-Tx vs 6w; Pre- Tx vs 6m FU | n.s.e,n.s.f;n. s.e,**f | |||||
| 42. Laurel Franklin et al., 2018 | Telephonebased CBT-I | In person CBT-I | Surface | Insomnia | Pre-Tx vs Post-Tx, 1,3m FU | n.s. d | |||||
| 54. Manber et al., 2019 | CBT-I | Modified pseudodesen sitization therapy for insomnia | Both | Insomnia | Pre-Tx vs 5w | ** | |||||
| 43. Margolies et al., 2013 | CBT-I, IRT | Wait-list | Both | Insomnia | Pre-Tx vs 6w | *** | n.s.a | ***a | ** | ||
| 44. Matthews et al., 2014 | CBT-I | Behavioral Placebo Treatment | None | Insomnia | Pre-Tx vs 6w; PreTx vs 3,6m FU | * | **b; *b | n.s.b; **b | |||
| 45. McCurry et al., 2016 | Telephonebased CBT-I | Menopause Education | Both | Insomnia | Pre-Tx vs 8w FU; Pre-Tx vs 24w FU | ***;*** | n.s.; n.s. | ***; ** | ***;** | ||
| 46. Palesh et al., 2018 | BBT-CI | Health Eating Education Learning | Both | Insomnia | Pre-Tx vs Post-Tx, 1m FU | *; * | |||||
| 47. Pigeon et al., 2017 | Brief CBT-I | Sleep Hygiene | Surface | Insomnia | Pre-Tx vs 4w; Pre- Tx vs 3m FU | n.s.; n.s. | n.s.b ; n.s. b | * b ; n.s. b | |||
| 53. Pigeon et al., 2019 | Brief CBT-I | Usual Care | Surface | Insomnia | Pre-Tx vs 6w | *** | |||||
| 48. Scogin et al., 2018 | Integrated CBTD+CBT-I | Treatment as usual | Both | Insomnia | Pre-Tx vs Post-Tx; 3m FUc | *;* | n.s.b, c | * b;- | ** ;-d | ||
| 49. Ulmer et al., 2011 | CBT-I, IRT | Usual Care | Both | Insomnia | Pre-Tx vs 12w | ** | * | * b | *** | ||
| Cognitive Processing Therapy | |||||||||||
| 78. Galovski et al., 2016 | CPT,H | Symptom monitoring control condition before beginning standard CPT | None | Insomnia | Pre-Tx vs 15w | * | n.s. | n.s. b | * | ||
| Complementary and Alternative Therapy | |||||||||||
| 55. Innes & Selfe, 2012 | Y | Educational film | Both | RLS | Pre-Tx vs 8w | * | *** b | * | |||
| 56. Irwin et al., 2017 | TCC | CBT-I | None | Insomnia | Pre-Tx vs 3m Post- Tx; PreTx vs 6, 15m FU | n.s.; n.s.; n.s. | n.s. b; n.s. b b ; n.s. | n.s. b; * b; n.s. b | n.s. | n.s.; n.s.; n.s. | |
| 58. Nakamura et al., 2011 | MBB | Sleep Hygiene | Surface | Poor sleep quality | Pre-Tx vs 2w | *; * | |||||
| 57. Nakamura et al., 2017 | MBB | Sleep Education | None | Poor sleep quality | Pre-Tx vs 2,3-w; Pre-Tx vs 3w; Pre- Tx vs 3m FU | n.s.; n.s.; * | |||||
| Imagery Rehearsal Therapy | |||||||||||
| 89. Cook et al., 2010 | IRT | Sleep and Nightmare Management Treatment | Both | Nightmares+ Poor sleep quality | Pre-Tx vs 6w | n.s. | n.s. | ||||
| 90. Harb et al., 2019 | IR,CBT-I | CBT-I | Both | Nightmares + Poor sleep quality | Pre-Tx vs 6m FU | n.s. | n.s. | ||||
| 88. Krakow et al., 2001 | IRT | Wait-list | None | Nightmares + Insomnia | Pre-Tx vs 3 or 6m FU | ** | ** | ||||
| Participant Population with Probable or Potential Sleep Disorder or Sleep Disturbance Diagnosis | |||||||||||
| Acceptance and Commitment Therapy | |||||||||||
| 75. Herbert et al., 2017 | VTC-ACT | In person ACT | Surface | - | Pre-Tx vs 8w; Pre- Tx vs 6m FU | n.s.; n.s. | |||||
| 76. Lang et al., 2017 | ACT | PresentCentered Therapy | None | - | Pre-tx vs 12w | * | |||||
| 77. Mosher et al., 2018 | ACT | Education/S upport | Both | - | Pre-Tx vs 8,12w FU | n.s.b; n.s.b | |||||
| Behavioral Therapy | |||||||||||
| 63,64. Berger et al., 2009a,b | BT | Healthy Eating | Both | - | Post-Tx vs 30,90,36 5d FU | *;**; n.s. | |||||
| 70. Lee et al., 2020 | BT | Attention control group (dietary strategies) | Surface | - | Pre-Tx vs. Post- Tx | n.s.b | *b | *b | |||
| 68. Martin et al., 2017 | BT | Sleep Education | Both | - | Pre-Tx vs 4w; Pre- Tx vs 4m FU | n.s.; n.s. | n.s.a; n.s.a | **a. a ; * | n.s.; n.s. | ||
| Cognitive Behavioral Therapy | |||||||||||
| 71. Brenes et al., 2016 | CBT | Nondirective Supportive TherapyTelephone | Surface | - | Pre-Tx vs 4,9,15m FU | **;**;** | |||||
| 72. Heapy et al., 2017 | IVR-CBT | In-person CBT | Surface | - | Pre-Tx vs 3,6,9m FU | n.s.; n.s.; n.s. | |||||
| 73. Stanley et al., 2016 | CBT | Community Resources and Emotional Support | Both | - | Pre-Tx vs 3m | n.s. | |||||
| 74. Stanley et al., 2018 | CBT | Enhanced Community Care with Resource Counseling | Both | - | Pre-Tx vs 6,9m FU | n.s.; n.s. | |||||
| Cognitive Processing Therapy | |||||||||||
| 79. Galovski et al., 2009 | CPT | Prolonged Exposure | None | - | Pre-Tx vs 6w | n.s. b | n.s. b | n.s. | |||
| 80. Gutner et al., 2013 | CPT | Prolonged Exposure | None | - | Pre-Tx vs 6w; 6w vs 6y FU | n.s.; n.s. | n.s.; n.s. | n.s.; n.s. | |||
| Complementary and Alternative Therapy | |||||||||||
| 61. Jamison et al., 2019 | CART | Wait-list | Surface | - | Pre-Tx vs 4w | n.s.b | n.s.b | ||||
| 59. Nidich et al., 2016 | TM | No- treatment | None | - | Pre-Tx vs 4m FU | *** | |||||
| 62. Porter et al., 2019 | Y | Social Support Group | Deep | - | Pre-Tx vs 8w; PreTx vs 3,6m FU | ns, *, ns | |||||
| 60. Stoerkel et al., 2018 | SCT | Treatment as usual | None | - | Pre-Tx vs Preoperat ive, 2w after surgery FU | n.s.; n.s. c | |||||
| Eclectic Therapy | |||||||||||
| 85. Alschuler et al., 2018 | RI | Wait-list | Surface | - | Pre-Tx vs Post-Tx | n.s. | |||||
| 86. Church et al., 2016 | TAU,EFT | Treatment as usual wait- list | None | - | Pre-Tx vs 6m | ** | |||||
| 87. Kahn et al., 2016 | MR | MR + PREP, PREP, waitlist | Both | - | Ctrl.1: Pre-Tx vs 8,16-w FU; Ctrl.2: Pre-Tx vs 8,16-w FU; Ctrl.3: Pre-Tx vs 8,16-w FU; | **,*; **, n.s.; *,* | |||||
| Mindfulness-based Stress Reduction | |||||||||||
| 81. Cash et al., 2015 | MBSR | Wait-list | None | - | Pre-Tx vs 8w; 8w vs 2m FU | *; n.s. | |||||
| 82. Lengacher et al., 2015 | MBSR | Usual Care | Both | - | Pre-Tx vs 6w; 6w vs 12w FU | n.s.a,b;n.s. a,b | n.s. a,b; *a, n.s.b | n.s.; n.s. | |||
| 83. Shapiro et al., 2003 | MBSR | Stress Management | Surface | - | Pre-Tx vs 6w | n.s.b | n.s. | ||||
| 84. Witek Janusek et al., 2019 | MBSR | Active control | Surface | - | Pre-Tx vs 8w-6m FU | * | |||||
| Problem-solving Therapy | |||||||||||
| 91. Bedford et al., 2018 | ePST | Minimal contact | None | - | Pre-Tx vs 6w | *** | |||||
Note: Reference number precedes author name in Article column. ACT=Acceptance and Commitment Therapy; BBT-CI=Brief Behavioral Therapy for Cancer-Related Insomnia; BT=Behavioral Therapy; BTI-MV=Brief Behavioral Treatment of Insomnia; CBT=Cognitive Behavioral Therapy; CBT-I=Cognitive Behavioral Therapy for Insomnia;
CPT=Cognitive Processing Therapy; EFT=Clinical Emotional Freedom Techniques; ePST=computer guided Problem-Solving Treatment; H=Hypnosis; IRT=Imagery Rehearsal
Therapy; Integrated CBT-D+CBT-I= Integrated CBT (CBT for depression and CBT for insomnia); IVR-CBT=Interactive voice response-based cognitive behavioral therapy;
MBB=Mind-body Bridging; M-BET=Multicomponent behavioral combined with exercise therapy; MBSR=Mindfulness-based stress reduction; MR=Mission Reconnect program; RI=Resilience intervention; SCT=Self-care toolkit; TAU=Treatment as usual ; TCC=Tai Chi Chih; TM=Transcendental Meditation; VTC-ACT=Video teleconferencing Acceptance and Commitment Therapy; Y=Yoga.
Definite= directly assessed for and confirmed the existence of a sleep disorder or sleep disturbance and included this in the inclusion criteria; Probable=directly assessed for any sleep disturbance but did not include endorsement of a sleep disturbance as part of the inclusion criteria. Potential= sleep did not assess for a sleep disturbance but based on the scientific literature would likely have a sleep disturbance concomitant with the co-morbid health or mental health condition.
Values reported are from Berger et al., 2009b
Objectively-measured sleep (i.e., actigraphy)
Subjectively-measured sleep (i.e., self-reported)
Group by time interaction instead of between group differences
Sleep quality measured using the PROMIS sleep disturbance scale
ESS daytime sleepiness
Diary-based sleepiness
Results of Culturally Adapted Intervention Studies among Participants with a Definite Sleep Disorder or Sleep Disturbance Diagnosis.
Overall, 20% of the RCTs of adapted behavioral treatments (BTs) included participants with a definite sleep disorder or sleep disturbance diagnosis and results documented reductions in insomnia symptoms but no other sleep outcomes compared to control conditions (Table 5). Specifically, a deep-level culturally adapted combined BT-IRT intervention and a deep-level culturally adapted BT intervention found significant improvement in insomnia symptoms versus pharmacotherapy among veterans with a definite endorsement of sleep disorder or disturbance (insomnia, nightmares and poor sleep quality) at enrollment, but no statistically significant group differences in nightmare frequency, sleep duration, or sleep efficiency (65, 66).
All of the RCTs of adapted CBT-I targeted adults with a definite sleep disorder or sleep disturbance, namely insomnia or insomnia symptoms assessed at enrollment. By and large, the majority of the surface-level and deep-level cultural adaptions were made to CBT-I. Of the 14 RCTs testing adapted CBT-I among participants with a definite insomnia diagnosis, 11studies documented significant improvement in insomnia symptoms among those who received a surface-level and/or deep-level culturally adapted CBT-I intervention (35, 40, 43, 45, 46, 48–51, 53, 54) compared to sleep hygiene education, healthy eating education condition, wait-list control, group prenatal visits, modified pseudodesensitization therapy for insomnia, or usual care controls. One did not find significant between group differences (47), and two did not measure insomnia symptoms (39, 42). Relatedly, two out of eight RCTs that tested an adapted version of CBT-I among women with breast cancer with an insomnia diagnosis and included sleep duration as a primary sleep outcome found statistically significant improvement in sleep duration favoring an adapted group-delivered CBT-I intervention (39), and an adapted CBT-I intervention with deep-level content adaptations (e.g., thoughts and fears related to the recurrence of cancer) (40). The remaining six studies that tested an adapted CBT-I intervention among those with insomnia did not find any statistically significant between group differences in sleep duration (43, 45, 47, 48, 51, 68). Ulmer et al. (49) combined CBT-I and IRT for veterans and found that the adapted intervention resulted in significant improvements in sleep duration and nightmare frequency compared to usual care.
Six out of the 14 RCTs that tested an adapted CBT-I intervention among participants with insomnia found significant improvements in self-reported or actigraphy-based sleep efficiency (35, 40, 43, 45, 47, 48). Similarly, seven out of 14 RCTs that tested adapted CBT-I found significant improvements in sleep quality (35, 39, 40, 43, 45, 48, 49). However, two studies did not find statistically significant differences in sleep quality by treatment condition (42, 50). Importantly, Alessi et al. (35), one of the highest quality studies (Table 3), found significant improvements in insomnia, actigraphy-based sleep efficiency, and sleep quality in favor of adapted CBT-I among older veterans with insomnia.
Half of the RCTs (n=2) of adapted CAM interventions were conducted with participants with a definite sleep disorder or sleep disturbance. These two culturally adapted CAM therapies generated significant between group differences that favored the adapted intervention among participants with restless legs syndrome or poor sleep quality. Specifically, a yoga intervention with both deep-level and surface-level cultural adaptations for older adults with restless legs syndrome, and a mind-body bridging intervention with surface-level cultural adaptations for veterans with poor sleep quality resulted in significant improvements favoring the adapted intervention over the control condition for insomnia reduction, longer sleep duration, and better sleep quality (55, 58). Similarly, all of RCTs of adapted IRT (n=2) were conducted with participants with a definite sleep disturbance. These two RCTs of deep-level culturally adapted IRT interventions among participants with a definite nightmares and poor sleep quality disturbances at enrollment did not report significant between group differences in nightmare frequency or sleep quality in veterans (89, 90). Of note, the only RCT of an adapted intervention to report effect on sleepiness found that an adapted CBT-I intervention did not generate significant between group differences in sleepiness reduction compared to usual care in participants with insomnia (51, 58).
Results of Culturally Adapted Intervention Studies among Participants with a Probable or Potential Sleep Disorder or Sleep Disturbance Diagnosis.
All of the RCTs testing adaptations of ACT were conducted among participants with a probable or potential sleep disturbance. None of these culturally adapted RCT studies (n=2) produced significant improvements in sleep efficiency (77) or sleep quality (75). Findings were mixed for the remaining three RCTs testing adapted BTs against various comparators among participants with a probable or potential sleep disturbance. Berger et al. (63, 64) found that a surface and deep-level cultural adaptation of BT versus a healthy eating lifestyle intervention significantly improved sleep quality for women with breast cancer and with potential sleep disturbances at enrollment at 30-days (63) and at 90-days (64) but not at 1-year (64). Similarly, in an RCT of a surface-level cultural adaptation of BT among adults diagnosed with HIV/AIDS with a probable or potential sleep disturbance, the adapted BT that included provision of materials (e.g., noise machine or fan to reduce noise in bedroom) compared to an attention control group did not generate significant between group differences in sleep duration; however, the adapted BT significantly improved sleep efficiency and sleep quality measured via self-report (70). Importantly, Martin et al. (68), one of the highest quality studies included in this review, did not find any statistically significant between group differences in insomnia, sleep duration, and sleep quality, though significant differences in self-reported sleep efficiency were found in favor of adapted BT versus the sleep education control condition in older veterans with a potential sleep disturbance diagnosis at enrollment.
All of the RCTs of adapted CBT were conducted among participants with a probable or potential sleep disturbance. Outcomes for these trials were also mixed, though most studies documented null effects. Specifically, Brenes et al. (71) documented that a surface-level culturally adapted telephone-delivered CBT versus non-directive telephone-delivered supportive therapy improved insomnia symptoms at four-, nine-, and 15-month follow-up (71) among rural older adults with a probable sleep disturbance at enrollment. However, a similarly adapted telephone-delivered CBT versus an in-person version did not generate significant between group differences in sleep quality in veterans with chronic back pain and with a potential sleep disturbance at enrollment (72). Similarly, a surface and deep-level cultural adaptation of a CBT program for rural older adults from low-income backgrounds compared to resource counseling (73, 74) did not generate significant group differences in insomnia among participants with a probable or potential sleep disturbances at enrollment.
The remaining half of RCTs of adapted CAM interventions were conducted with participants with probable or potential sleep disturbance and yielded largely null or inconsistent effects. For example, a surface-level adapted biofeedback intervention for veterans compared to a wait-list control did not generate significant treatment group differences in sleep duration or sleep quality (61). Moreover, a yoga intervention with deep-level cultural adaptations targeting women with metastatic breast cancer resulted in worse sleep quality scores at three months compared to a social support intervention, but did not generate significant treatment differences at eight weeks or six months follow-up (62).
All of the RCTs testing adapted eclectic therapies (n=2) were conducted among participants with a probable or potential sleep disturbance. Of these, a positive psychology intervention culturally adapted for group and telephone-delivery for adults with multiple sclerosis compared to a wait-list control did not generate significant group differences in sleep quality (85). In contrast, a mindfulness, support, and massage-based dyadic intervention that included surface-level (online delivered) and deep-level (involvement of family) cultural adaptations resulted in significant improvements in sleep quality at 8 week follow-up among a veteran sample (87).
Similarly all of the RCTs testing adapted MBSR interventions were conducted with participants with a probable or potential sleep disturbance. Results largely indicate no statistically significant between intervention group differences in sleep duration, sleep efficiency, or sleep quality (82, 83). However, a surface-level and deep-level culturally adapted MBSR intervention resulted in significant improvements in sleep efficiency at 12 weeks compared to usual care among women with breast cancer and a probable/potential sleep disturbance diagnosis (82). An additional surface-level cultural adaptation of MBSR compared to an active control condition resulted in significant improvements in sleep quality among women with breast cancer and a probable or potential sleep disturbance (84).
DISCUSSION
Our systematic review of the peer-reviewed English-language literature on RCTs conducted in the United States that tested psychological interventions for prevalent sleep-wake disorders and sleep disturbances found that only 6.97% of the published literature or 56 studies out of 803 full-text articles assessed for eligibility targeted an underserved or high risk adult group, defined as racial/ethnic minorities, women, low socioeconomic status groups (those who are unemployed, low income, low educational attainment), immigrants, sexual orientation minorities, veterans, or those with disabilities, and included a primary sleep outcome. Of included RCTs, 64.29% conducted a surface-level or deep-level cultural adaptation of a psychological intervention for prevalent sleep-wake disorders or sleep disturbances to address the target underserved or high risk population. The effectiveness of the culturally adapted interventions versus control conditions on important sleep outcomes varied by the type of psychological intervention and by the participant population, specifically whether participants had a definite, probable, or potential sleep disorder or sleep disturbance diagnosis at enrollment. We summarize key findings below and offer recommendations for future intervention research in this area.
Our first aim was to examine the extent to which sociodemographic characteristics were reported in RCTs of psychological interventions for prevalent sleep-wake disorders that targeted seven specified underserved groups. Overall, there was a lack of racial/ethnic, socioeconomic, sexual orientation, and linguistic diversity in the sample composition. Specifically, most studies were conducted with majority White samples (64.86%) and with a small representation of Asians (only 4.06%), Latina/os (9.45%), and American Indians/Alaska Natives (percentage unknown because often collapsed into the Other Ethnicity category, 9.23%), percentages that do not reflect the demographic composition of the United States (92). Of note, while on average, 28.16% of RCT participants were Black, which is more reflective of the composition of Black Americans in the United States (92), this percentage is driven by a few studies that recruited large samples of Black participants (42, 51, 73, 74). Importantly, some studies did not report the racial/ethnic composition of the sample in the published manuscript (44, 58, 81, 86). About half of the sample (55.35%) was composed of women (arguably mostly non-Hispanic White women), and similarly 44.64% of included trials targeted veterans. Most of the trial participants had high levels of educational attainment (average was 14.42 years), though there was more representation of lower income categories (<$20,000) across included studies than what is typically observed in RCTs. Six studies reported disability status (48, 69, 72, 73, 83, 85) and two studies targeted unemployed populations (68, 70). Two trials included rural adults (48, 71). By and large, none of the included RCTs explicitly focused on or reported data on sexual orientation or gender minorities, immigrant groups, or linguistic minorities, with two exceptions (54, 70). While it is possible that we may have missed RCTs that may have included immigrants, non-English speakers, and sexual or gender minorities but did not report these sociodemographic data, these omissions in data collection and data reporting reflect important limitations. The lack of RCTs directly testing interventions in linguistic minority communities in the US is particularly problematic in light of the fact that 85% of US immigrants aged 5 years or older speak a language other than English at home, and that 28.9% do not speak English at all or not well (93). Consistent with calls for increasing the diversity of psychological trials more generally as a means to enhance generalizability (94), future RCTs of psychological interventions in behavioral sleep medicine should seek to expand the representativeness of the participant sample to be more inclusive of underserved groups, and thereby increase the percentage of racial/ethnic minorities, those who are unemployed, sexual orientation and gender minorities, linguistic minorities, immigrants, those with disabilities, and those living in rural settings who participate in these trials. Moreover, while inclusion of racial/ethnic minorities and women is required for federally-funded research per the 1993 National Institute of Health Revitalization Act and its subsequent policy derivatives (95), and the CONSORT statement (96) instructs investigators to include a table showing baseline demographic and clinical characteristics for each intervention group, there were several studies that did not provide this information. Stronger enforcement of the NIH reporting policies and CONSORT statement is needed in order to assess sample representativeness, ensure inclusiveness, and evaluate generalizability.
Our second aim was to identify the extent to which investigators made surface-level or deep-level cultural adaptations to psychological interventions for prevalent sleep-wake disorders or sleep disturbances to address important cultural and contextual factors. To that end, 64.29% of included RCTs conducted either a surface- and/or deep-level cultural adaptation. By far, the most common surface-level cultural adaptations included changes to the delivery modality (from in-person one-on-one to telephone, online, virtual, video-based, or group-based models) and setting (in home-delivery, primary care offices, community sites). Other surface-level cultural adaptations included changes to the treatment dose (e.g., shortening the treatment duration, alterations to the timing of intervention or intensity), and constituent –involving strategies (delivery of intervention by non-clinicians). Deep-level cultural adaptations mostly included content-level adaptations, such as the integration of cancer related experiences and fears of recurrence, or menopause-related sleep changes, or psycho-education about trauma, or infant development into the intervention. Deep-level cultural adaptations to core treatment components often included the combination of two treatments (e.g., CBT-I or BT and IRT), or modifying stimulus control instructions or sleep restriction therapy to better suit the health needs or limitations of the target populations (e.g., limiting sleep restriction to five hours for pregnant women). Other deep-level cultural adaptations such as the involvement of family, the incorporation of sociocultural strategies and cultural values into the psychological interventions were disproportionately less common, and occurred in only four studies (48, 73, 74, 87). Similarly, deep-level linguistic strategies such as revising the intervention materials to match the literacy level of participants were also less common, occurring in only two studies (48, 74). None of the RCTs that conducted deep-level cultural adaptations actively targeted social support and social networks into the intervention, which represent an important gap. While an estimated 51% of the US population demonstrate low level English literacy skills that are indicative of difficulties with performing complex tasks that require higher level reading skills or problem solving (97) the literacy level of intervention content is rarely reported in RCTs of psychological interventions. Further, the average educational attainment of RCT participants in this review was over 14 years. In order to ensure access to high quality mental health care for all, and adequately address mental health care disparities, it will be imperative that psychological interventions are developed with greater attention to general literacy, numeracy, and health literacy.
Our third aim was to describe the effectiveness of the culturally adapted psychological interventions for prevalent sleep-wake disorders and sleep disturbances on important primary sleep outcomes by participant population and intervention type. Of adapted RCTs, roughly 56% included participants with a definite diagnosis of a sleep disorder or endorsement of a sleep disturbance, 14% included participants with a probable sleep disturbance, and 31% included participants with a potential sleep disturbance at enrollment. Most of the surface-level and deep-level cultural adaptations were made to CBT-I, BT, CBT, and CAM interventions. Importantly, all of the RCTs of adapted CBT-I and IRT were conducted among participants with a definite sleep disorder or sleep disturbance. In contrast, all of RCTs on adapted ACT, CBT, MBSR, and eclectic therapies were conducted among participants with a probable or potential sleep disturbance. Overall, participant population appeared to pattern intervention effectiveness. There were more consistent and statistically significant between group differences documented favoring the adapted intervention against comparators in RCTs conducted among participants with a definite sleep disorder or sleep disturbance, whereas a preponderance of null and inconsistent findings were documented in RCTs of adapted interventions conducted among participants with a probable/potential sleep disorder or sleep disturbance, For example, 78% of RCTs testing an adapted CBT-I intervention among participants with a definite insomnia diagnosis showed significant reductions in insomnia (and to a lesser extent sleep quality) in favor of the culturally adapted intervention versus other comparators. Similarly, RCTs of adapted BT and CAM interventions conducted in participant populations with a definite sleep disorder or sleep disturbance diagnosis also showed significant improvements in insomnia and sleep quality compared to various controls. Yet, the results were markedly less consistent for RCTs of adapted BTs, CBTs, and CAM interventions (e.g., yoga, mind-body bridging) conducted among participants with a probable or potential sleep disorder or sleep disturbance. Further, cultural adaptations of IRT, MBSR, ACT, and eclectic therapies did not produce consistent between intervention group differences in primary sleep outcomes (75, 77, 82, 83, 85, 87, 89), with two exceptions (82, 87). None of the RCTs tested cultural adaptations of CPT and PST, and this represents a significant gap and important area for future research. Moreover, only three RCTs (42, 72, 75) (5.3%) tested the effectiveness of a surface-level culturally adapted behavioral intervention (i.e., CBT-I, ACT, and CBT) against the same un-adapted intervention documenting no statistically between group differences in sleep quality. Future research should test the incremental effects of cultural adaptations against un-adapted treatments where possible (23). This type of comparative research will also help identify the surface or deep-level cultural adaptations required to improve effectiveness for all behavioral sleep interventions compared to un-adapted interventions, and identify which aspects of sleep interventions require adaptation.
Importantly, knowledge into these cultural adaptation factors and their effect on outcomes will help inform broad behavioral sleep medicine implementation and dissemination efforts. Collectively, these findings suggest that, on balance, psychological interventions for sleep disorders with cultural adaptations generally resulted in improved sleep outcomes particularly for interventions that targeted participant populations with a definite sleep disorder or sleep disturbance diagnosis determined by systematic assessment at study enrollment. The evidence of improved sleep outcomes, particularly insomnia and sleep quality for culturally adapted CBT-I, BT, and CAM interventions against comparators in RCTs with participants with a definite insomnia diagnosis or poor sleep quality appears to be quite strong and consistent. Herein, we reviewed both surface-level and deep-level cultural adaptations and did not prioritize one type of cultural adaptation over another. Future research should examine whether specific types of cultural adaptations or the number of cultural adaptations modify the effectiveness of a psychological intervention targeting sleep-wake disorders and sleep disturbances among underserved populations.
Our systematic review has several limitations. First, the heterogeneity in interventions, analytic method, article quality, types of cultural adaptations, and outcome measures prevented us from conducting a meta-analysis. We also included only English-language articles, which may contribute to publication bias. Second, as with any systematic review, we may have missed articles that met our inclusion criteria and particularly those that appeared in the grey literature. While we used multiple databases including those that identify conference proceedings and ongoing trials, as well as multiple coders to mitigate this limitation, our findings could be influenced by the absence of a more exhaustive search of the grey literature (e.g., search of National Technical Information Service (NTIS) database and PsycEXTRA). Relatedly, none of the included RCTs targeted obstructive sleep apnea, or circadian-rhythm sleep-wake disorder shift-work type, despite our explicit inclusion in our search strategy; only one study included participants with a sleep disorder diagnosis of restless legs syndrome. As such, research that explores the development and testing of RCTs of cultural adaptations of psychological interventions targeting these sleep disorders is warranted. Third, our measure of methodological quality or risk of bias did not capture the variance in analytic methods across studies and relied on subjective assessment of risk domains. Relatedly, the risk of bias tool we used did not assess for fidelity to the study protocol when assessing performance bias, which may influence intervention effectiveness. However, we used multiple coders and a well-validated assessment tool to evaluate risk of bias to mitigate this limitation.
CONCLUSION
While access to high quality, patient-centered health care that is linguistically and culturally sensitive is a national health priority (98), disparities in access to high quality mental health care persist (99), including mental health care for the resolution of sleep-wake disorders and sleep disturbances. The development, testing, and dissemination of cultural adaptations of efficacious and effective psychological interventions for prevalent sleep-wake disorders and sleep disturbances are one means to address these disparities in mental health care for prevalent sleep-wake disorders and sleep disturbances. However, our systematic review found that only 6.97% of RCTs of psychological interventions targeted an underserved adult group in the United States and met the remaining inclusion criteria. None of the extant RCTs explicitly targeted linguistic minorities, immigrants, or LGBTQ adults, and there was limited racial/ethnic and socioeconomic diversity in the sample composition. Of those trials, 64.24% conducted either a surface-level or deep-level cultural adaptation. Most RCTs that conducted surface-level cultural adaptations made changes to the delivery modality or setting, whereas most RCTs that conducted deep-level cultural adaptations made changes to the content or the core components of the intervention. While RCTs testing adapted CBT-I interventions showed significant improvements in sleep outcomes in favor of the culturally adapted interventions versus other comparators, the results were less consistent for RCTs of adapted BTs, CBTs, and CAM interventions, and potentially dependent on participant population, specifically whether there was the presence of a definite, probable, or potential sleep disorder or disturbance at enrollment. In order to meet the demand for behavioral health specialists who can adequately treat sleep-wake disorders and sleep disturbances in an increasingly diverse United States of America, it will become imperative for intervention scientists to better leverage evidence-based approaches to cultural adaptations of behavioral health interventions (21) for prevalent sleep-wake disorders and sleep disturbances.
Supplementary Material
Figure 2.
Legend. Types of Surface-Level and Deep-Level Cultural Adaptations of Psychological Interventions for Prevalent Sleep Disorders and Sleep Disturbances. Percentages reflect the ratio of the number of times a specific type of cultural adaptation was made to the total number of studies testing adapted interventions multiplied by 100.
Practice Points.
Only 6.97% of randomized controlled trials of psychological interventions for prevalent sleep-wake disorders or sleep disturbances ever published targeted an underserved adult subgroup (i.e., veterans, women, racial/ethnic minorities, low socioeconomic status, those with a disability).
None of the included studies explicitly targeted linguistic minorities, immigrants, or LGBTQ adults, and there was limited racial/ethnic and socioeconomic diversity in the sample composition.
Nearly 65% of randomized controlled trials tested psychological interventions with surface-level or deep-level cultural adaptations. Surface-level cultural adaptations to delivery modality and setting were most common.
On balance, psychological interventions for sleep disorders with cultural adaptations generally resulted in improved sleep outcomes, though the evidence appears to be more consistent for culturally adapted CBT-I interventions among participants with a definite sleep disorder or sleep disturbance.
Research Agenda.
Future RCTs of psychological interventions in behavioral sleep medicine should seek to expand the diversity of the participant sample, and thereby enhance generalizability.
The average educational attainment of RCT participants in this review was over 14 years. In order to ensure access to high quality mental health care for all, it will be imperative that psychological interventions are developed with greater attention to general literacy, numeracy, and health literacy concerns.
Future research should examine whether specific types of cultural adaptations or the number of cultural adaptations modify the effectiveness of a psychological intervention targeting sleep disorders among underserved populations.
Acknowledgements:
This work was supported by the National Heart Lung and Blood Institute grant number HL125748, and grant number R01HS024274 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Abbreviations
- ACT
Acceptance and commitment therapy
- BT
Behavioral therapy
- CAM
Complementary and alternative medicine
- CBT-I
Cognitive behavioral therapy
- CBT-I
Cognitive behavioral therapy for insomnia
- CPT
Cognitive processing therapy
- IRT
Imagery rehearsal therapy
- LGBTQ
Lesbian, gay, bisexual, transgender, and queer
- MBSR
Mindfulness-based stress reduction
- PST
Problem-solving therapy
- RCT
Randomized Controlled Trial
Footnotes
Conflicts of Interest: The authors do not have any conflicts of interest to disclose.
This systematic review protocol is registered as:
PROSPERO2016:CRD42016039070 (www.crd.york.ac.uk/PROSPERO/display_record.php?ID =CRD42016039070)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–58. [DOI] [PubMed] [Google Scholar]
- 2.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Wheaton AG, Chapman DP, Croft JB. Sleep duration and chronic diseases among U.S. adults age 45 years and older: evidence from the 2010 Behavioral Risk Factor Surveillance System. Sleep. 2013;36(10):1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depression and anxiety. 2015;32(9):664–70. [DOI] [PubMed] [Google Scholar]
- 5.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1):10–9. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of Occupational Accidents in Workers with Obstructive Sleep Apnea: Systematic Review and Meta-analysis. Sleep. 2016;39(6):1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychological bulletin. 2007;133(3):482–528. [DOI] [PubMed] [Google Scholar]
- 9.Luyster FS, Strollo PJ Jr., Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35(6):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Morselli PL, Guilleminault C. Prevalence of nightmares and their relationship to psychopathology and daytime functioning in insomnia subjects. Sleep. 1997;20(5):340–8. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental health disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 12.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: The return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. [DOI] [PubMed] [Google Scholar]
- 13.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Hampton LM, Daubresse M, Chang HY, Alexander GC, Budnitz DS. Emergency department visits by adults for psychiatric medication adverse events. JAMA psychiatry. 2014;71(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healthy People 2030 Sleep Overview and Objectives [Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/sleep.
- 17.Lettieri CJ, Williams SG, Collen JF, Wickwire EM. Treatment of Obstructive Sleep Apnea: Achieving Adherence to Positive Airway Pressure Treatment and Dealing with Complications. Sleep medicine clinics. 2017;12(4):551–64. [DOI] [PubMed] [Google Scholar]
- 18.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004). Sleep. 2006;29(11):1398–414. [DOI] [PubMed] [Google Scholar]
- *19.Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–9. [DOI] [PubMed] [Google Scholar]
- 20.Priday LJ, Byrne C, Totsika V. Behavioural interventions for sleep problems in people with an intellectual disability: a systematic review and meta-analysis of single case and group studies. J Intellect Disabil Res. 2017;61(1):1–15. [DOI] [PubMed] [Google Scholar]
- *21.Barrera M Jr., Castro FG, Strycker LA, Toobert DJ. Cultural adaptations of behavioral health interventions: a progress report. J Consult Clin Psychol. 2013;81(2):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernal G, Jiménez-Chafey MI, Domenech Rodríguez MMJPPR, Practice. Cultural adaptation of treatments: A resource for considering culture in evidence-based practice. Professional Psychology: Research and Practice. 2009;40(4):361. [Google Scholar]
- *23.Hall GC, Ibaraki AY, Huang ER, Marti CN, Stice E. A Meta-Analysis of Cultural Adaptations of Psychological Interventions. Behavior therapy. 2016;47(6):993–1014. [DOI] [PubMed] [Google Scholar]
- *24.Healey P, Stager ML, Woodmass K, Dettlaff AJ, Vergara A, Janke R, et al. Cultural adaptations to augment health and mental health services: a systematic review. BMC Health Serv Res. 2017;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griner D, Smith TB. Culturally adapted mental health intervention: A meta-analytic review. Psychotherapy (Chicago, Ill). 2006;43(4):531–48. [DOI] [PubMed] [Google Scholar]
- 26.Antoniades JM D; Brijnath B Efficacy of depression treatments for immigrant patients: Results from a systematic review. BioMed Central Psychiatry. 2014;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A critical review of culturally sensitive treatments for depression: Recommendations for intervention and research [press release]. US: Educational Publishing Foundation; 2014. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- *29.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health education & behavior : the official publication of the Society for Public Health Education. 2003;30(2):133–46. [DOI] [PubMed] [Google Scholar]
- 31.Mier N, Ory MG, Medina AA. Anatomy of culturally sensitive interventions promoting nutrition and exercise in hispanics: a critical examination of existing literature. Health promotion practice. 2010;11(4):541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnicow K, Baranowski T, Ahluwalia JS, Braithwaite RL. Cultural sensitivity in public health: defined and demystified. Ethnicity & disease. 1999;9(1):10–21. [PubMed] [Google Scholar]
- 33.Wilson BD, Miller RL. Examining strategies for culturally grounded HIV prevention: a review. AIDS education and prevention : official publication of the International Society for AIDS Education. 2003;15(2):184–202. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Green S. Cochrane handbook for systematic review of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. 2011. [Google Scholar]
- 35.Alessi C, Martin JL, Fiorentino L, Fung CH, Dzierzewski JM, Rodriguez Tapia JC, et al. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. Journal of the American Geriatrics Society. 2016;64(9):1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake CL, Kalmbach DA, Arnedt JT, Cheng P, Tonnu CV, Cuamatzi-Castelan A, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–82. [DOI] [PubMed] [Google Scholar]
- 38.Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncology nursing forum. 2007;34(5):E51–9. [DOI] [PubMed] [Google Scholar]
- 40.Fiorentino L, McQuaid JR, Liu L, Natarajan L, He F, Cornejo M, et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover pilot study. Nature and science of sleep. 2010;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung CH, Martin JL, Josephson K, Fiorentino L, Dzierzewski JM, Jouldjian S, et al. Efficacy of Cognitive Behavioral Therapy for Insomnia in Older Adults With Occult Sleep-Disordered Breathing. Psychosomatic medicine. 2016;78(5):629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurel Franklin C, Walton JL, Raines AM, Chambliss JL, Corrigan SA, Cuccurullo LJ, et al. Pilot study comparing telephone to in-person delivery of cognitive-behavioural therapy for trauma-related insomnia for rural veterans. Journal of telemedicine and telecare. 2018;24(9):629–35. [DOI] [PubMed] [Google Scholar]
- 43.Margolies SO, Rybarczyk B, Vrana SR, Leszczyszyn DJ, Lynch J. Efficacy of a cognitive-behavioral treatment for insomnia and nightmares in Afghanistan and Iraq veterans with PTSD. Journal of clinical psychology. 2013;69(10):1026–42. [DOI] [PubMed] [Google Scholar]
- 44.Matthews EE, Berger AM, Schmiege SJ, Cook PF, McCarthy MS, Moore CM, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncology nursing forum. 2014;41(3):241–53. [DOI] [PubMed] [Google Scholar]
- 45.McCurry SM, Guthrie KA, Morin CM, Woods NF, Landis CA, Ensrud KE, et al. Telephone-Based Cognitive Behavioral Therapy for Insomnia in Perimenopausal and Postmenopausal Women With Vasomotor Symptoms: A MsFLASH Randomized Clinical Trial. JAMA internal medicine. 2016;176(7):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palesh O, Scheiber C, Kesler S, Janelsins MC, Guido JJ, Heckler C, et al. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. British journal of cancer. 2018;119(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pigeon WR, Funderburk J, Bishop TM, Crean HF. Brief cognitive behavioral therapy for insomnia delivered to depressed veterans receiving primary care services: A pilot study. J Affect Disord. 2017;217:105–11. [DOI] [PubMed] [Google Scholar]
- 48.Scogin F, Lichstein K, DiNapoli EA, Woosley J, Thomas SJ, LaRocca MA, et al. Effects of Integrated Telehealth-Delivered Cognitive-Behavioral Therapy for Depression and Insomnia in Rural Older Adults. Journal of psychotherapy integration. 2018;28(3):292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulmer CS, Edinger JD, Calhoun PS. A multi-component cognitive-behavioral intervention for sleep disturbance in veterans with PTSD: a pilot study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011;7(1):57–68. [PMC free article] [PubMed] [Google Scholar]
- 50.Cain MA, Brumley J, Louis-Jacques A, Drerup M, Stern M, Louis JM. A Pilot Study of a Sleep Intervention Delivered through Group Prenatal Care to Overweight and Obese Women. Behavioral sleep medicine. 2019:1–11. [DOI] [PubMed] [Google Scholar]
- 51.Chakravorty S, Morales KH, Arnedt JT, Perlis ML, Oslin DW, Findley JC, et al. Cognitive Behavioral Therapy for Insomnia in Alcohol Dependent Veterans: A Randomized, Controlled Pilot Study. Alcoholism: Clinical and Experimental Research. 2019;43(6):1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalmbach DA, Cheng P, Arnedt JT, Cuamatzi-Castelan A, Atkinson RL, Fellman-Couture C, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Journal of Clinical Sleep Medicine. 2019;15(7):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pigeon WR, Funderburk JS, Cross W, Bishop TM, Crean HF. Brief CBT for insomnia delivered in primary care to patients endorsing suicidal ideation: a proof-of-concept randomized clinical trial. Translational behavioral medicine. 2019;9(6):1169–77. [DOI] [PubMed] [Google Scholar]
- 54.Manber R, Bei B, Simpson N, Asarnow L, Rangel E, Sit A, et al. Cognitive behavioral therapy for prenatal insomnia: A randomized controlled trial. Obstetrics and gynecology. 2019;133(5):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Innes KE, Selfe TK. The Effects of a Gentle Yoga Program on Sleep, Mood, and Blood Pressure in Older Women with Restless Legs Syndrome (RLS): A Preliminary Randomized Controlled Trial. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:294058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Nicassio P, Ganz PA, et al. Tai Chi Chih Compared With Cognitive Behavioral Therapy for the Treatment of Insomnia in Survivors of Breast Cancer: A Randomized, Partially Blinded, Noninferiority Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(23):2656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura Y, Lipschitz DL, Donaldson GW, Kida Y, Williams SL, Landward R, et al. Investigating Clinical Benefits of a Novel Sleep-Focused Mind-Body Program on Gulf War Illness Symptoms: A Randomized Controlled Trial. Psychosomatic medicine. 2017;79(6):706–18. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura Y, Lipschitz DL, Landward R, Kuhn R, West G. Two sessions of sleepfocused mind-body bridging improve self-reported symptoms of sleep and PTSD in veterans: A pilot randomized controlled trial. Journal of psychosomatic research. 2011;70(4):335–45. [DOI] [PubMed] [Google Scholar]
- 59.Nidich S, O’Connor T, Rutledge T, Duncan J, Compton B, Seng A, et al. Reduced Trauma Symptoms and Perceived Stress in Male Prison Inmates through the Transcendental Meditation Program: A Randomized Controlled Trial. The Permanente journal. 2016;20(4):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoerkel E, Bellanti D, Paat C, Peacock K, Aden J, Setlik R, et al. Effectiveness of a Self-Care Toolkit for Surgical Breast Cancer Patients in a Military Treatment Facility. Journal of alternative and complementary medicine (New York, NY). 2018;24(9–10):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamison AL, Slightam C, Bertram F, Kim S, Roth WT. Randomized clinical trial of capnometry-assisted respiratory training in veterans with posttraumatic stress disorder hyperarousal. Psychological Trauma: Theory, Research, Practice, and Policy. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter LS, Carson JW, Olsen M, Carson KM, Sanders L, Jones L, et al. Feasibility of a mindful yoga program for women with metastatic breast cancer: results of a randomized pilot study. Supportive Care in Cancer. 2019;27(11):4307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger AM, Kuhn BR, Farr LA, Lynch JC, Agrawal S, Chamberlain J, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho-oncology. 2009a;18(6):634–46. [DOI] [PubMed] [Google Scholar]
- 64.Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009b;27(35):6033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Germain A, Richardson R, Moul DE, Mammen O, Haas G, Forman SD, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. Journal of psychosomatic research. 2012;72(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Germain A, Richardson R, Stocker R, Mammen O, Hall M, Bramoweth AD, et al. Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: preliminary randomized controlled trial. Behaviour research and therapy. 2014;61:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson TM 2nd, Vaughan CP, Goode PS, Bliwise DL, Markland AD, Huisingh C, et al. Pilot Results from a Randomized Trial in Men Comparing Alpha-Adrenergic Antagonist versus Behavior and Exercise for Nocturia and Sleep. Clinical therapeutics. 2016. [DOI] [PubMed] [Google Scholar]
- 68.Martin JL, Song Y, Hughes J, Jouldjian S, Dzierzewski JM, Fung CH, et al. A Four-Session Sleep Intervention Program Improves Sleep for Older Adult Day Health Care Participants: Results of a Randomized Controlled Trial. Sleep. 2017;40(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gebara MA, DiNapoli EA, Lederer LG, Bramoweth AD, Germain A, Kasckow JW, et al. Brief behavioral treatment for insomnia in older adults with late-life treatment-resistant depression and insomnia: a pilot study. Sleep and biological rhythms. 2019;17(3):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee KA, Jong S, Gay CL. Fatigue management for adults living with HIV: A randomized controlled pilot study. Research in nursing & health. 2020;43(1):56–67. [DOI] [PubMed] [Google Scholar]
- 71.Brenes GA, Danhauer SC, Lyles MF, Anderson A, Miller ME. Effects of Telephone-Delivered Cognitive-Behavioral Therapy and Nondirective Supportive Therapy on Sleep, Health-Related Quality of Life, and Disability. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2016;24(10):846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heapy AA, Higgins DM, Goulet JL, LaChappelle KM, Driscoll MA, Czlapinski RA, et al. Interactive Voice Response-Based Self-management for Chronic Back Pain: The COPES Noninferiority Randomized Trial. JAMA internal medicine. 2017;177(6):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanley MA, Wilson N, Shrestha S, Amspoker AB, Armento M, Cummings JP, et al. Calmer life: A culturally tailored intervention for anxiety in underserved older adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2016;24(8):648–58. [DOI] [PubMed] [Google Scholar]
- 74.Stanley MA, Wilson NL, Shrestha S, Amspoker AB, Wagener P, Bavineau J, et al. Community-Based Outreach and Treatment for Underserved Older Adults With Clinically Significant Worry: A Randomized Controlled Trial. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2018;26(11):1147–62. [DOI] [PubMed] [Google Scholar]
- 75.Herbert MS, Afari N, Liu L, Heppner P, Rutledge T, Williams K, et al. Telehealth Versus In-Person Acceptance and Commitment Therapy for Chronic Pain: A Randomized Noninferiority Trial. The journal of pain 2017;18(1528–8447 (Electronic)):200–11. [DOI] [PubMed] [Google Scholar]
- 76.Lang AJ, Schnurr PP, Jain S, He F, Walser RD, Bolton E, et al. Randomized controlled trial of acceptance and commitment therapy for distress and impairment in OEF/OIF/OND veterans. Psychological trauma : theory, research, practice and policy. 2017;9(Suppl 1), (1942–969X (Electronic)):74–84. [DOI] [PubMed] [Google Scholar]
- 77.Mosher CE, Secinti E, Li R, Hirsh AT, Bricker J, Miller KD, et al. Acceptance and commitment therapy for symptom interference in metastatic breast cancer patients: a pilot randomized trial. Supportive Care in Cancer. 2018;26(6):1993–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galovski TE, Harik JM, Blain LM, Elwood L, Gloth C, Fletcher TD. Augmenting cognitive processing therapy to improve sleep impairment in PTSD: A randomized controlled trial. J Consult Clin Psychol. 2016;84(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galovski TE, Monson C, Bruce SE, Resick PA. Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? Journal of traumatic stress. 2009;22(3):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutner CA, Casement MD, Stavitsky Gilbert K, Resick PA. Change in sleep symptoms across Cognitive Processing Therapy and Prolonged Exposure: a longitudinal perspective. Behaviour research and therapy. 2013;51(12):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cash E, Salmon P, Weissbecker I, Rebholz WN, Bayley-Veloso R, Zimmaro LA, et al. Mindfulness meditation alleviates fibromyalgia symptoms in women: results of a randomized clinical trial. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2015;49(3):319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lengacher CA, Reich RR, Paterson CL, Jim HS, Ramesar S, Alinat CB, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psycho-oncology. 2015;24(4):424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: an exploratory study. Journal of psychosomatic research. 2003;54(1):85–91. [DOI] [PubMed] [Google Scholar]
- 84.Witek Janusek L, Tell D, Mathews HL. Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: a randomized trial with active control. Brain, Behavior, and Immunity. 2019;80:358–73. [DOI] [PubMed] [Google Scholar]
- 85.Alschuler KN, Arewasikporn A, Nelson IK, Molton IR, Ehde DM. Promoting resilience in individuals aging with multiple sclerosis: Results from a pilot randomized controlled trial. Rehabilitation psychology. 2018;63(3):338–48. [DOI] [PubMed] [Google Scholar]
- 86.Church D, Sparks T, Clond M. EFT (Emotional Freedom Techniques) and Resiliency in Veterans at Risk for PTSD: A Randomized Controlled Trial. Explore (New York, NY). 2016;12(5):355–65. [DOI] [PubMed] [Google Scholar]
- 87.Kahn JR, Collinge W, Soltysik R. Post-9/11 Veterans and Their Partners Improve Mental Health Outcomes with a Self-directed Mobile and Web-based Wellness Training Program: A Randomized Controlled Trial. Journal of medical Internet research. 2016;18(9):e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. Jama. 2001;286(5):537–45. [DOI] [PubMed] [Google Scholar]
- 89.Cook JM, Harb GC, Gehrman PR, Cary MS, Gamble GM, Forbes D, et al. Imagery rehearsal for posttraumatic nightmares: a randomized controlled trial. Journal of traumatic stress. 2010;23(5):553–63. [DOI] [PubMed] [Google Scholar]
- 90.Harb GC, Cook JM, Phelps AJ, Gehrman PR, Forbes D, Localio R, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. Journal of Clinical Sleep Medicine. 2019;15(05):757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bedford LA, Dietch JR, Taylor DJ, Boals A, Zayfert C. Computer-Guided Problem-Solving Treatment for Depression, PTSD, and Insomnia Symptoms in Student Veterans: A Pilot Randomized Controlled Trial. Behavior therapy. 2018;49(5):756–67. [DOI] [PubMed] [Google Scholar]
- 92.United States Census Bureau Population Division. Annual Estimate of the Resident Population by Sex, Age, Race Alone, and Hispanic Origin for the United States and States: April 1, 2010 to July 1, 2017. [updated June 2018. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk#.
- 93.Gambino CP, Acosta YD, Grieco EM. English-Speaking Ability of the Foreign-Born Population in the United States: 2012. Washington, D.C., Bureau USC; 2014. Report No.: ACS-26. [Google Scholar]
- *94.Polo AJ, Makol BA, Castro AS, Colón-Quintana N, Wagstaff AE, Guo S. Diversity in randomized clinical trials of depression: A 36-year review. Clinical Psychology Review. 2019;67:22–35. [DOI] [PubMed] [Google Scholar]
- 95.National Institute of Health. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research [Available from: https://grants.nih.gov/grants/funding/women_min/guidelines.htm.
- 96.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC medicine. 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirsch IS, Jungeblut A, Jenkins L, Kolstad A. Adult literacy in America: A first look at the results of the National Adult Literacy Survey. Third Edition ed. Washington D.C.: National Center for Education Statistics; 2002. [Google Scholar]
- *98.2017 National Healthcare Quality and Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; September 2018. AHRQ Pub No. 18–00330EF. [PubMed] [Google Scholar]
- *99.Cook BL, Trinh NH, Li Z, Hou SS, Progovac AM. Trends in Racial-Ethnic Disparities in Access to Mental Health Care, 2004–2012. Psychiatric services (Washington, DC). 2017;68(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.