Abstract
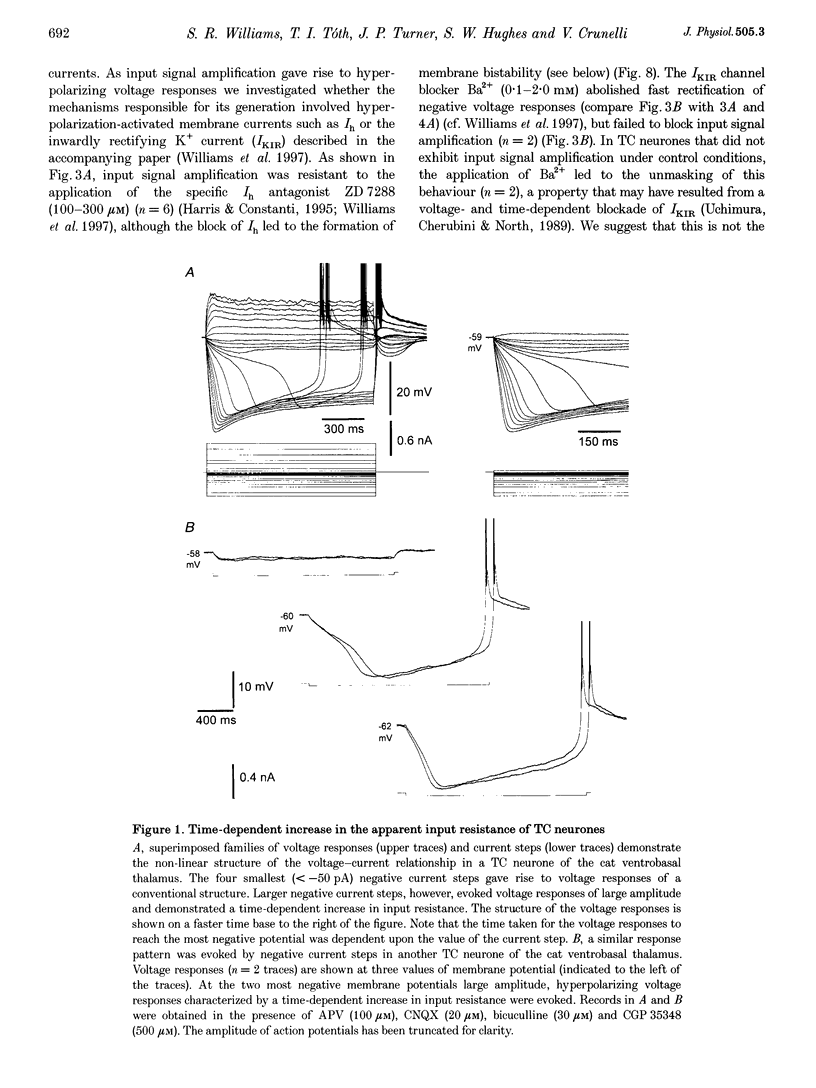
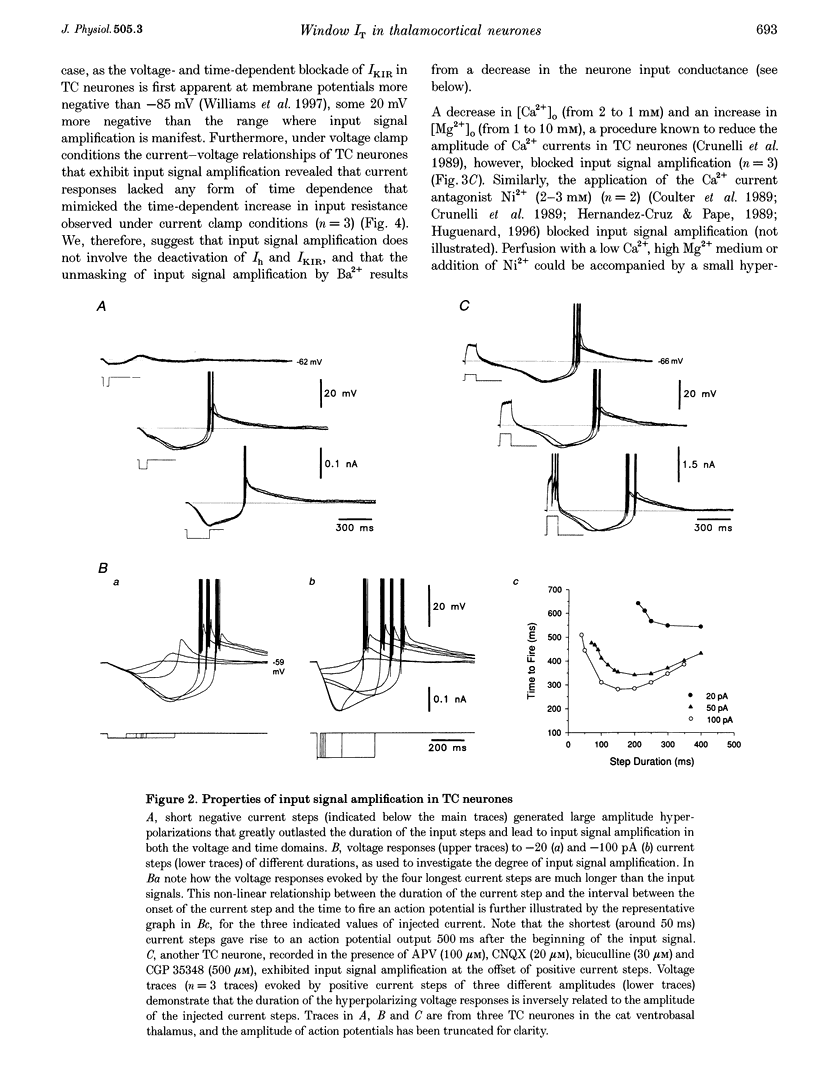
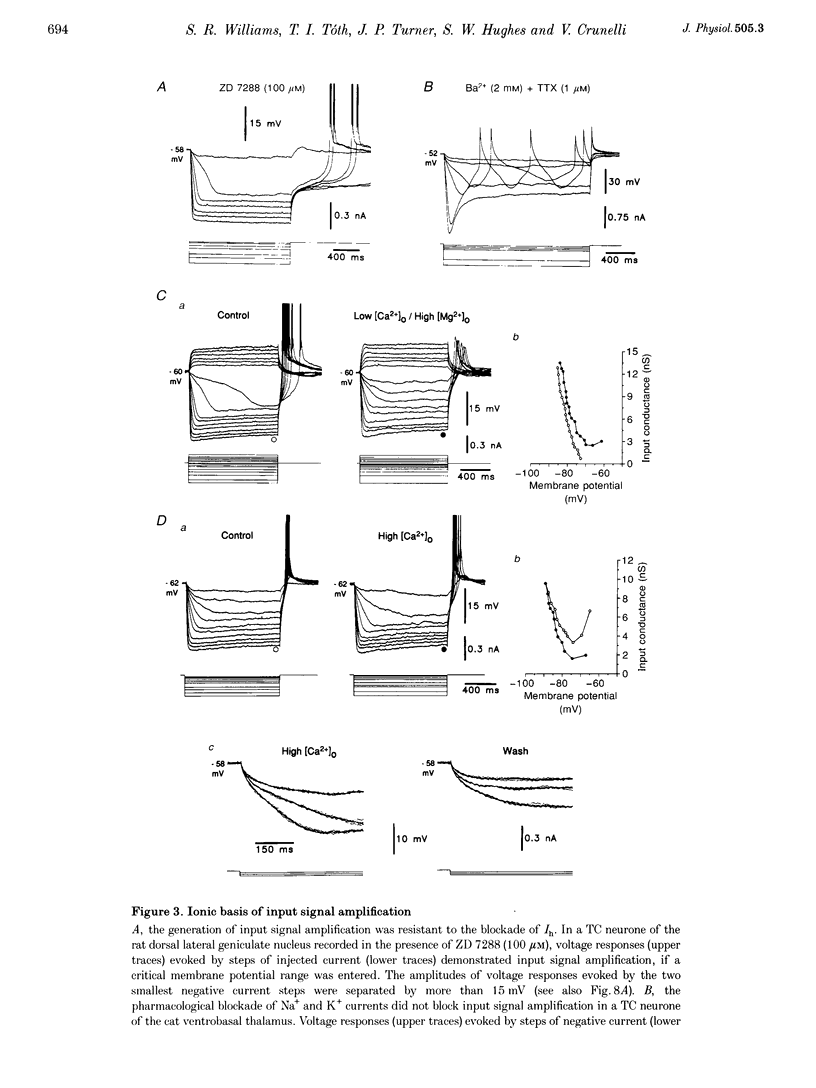
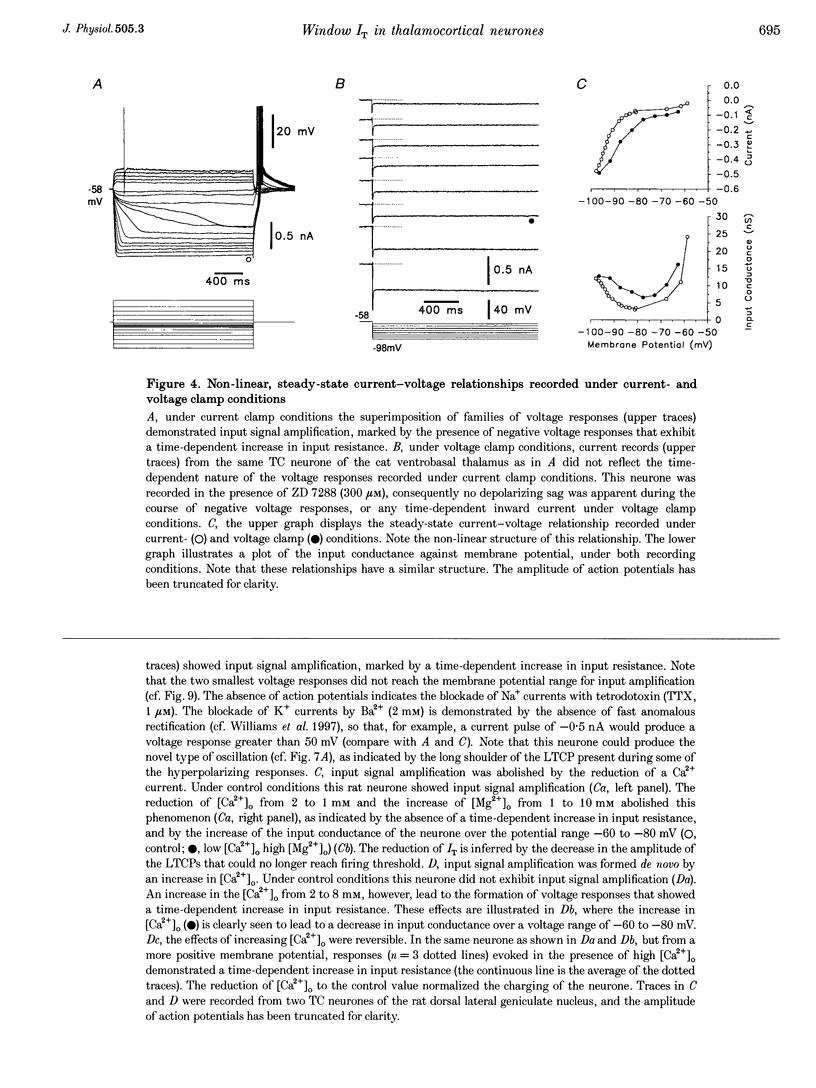
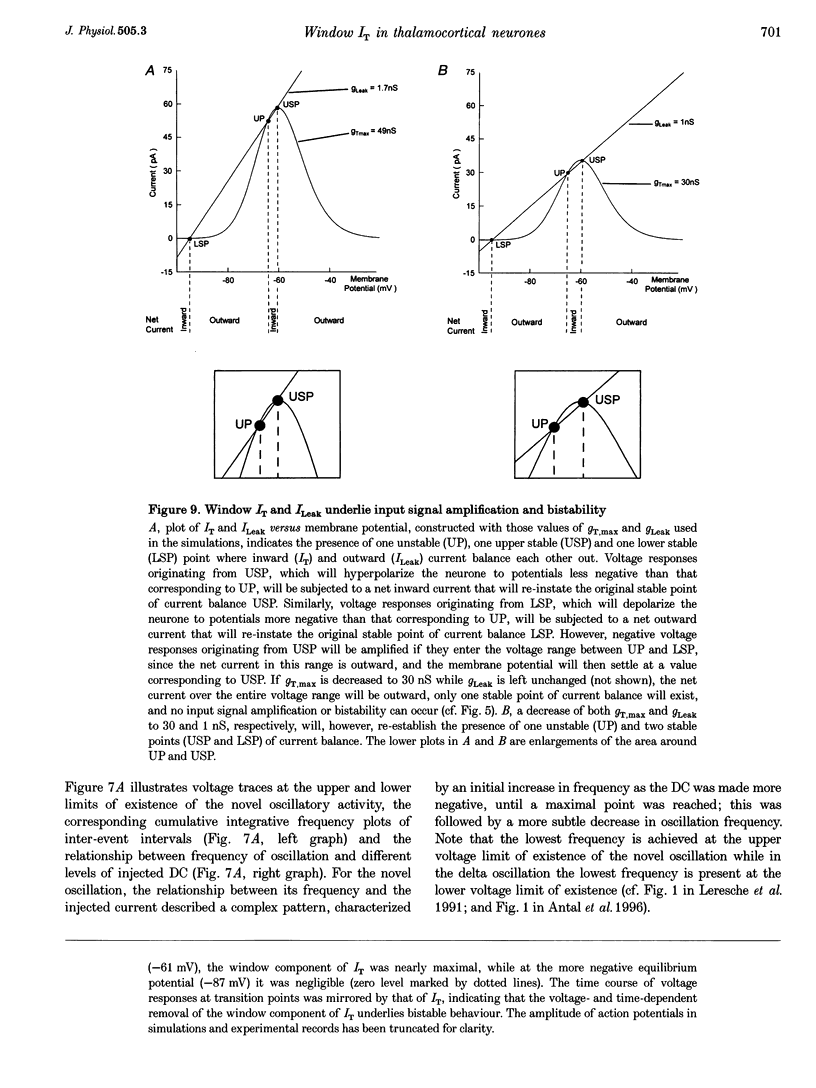
1. The mechanism underlying a novel form of input signal amplification and bistability was investigated by intracellular recording in rat and cat thalamocortical (TC) neurones maintained in slices and by computer simulation with a biophysical model of these neurones. 2. In a narrow membrane potential range centred around -60 mV, TC neurones challenged with small (10-50 pA), short (50-200 ms) current steps produced a stereotyped, large amplitude hyperpolarization (> 20 mV) terminated by the burst firing of action potentials, leading to amplification of the duration and amplitude of the input signal, that is hereafter referred to as input signal amplification. 3. In the same voltage range centred around -60 mV, single evoked EPSPs and IPSPs also produced input signal amplification, indicating that this behaviour can be triggered by physiologically relevant stimuli. In addition, a novel, intrinsic, low frequency oscillation, characterized by a peculiar voltage dependence of its frequency and by the presence of plateau potentials on the falling phase of low threshold Ca2+ potentials, was recorded. 4. Blockade of pure Na+ and K+ currents by tetrodotoxin (1 microM) and Ba2+ (0.1-2.0 mM), respectively, did not affect input signal amplification, neither did the presence of excitatory or inhibitory amino acid receptor antagonists in the perfusion medium. 5. A decrease in [Ca2+]o (from 2 to 1 mM) and an increase in [Mg2+]o (from 2 to 10 mM), or the addition of Ni2+ (2-3 mM), abolished input signal amplification, while an increase in [Ca2+]o (from 2 to 8 mM) generated this behaviour in neurones where it was absent in control conditions. These results indicate the involvement of the low threshold Ca2+ current (IT) in input signal amplification, since the other Ca2+ currents of TC neurones are activated at potentials more positive than -40 mV. 6. Blockade of the slow inward mixed cationic current (Ih) by 4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)-pyrimidinium++ + chloride (ZD 7288)(100-300 microM) did not affect the expression of the large amplitude hyperpolarization, but abolished the subsequent repolarization to the original membrane potential. In this condition, therefore, input signal amplification was replaced by bistable membrane behaviour, where two stable membrane potentials separated by 15-30 mV could be switched between by small current steps. 7. Computer simulation with a model of a TC neurone, which contained only IT, Ih, K+ leak current (ILeak) and those currents responsible for action potentials, accurately reproduced the qualitative and quantitative properties of input signal amplification, bistability and low frequency oscillation, and indicated that these phenomena will occur at some value of the injected DC if, and only if, the 'window' component of IT (IT,Window) and the leak conductance (gLeak) satisfy the relation (dIT,Window/dV)max > gLeak. 8. The physiological implications of these findings for the electroresponsiveness of TC neurones are discussed, and, as IT is widely expressed in the central nervous system, we suggest that 'window' IT will markedly affect the integrative properties of many neurones.
Full text
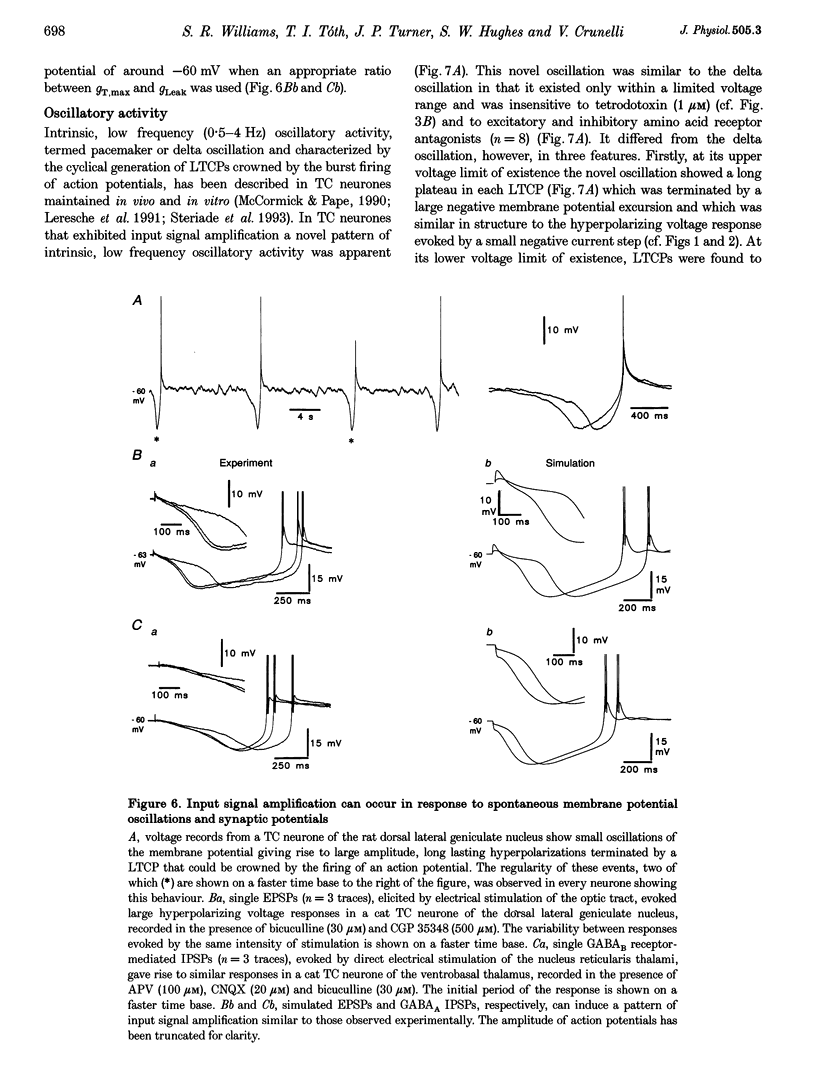
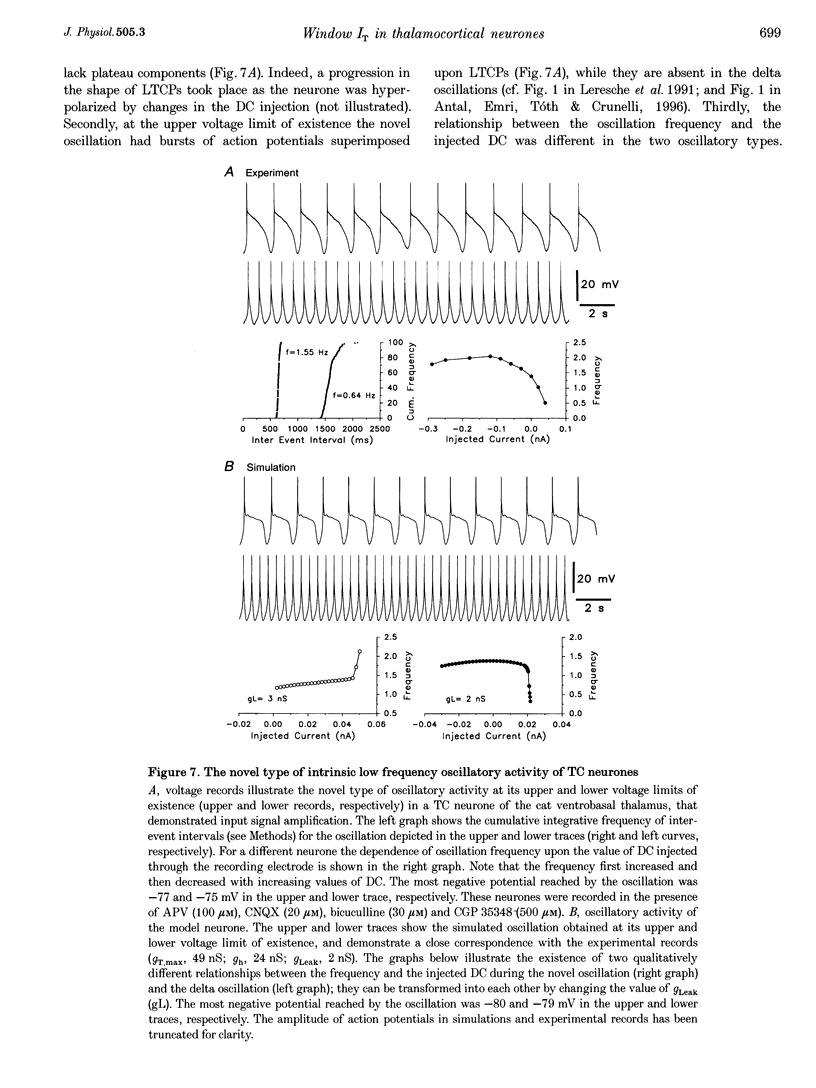
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
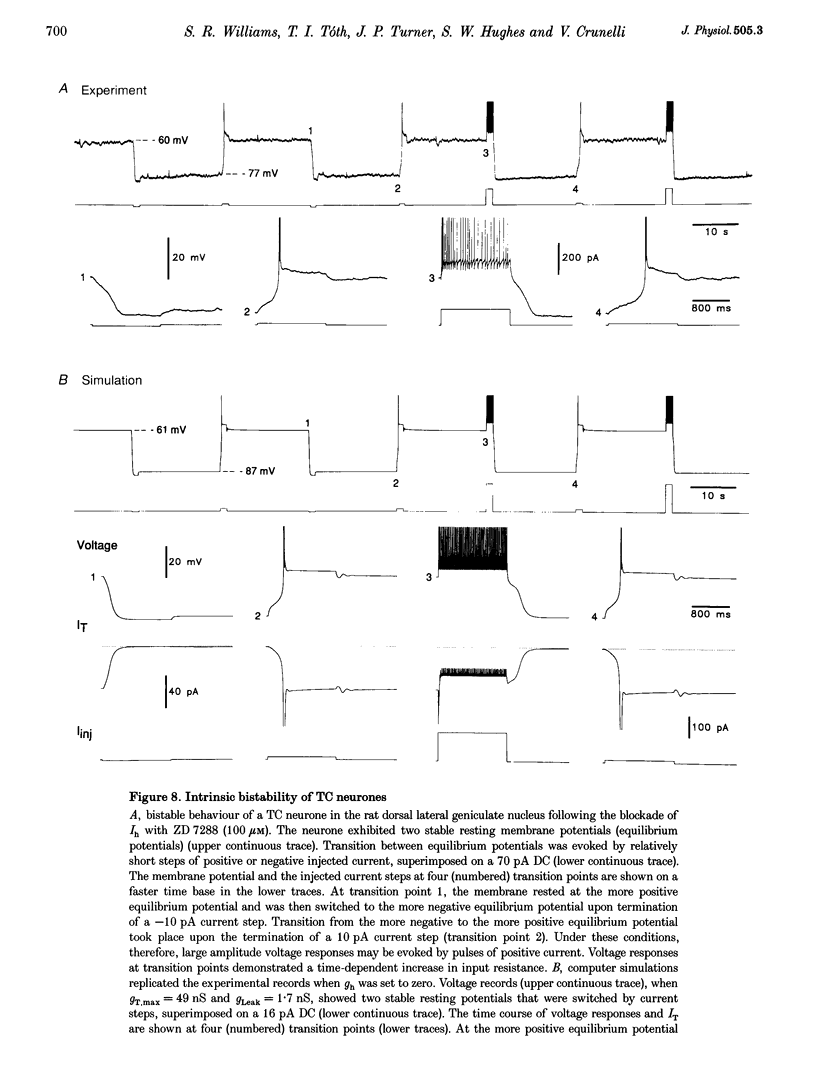
- Antal K., Emri Z., Tóth T. I., Crunelli V. Model of a thalamocortical neurone with dendritic voltage-gated ion channels. Neuroreport. 1996 Nov 4;7(15-17):2655–2658. doi: 10.1097/00001756-199611040-00049. [DOI] [PubMed] [Google Scholar]
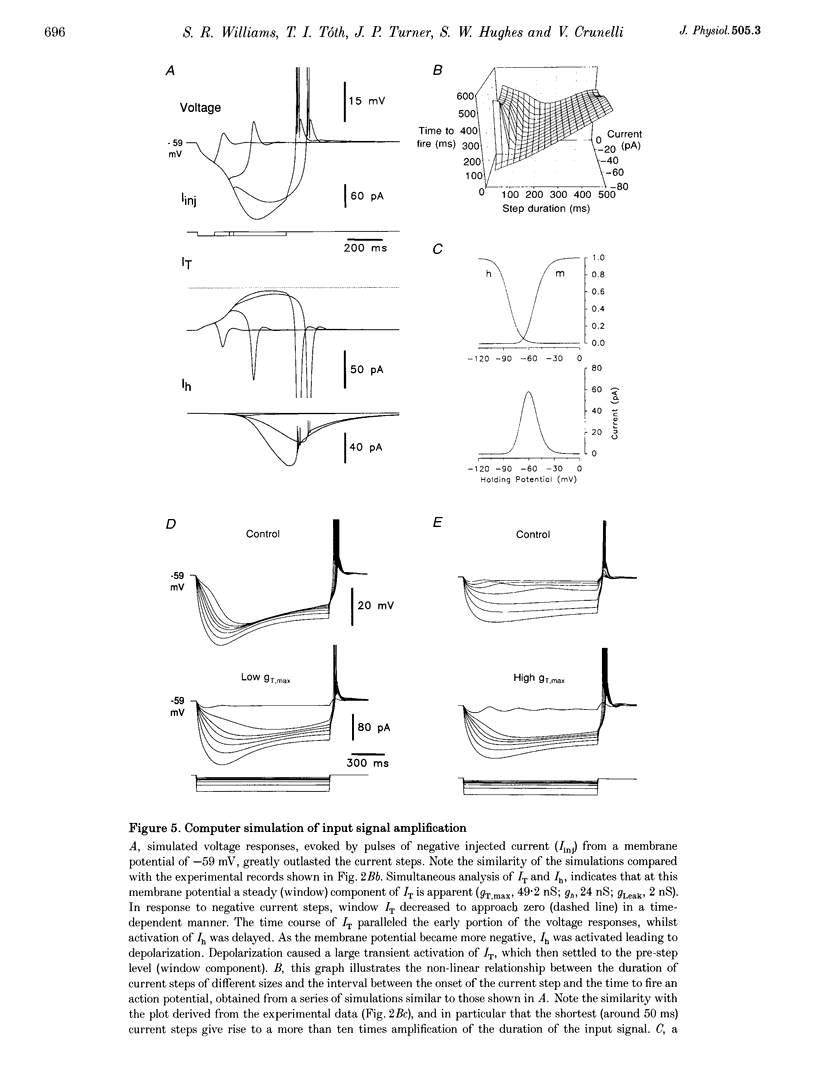
- Bal T., von Krosigk M., McCormick D. A. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995 Mar 15;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. Cumulative frequency curves in population analysis. Trends Pharmacol Sci. 1990 Oct;11(10):404–406. doi: 10.1016/0165-6147(90)90146-y. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989 Jul;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987 Mar;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 1991 Jan;14(1):16–21. doi: 10.1016/0166-2236(91)90178-w. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. Membrane properties of morphologically identified X and Y cells in the lateral geniculate nucleus of the cat in vitro. J Physiol. 1987 Sep;390:243–256. doi: 10.1113/jphysiol.1987.sp016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991 Feb;65(2):371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Ebenezer I. S. Effects of the 5HT1A agonist, 8-OH-DPAT, on operant food intake in non-deprived rats. Neuroreport. 1992 Jan;3(1):62–64. doi: 10.1097/00001756-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Guido W., Weyand T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol. 1995 Oct;74(4):1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- Guyon A., Leresche N. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. J Physiol. 1995 May 15;485(Pt 1):29–42. doi: 10.1113/jphysiol.1995.sp020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. C., Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol. 1995 Dec;74(6):2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A., Pape H. C. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J Neurophysiol. 1989 Jun;61(6):1270–1283. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- Holmes W. R., Rall W. Electrotonic length estimates in neurons with dendritic tapering or somatic shunt. J Neurophysiol. 1992 Oct;68(4):1421–1437. doi: 10.1152/jn.1992.68.4.1421. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989 Jul;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche N., Lightowler S., Soltesz I., Jassik-Gerschenfeld D., Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. C., Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995 Apr 14;268(5208):301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991 Oct;66(4):1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Huguenard J. R. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992 Oct;68(4):1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate "metabotropic" receptors. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong T. W., Stea A., Hodson C. D., Dubel S. J., Vincent S. R., Snutch T. P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993 May 21;260(5111):1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Spruston N., Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992 Mar;67(3):508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Otis T. S., Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992 May;67(5):1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Steriade M., Apostol V., Oakson G. Control of unitary activities in cerebellothalamic pathway during wakefulness and synchronized sleep. J Neurophysiol. 1971 May;34(3):389–413. doi: 10.1152/jn.1971.34.3.389. [DOI] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991 Oct;11(10):3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K., Miles R., Michelson H. A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J Neurophysiol. 1991 Aug;66(2):635–650. doi: 10.1152/jn.1991.66.2.635. [DOI] [PubMed] [Google Scholar]
- Turner J. P., Anderson C. M., Williams S. R., Crunelli V. Morphology and membrane properties of neurones in the cat ventrobasal thalamus in vitro. J Physiol. 1997 Dec 15;505(Pt 3):707–726. doi: 10.1111/j.1469-7793.1997.707ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. P., Leresche N., Guyon A., Soltesz I., Crunelli V. Sensory input and burst firing output of rat and cat thalamocortical cells: the role of NMDA and non-NMDA receptors. J Physiol. 1994 Oct 15;480(Pt 2):281–295. doi: 10.1113/jphysiol.1994.sp020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N., Cherubini E., North R. A. Inward rectification in rat nucleus accumbens neurons. J Neurophysiol. 1989 Dec;62(6):1280–1286. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Turner J. P., Anderson C. M., Crunelli V. Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. J Physiol. 1996 Jan 1;490(Pt 1):129–147. doi: 10.1113/jphysiol.1996.sp021131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. R., Turner J. P., Hughes S. W., Crunelli V. On the nature of anomalous rectification in thalamocortical neurones of the cat ventrobasal thalamus in vitro. J Physiol. 1997 Dec 15;505(Pt 3):727–747. doi: 10.1111/j.1469-7793.1997.727ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


