Abstract
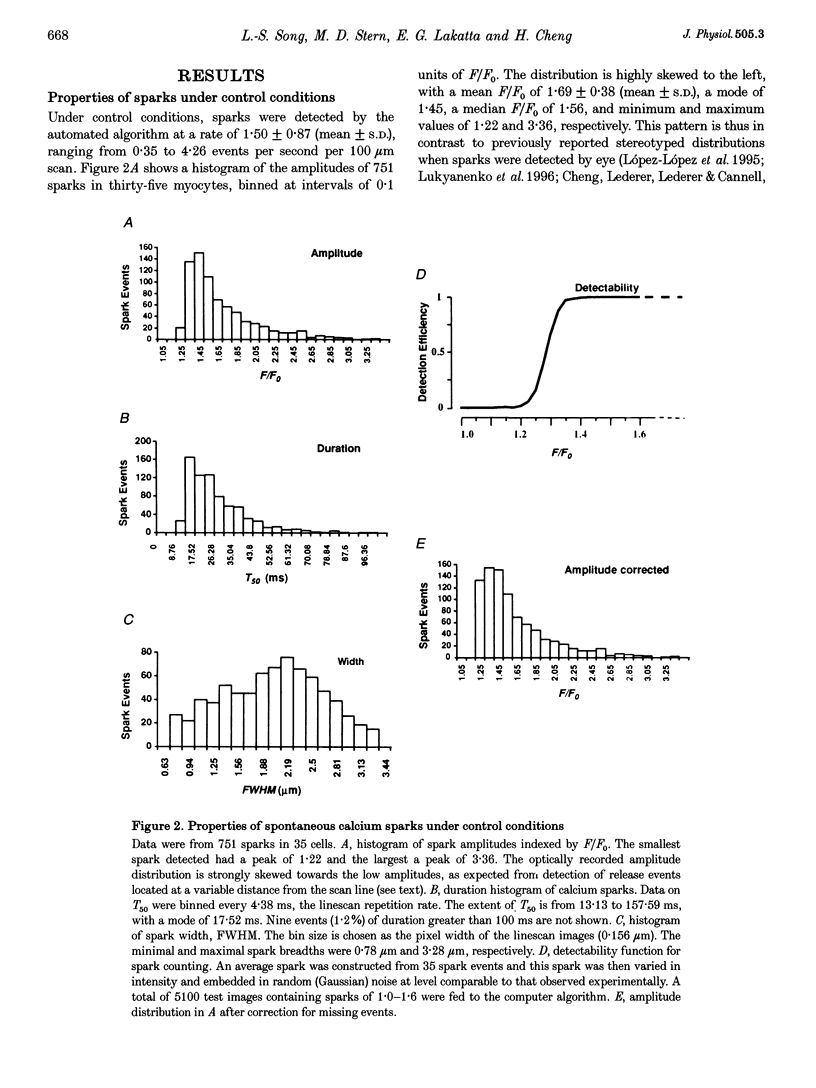
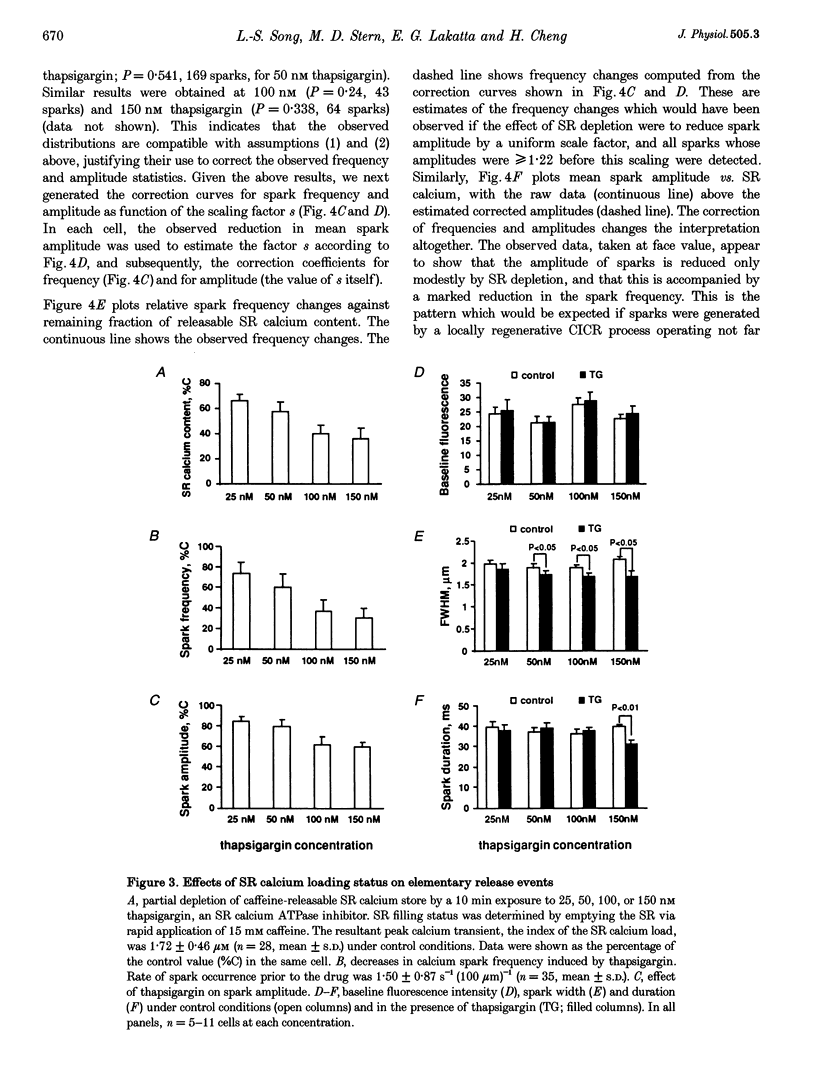
1. The exact nature of calcium sparks in the heart remains highly controversial. We sought to determine whether calcium sparks arise from a single or multiple calcium release channels/ ryanodine receptors in the sarcoplasmic reticulum (SR). If their genesis involves a calcium-coupled recruitment of multiple channels, calcium sparks might be abolished by a modest depletion of SR calcium (because of the decrease in unitary calcium flux and hence a decrease in the gain of local calcium-induced calcium release). If, on the other extreme, calcium sparks are produced despite severe SR depletion, the single-channel origin will be preferred. 2. Spontaneous calcium sparks were studied in rat ventricular myocytes using confocal microscopy and the fluorescent calcium probe fluo-3. A computer algorithm was developed to count and measure objectively calcium sparks in linescan images. 3. Thapsigargin (25-150 nM) depleted caffeine-releasable SR calcium by up to 64%, in a dose- and time-dependent manner, without altering the resting cytosolic calcium level. During SR depletion, calcium sparks were robustly observed, albeit at reduced frequency (> or = 30% of control) and amplitude (> or = 60% of control). 4. Due to the reduced detectability of small sparks against noise background, the observed data would overestimate reduction in spark frequency but underestimate amplitude reduction. After correction for this detection bias, we found that the spark frequency was independent of SR load, whereas the amplitude was proportional to load. 5. We conclude that, although spark amplitude depends on SR filling status, the frequency of spark generation is independent of SR calcium load, and therefore independent of the local calcium release rate. This implies that sparks are single-channel events, or collective events that are well above threshold for local regeneration. Additionally, our results suggest that intraluminal SR calcium, at normal or low loads, does not play a major role in the regulation of on-gating of the ryanodine receptor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandes R. P., Barton M., Philippens K. M., Schweitzer G., Mügge A. Endothelial-derived superoxide anions in pig coronary arteries: evidence from lucigenin chemiluminescence and histochemical techniques. J Physiol. 1997 Apr 15;500(Pt 2):331–342. doi: 10.1113/jphysiol.1997.sp022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr, Vaghy P. L., Meissner G., Ferguson D. G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995 May;129(3):673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Xiao R. P., Gómez A. M., Zhou Y. Y., Ziman B., Spurgeon H., Lakatta E. G., Lederer W. J. Excitation-contraction coupling in heart: new insights from Ca2+ sparks. Cell Calcium. 1996 Aug;20(2):129–140. doi: 10.1016/s0143-4160(96)90102-5. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Györke S., Lukyanenko V., Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997 Apr 15;500(Pt 2):297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N., Ronjat M., Mészáros L. G., Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989 Aug 8;28(16):6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Janczewski A. M., Lakatta E. G. Thapsigargin inhibits Ca2+ uptake, and Ca2+ depletes sarcoplasmic reticulum in intact cardiac myocytes. Am J Physiol. 1993 Aug;265(2 Pt 2):H517–H522. doi: 10.1152/ajpheart.1993.265.2.H517. [DOI] [PubMed] [Google Scholar]
- Janczewski A. M., Spurgeon H. A., Stern M. D., Lakatta E. G. Effects of sarcoplasmic reticulum Ca2+ load on the gain function of Ca2+ release by Ca2+ current in cardiac cells. Am J Physiol. 1995 Feb;268(2 Pt 2):H916–H920. doi: 10.1152/ajpheart.1995.268.2.H916. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kasai M. Regulation of calcium channel in sarcoplasmic reticulum by calsequestrin. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1120–1127. doi: 10.1006/bbrc.1994.1347. [DOI] [PubMed] [Google Scholar]
- Kirby M. S., Sagara Y., Gaa S., Inesi G., Lederer W. J., Rogers T. B. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem. 1992 Jun 25;267(18):12545–12551. [PubMed] [Google Scholar]
- Langer G. A., Peskoff A. Calcium concentration and movement in the diadic cleft space of the cardiac ventricular cell. Biophys J. 1996 Mar;70(3):1169–1182. doi: 10.1016/S0006-3495(96)79677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996 Apr 1;492(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke I., Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996 Oct;432(6):1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Parker I., Zang W. J., Wier W. G. Ca2+ sparks involving multiple Ca2+ release sites along Z-lines in rat heart cells. J Physiol. 1996 Nov 15;497(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Stern M. D. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct. 1997;26:47–82. doi: 10.1146/annurev.biophys.26.1.47. [DOI] [PubMed] [Google Scholar]
- Santana L. F., Cheng H., Gómez A. M., Cannell M. B., Lederer W. J. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996 Jan;78(1):166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Satoh H., Blatter L. A., Bers D. M. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997 Feb;272(2 Pt 2):H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Capogrossi M. C., Lakatta E. G. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988 Dec;9(5-6):247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A., Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996 Jun;70(6):2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]




