Abstract
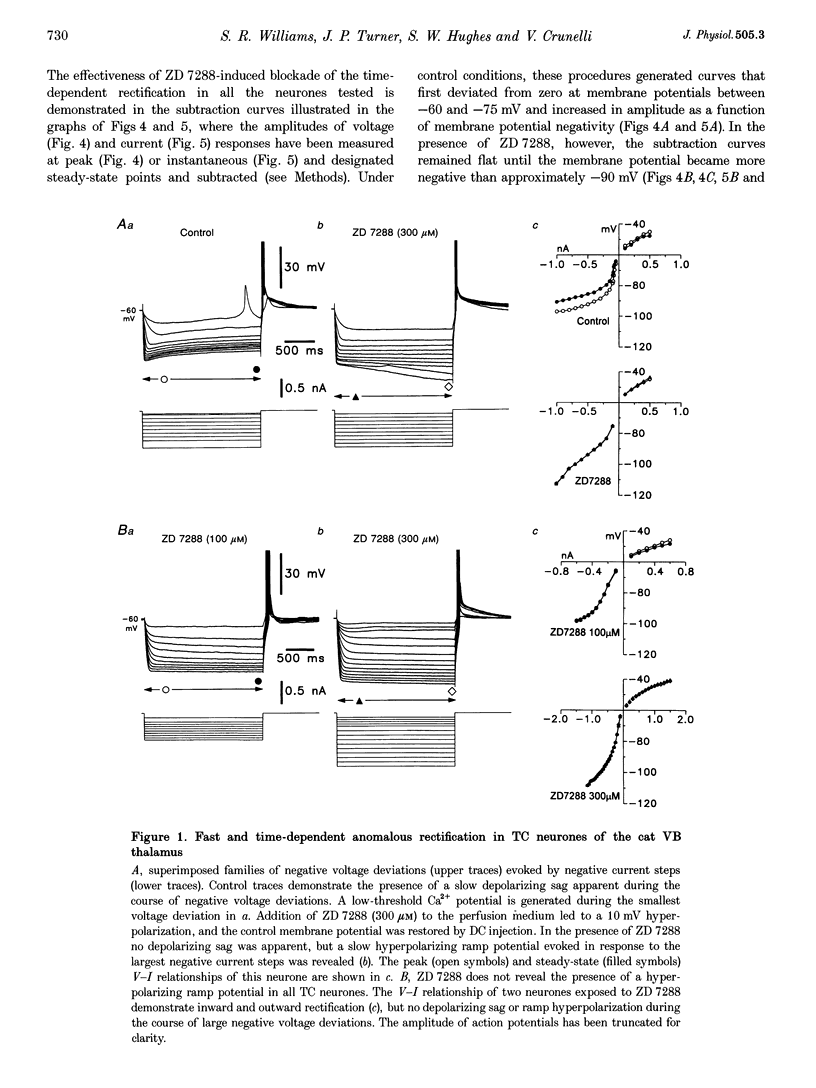
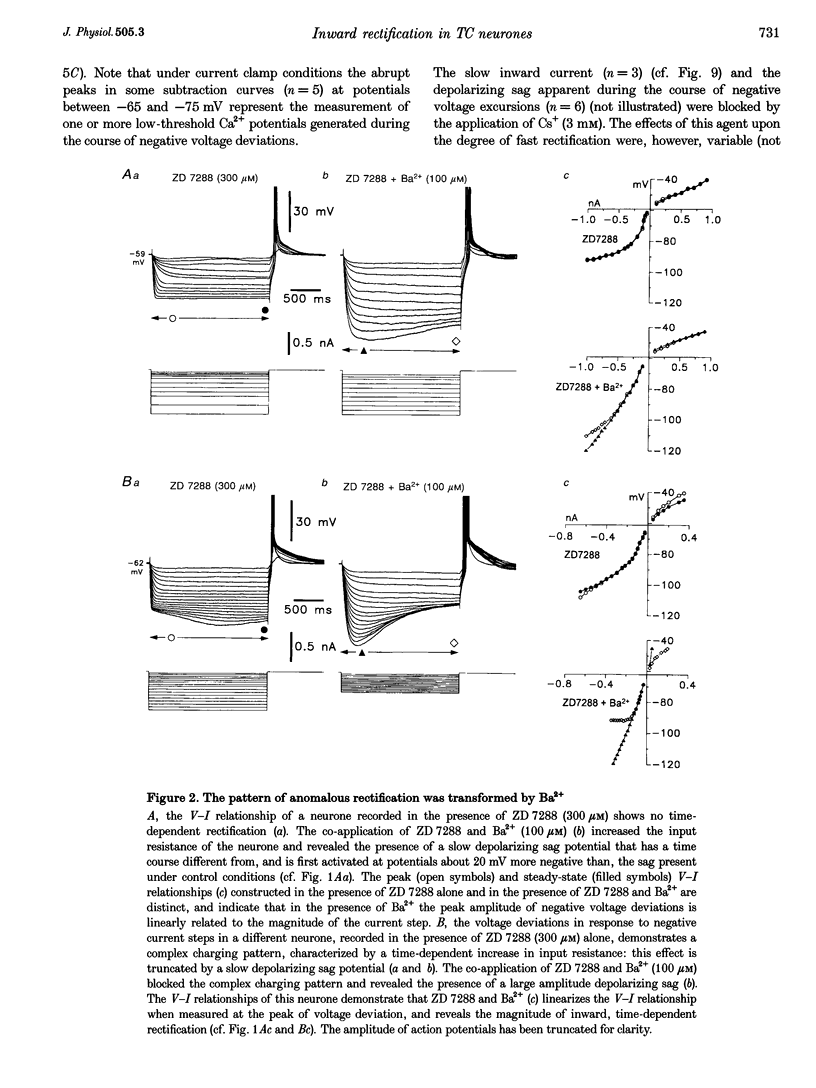
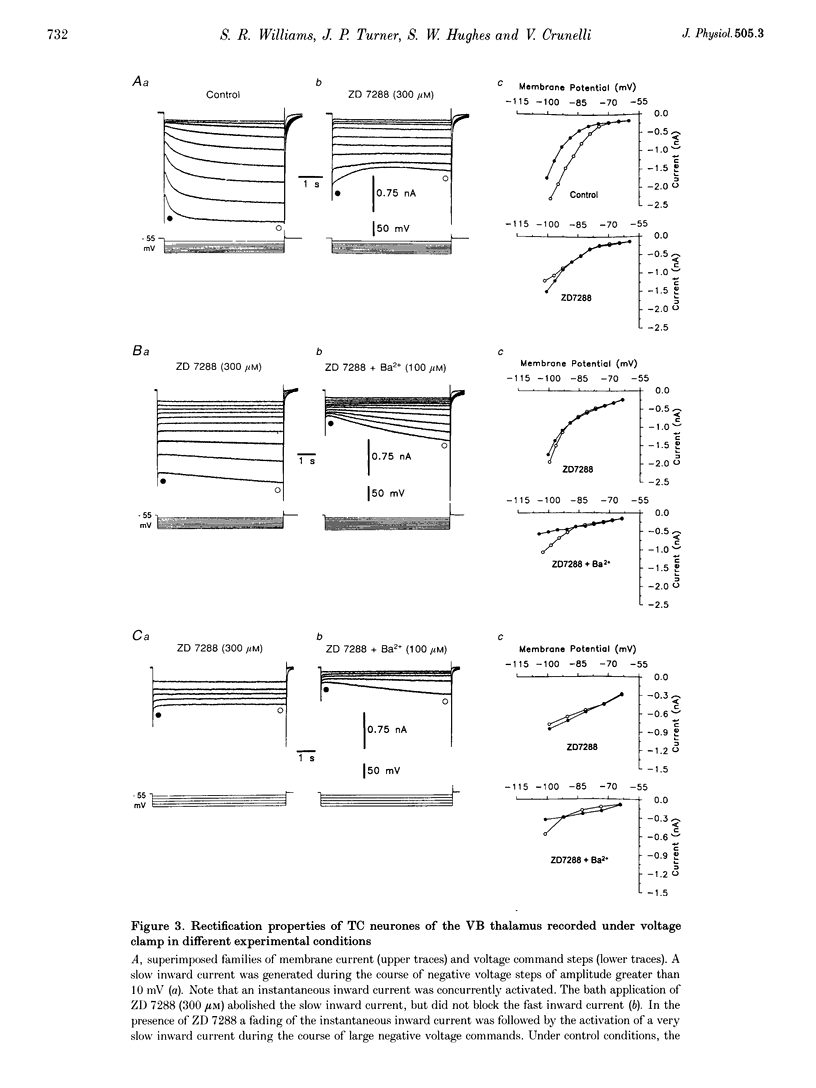
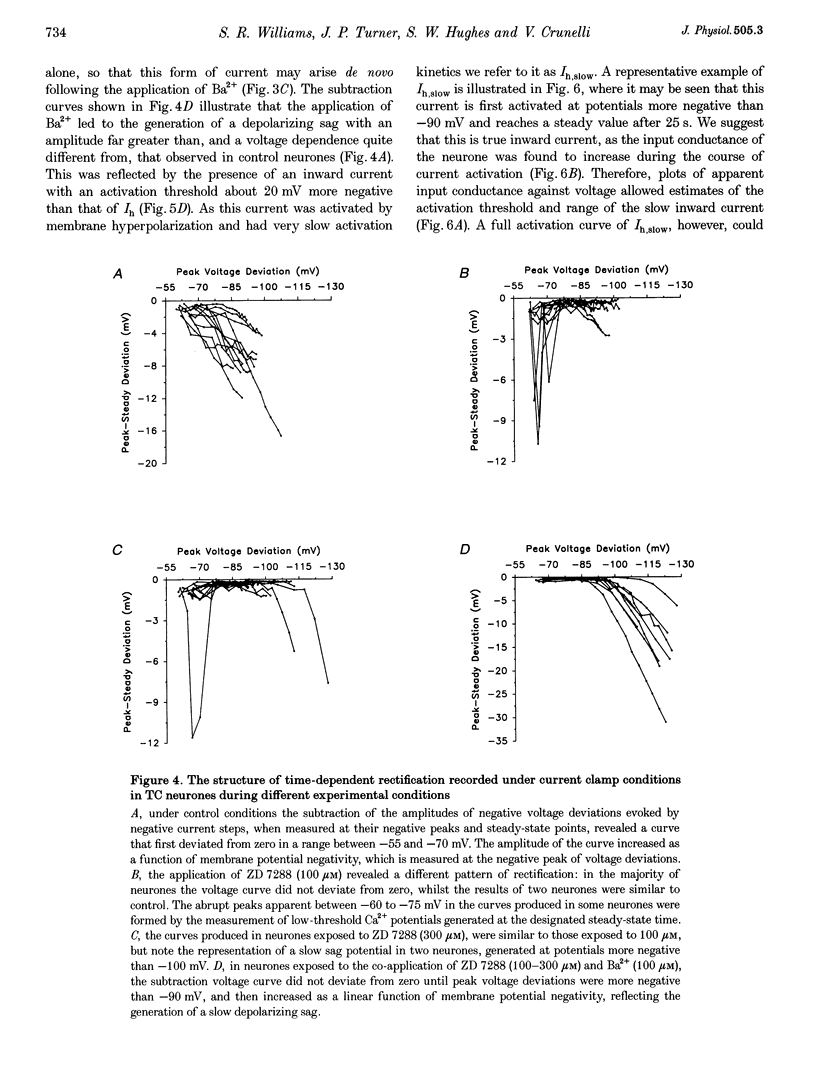
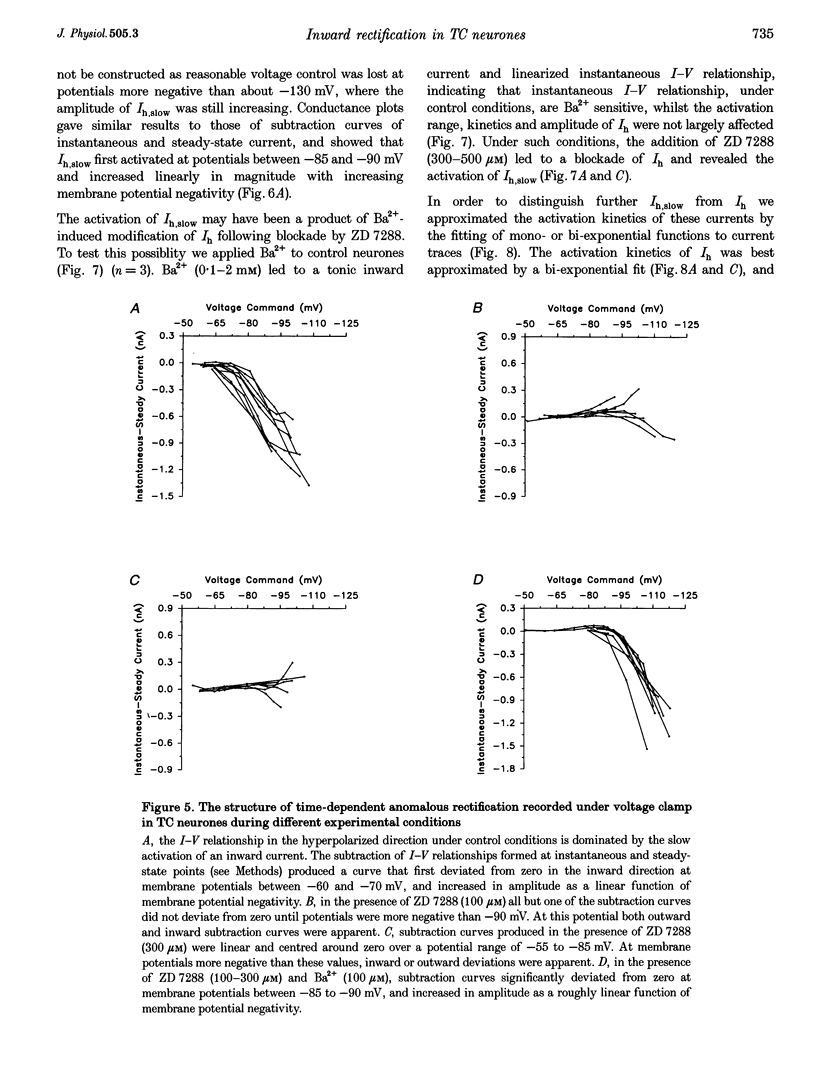
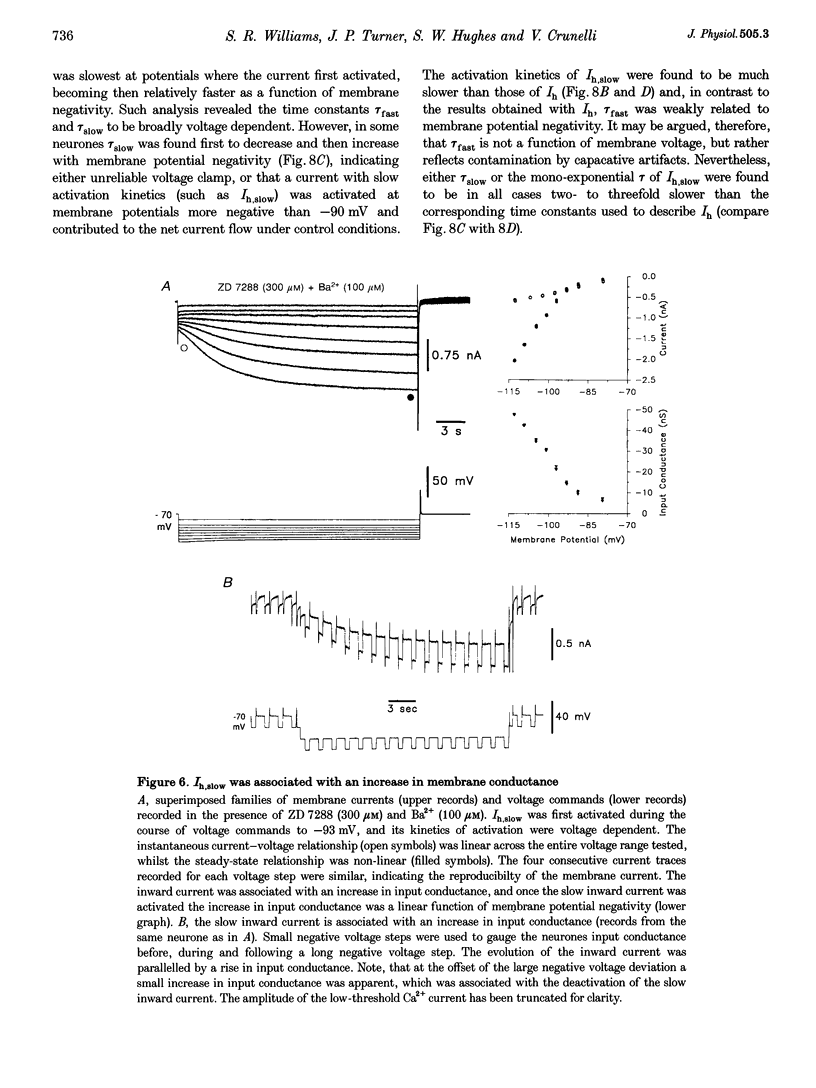
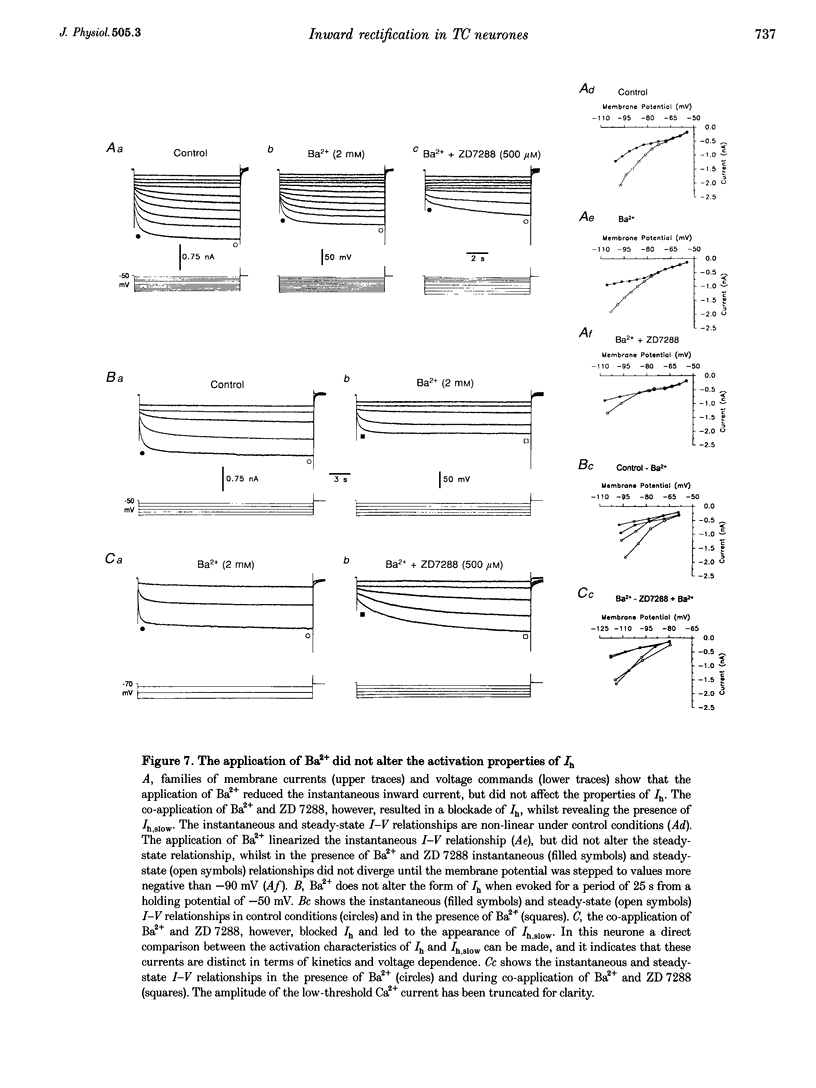
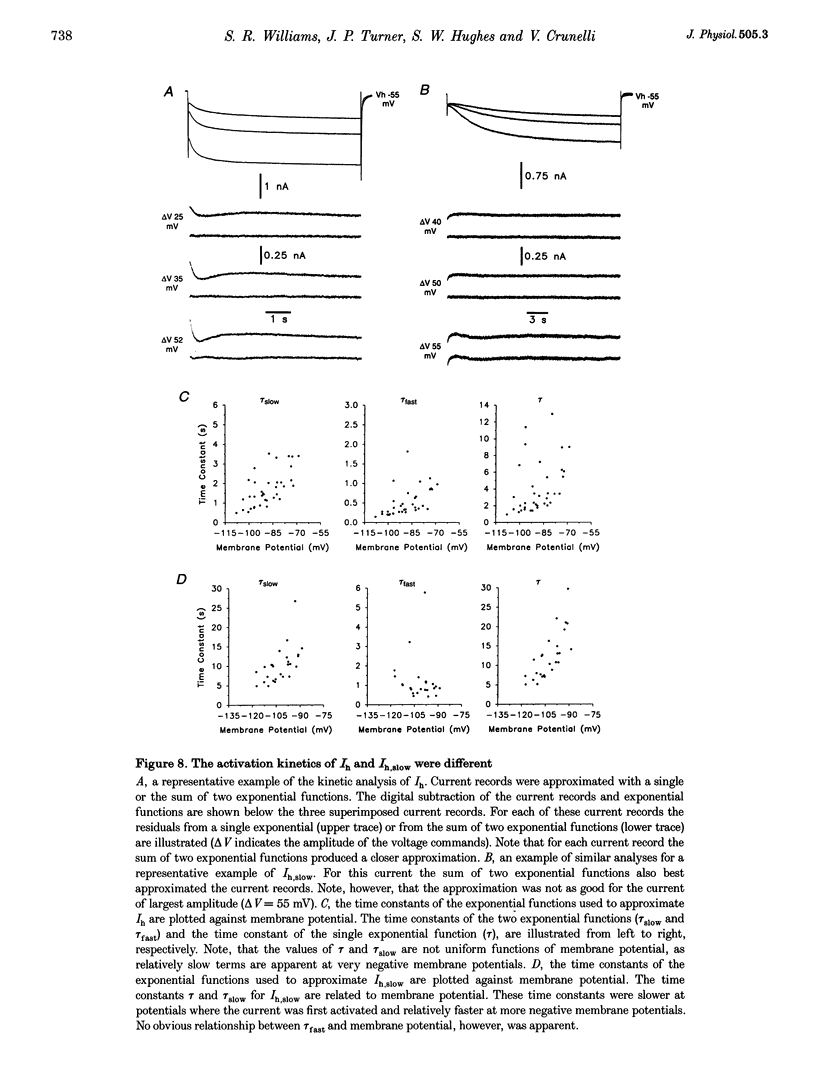
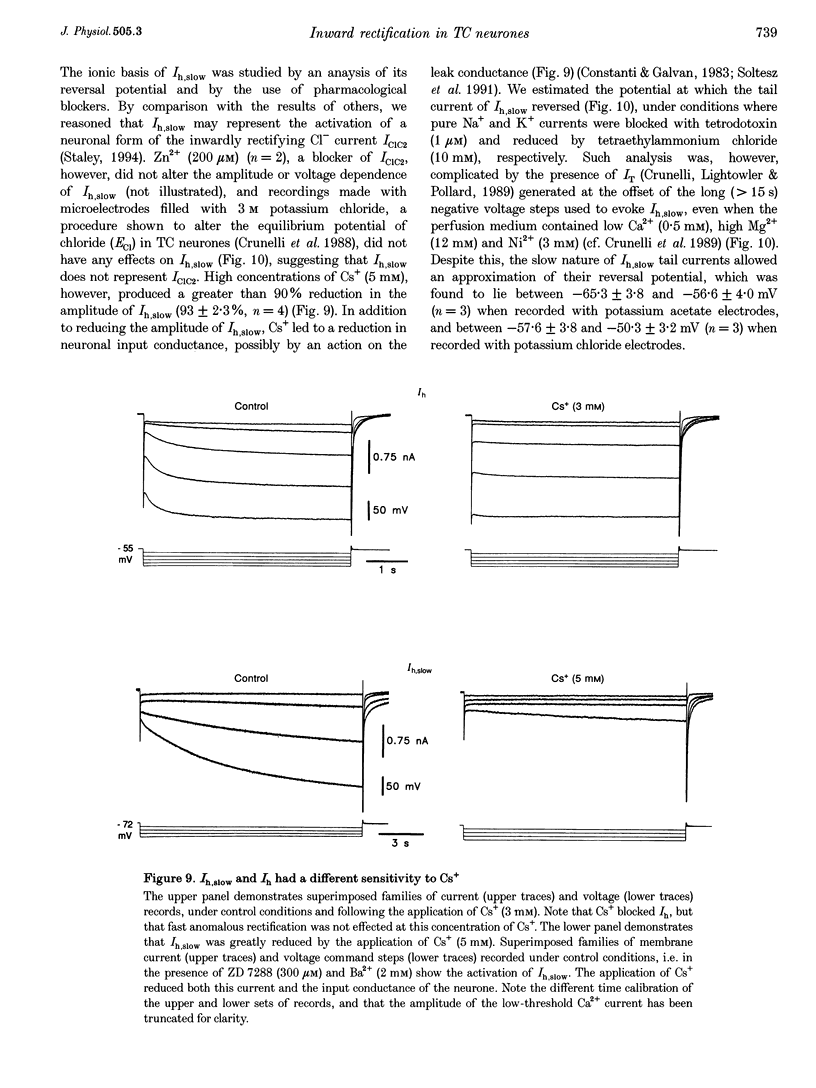
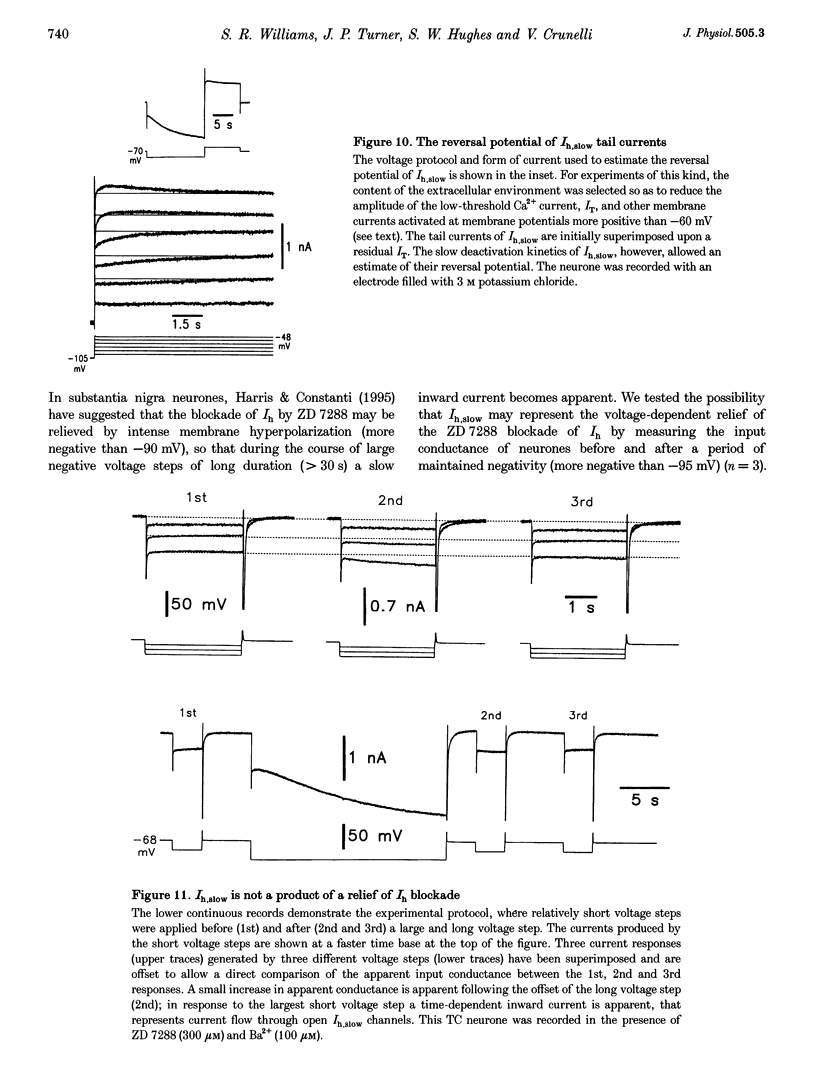
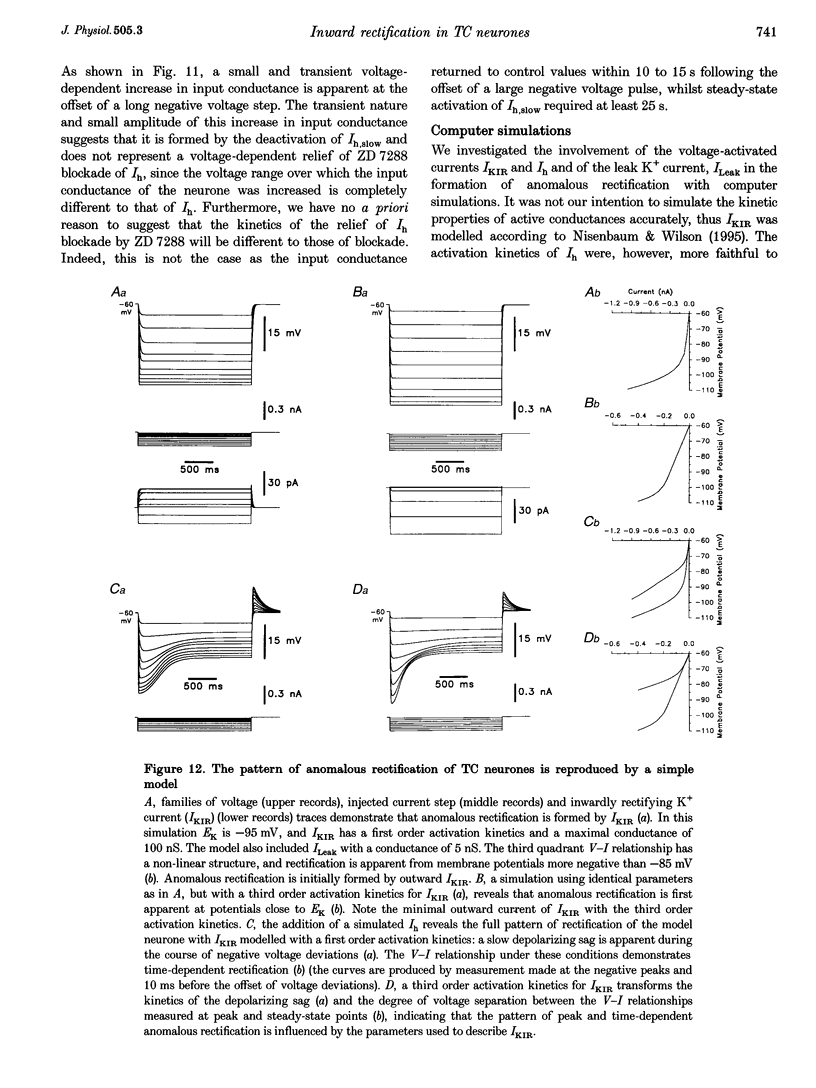
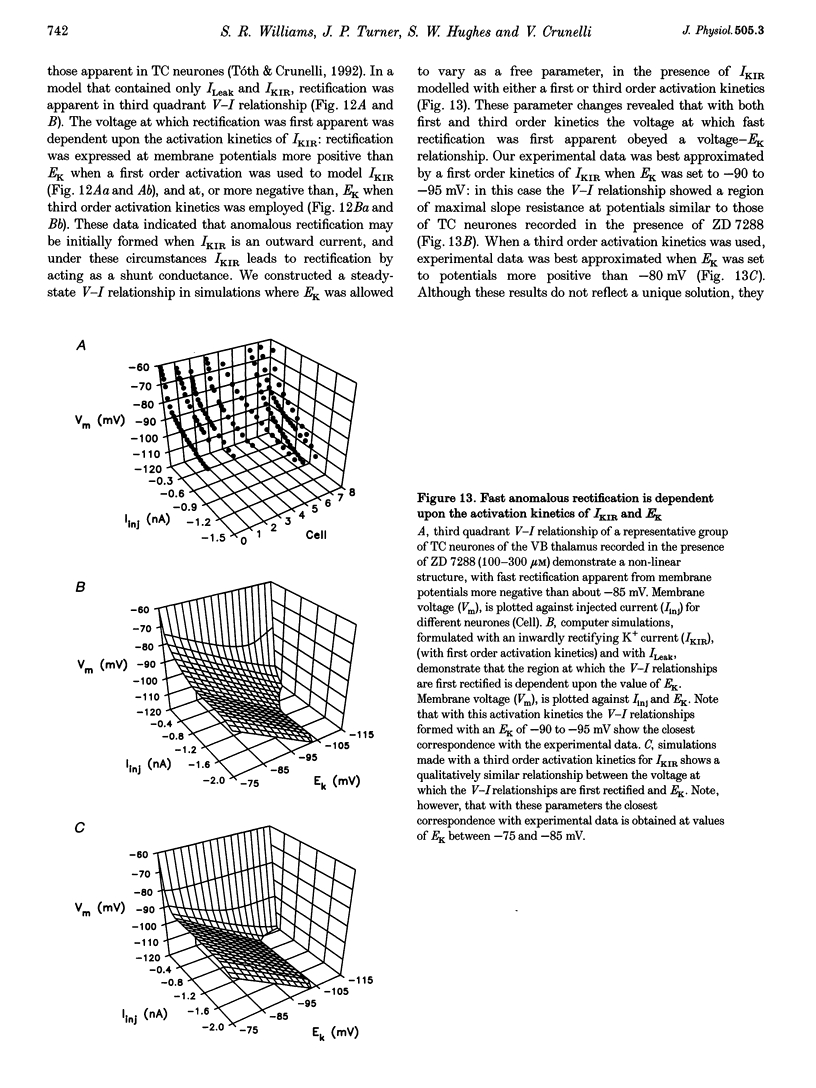
1. Intracellular sharp electrode current clamp and discontinuous single electrode voltage clamp recordings were made from thalamocortical neurones (n = 57) of the cat ventrobasal thalamus in order to investigate the mechanism underlying anomalous rectification. 2. Under current clamp conditions, voltage-current (V-I) relationships in a potential range of -55 to -110 mV demonstrated anomalous rectification with two components: fast rectification, which controlled the peak of negative voltage deviations, and time-dependent rectification. Time-dependent rectification was apparent as a depolarizing sag generated during the course of negative voltage deviations, was first formed at potentials in the range -60 to -70 mV, and was sensitive to 3 mM Cs+ (n = 6). Similarly, under voltage clamp conditions, instantaneous and steady-state I-V relationships demonstrated anomalous rectification. A slowly activating inward current with an activation threshold in the range of -65 to -70 mV formed time-dependent rectification. This current was sensitive to Cs+ (3 mM) (n = 3) and had properties similar to the slow inward mixed cationic current (Ih). 3. 4-(N-Ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino)-pyrimidinium++ + chloride (ZD 7288) (100-300 microM) irreversibly blocked time-dependent rectification mediated by Ih (n = 23 of 25 neurones), and led to a hyperpolarization of the resting membrane potential (6.8 +/- 0.5 mV). In the presence of ZD 7288, V-I and I-V relationships, exhibited fast anomalous rectification, first activated from potential more negative than -80 mV. 4. Ba2+ (100 microM) (n = 8), in the continuous presence of ZD 7288, reversibly linearized peak V-I and instantaneous I-V relationships over a potential range of -70 to -120 mV, and led to a membrane depolarization (13.3 +/- 4.2 mV) or tonic inward current (192 +/- 36 pA). 5. The co-application of ZD 7288 and Ba2+ revealed a depolarizing sag in negative voltage deviations under current clamp conditions, or a large inward current with kinetics two to three times slower than those of Ih under voltage clamp conditions. This novel form of time-dependent rectification was first apparent at potentials more negative than about -85 mV, was sensitive to 5 mM Cs+ (n = 4), and is termed Ih,slow. Ih,slow tail currents reversed between -65.3 and -56.6 mV (with potassium acetate electrodes, n = 3) or -57.6 and -50.3 mV (with KCl electrodes, n = 3). 6. Computer simulations confirmed that the pattern of anomalous rectification in thalamocortical neurones of the cat ventrobasal thalamus is mediated by the concerted action of Ih and a Ba(2+)-sensitive current with properties similar to an inwardly rectifying K+ current (IKIR).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal T., von Krosigk M., McCormick D. A. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995 Mar 15;483(Pt 3):665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks M. I., Pearce R. A., Smith P. H. Hyperpolarization-activated cation current (Ih) in neurons of the medial nucleus of the trapezoid body: voltage-clamp analysis and enhancement by norepinephrine and cAMP suggest a modulatory mechanism in the auditory brain stem. J Neurophysiol. 1993 Oct;70(4):1420–1432. doi: 10.1152/jn.1993.70.4.1420. [DOI] [PubMed] [Google Scholar]
- BoSmith R. E., Briggs I., Sturgess N. C. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993 Sep;110(1):343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J., Werblin F. S. Inwardly rectifying potassium conductance can accelerate the hyperpolarizing response in retinal horizontal cells. J Neurophysiol. 1995 Dec;74(6):2258–2265. doi: 10.1152/jn.1995.74.6.2258. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. C., Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol. 1995 Dec;74(6):2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hu B. Cellular basis of temporal synaptic signalling: an in vitro electrophysiological study in rat auditory thalamus. J Physiol. 1995 Feb 15;483(Pt 1):167–182. doi: 10.1113/jphysiol.1995.sp020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C., Dissmann E., Stühmer W., Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996 Jun 1;16(11):3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman P. M., Kelly J. S. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol. 1992 Oct;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994 Sep 15;371(6494):243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum E. S., Wilson C. J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995 Jun;15(6):4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H. C. Specific bradycardic agents block the hyperpolarization-activated cation current in central neurons. Neuroscience. 1994 Mar;59(2):363–373. doi: 10.1016/0306-4522(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63(1):1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Solomon J. S., Doyle J. F., Burkhalter A., Nerbonne J. M. Differential expression of hyperpolarization-activated currents reveals distinct classes of visual cortical projection neurons. J Neurosci. 1993 Dec;13(12):5082–5091. doi: 10.1523/JNEUROSCI.13-12-05082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J. S., Nerbonne J. M. Two kinetically distinct components of hyperpolarization-activated current in rat superior colliculus-projecting neurons. J Physiol. 1993 Sep;469:291–313. doi: 10.1113/jphysiol.1993.sp019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Staley K. The role of an inwardly rectifying chloride conductance in postsynaptic inhibition. J Neurophysiol. 1994 Jul;72(1):273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. J Physiol. 1979 Sep;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991 Oct;11(10):3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. Influence of barium on rectification in rat neocortical neurons. Neurosci Lett. 1993 Jul 9;157(1):62–66. doi: 10.1016/0304-3940(93)90643-y. [DOI] [PubMed] [Google Scholar]
- Turner J. P., Anderson C. M., Williams S. R., Crunelli V. Morphology and membrane properties of neurones in the cat ventrobasal thalamus in vitro. J Physiol. 1997 Dec 15;505(Pt 3):707–726. doi: 10.1111/j.1469-7793.1997.707ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth T., Crunelli V. Computer simulation of the pacemaker oscillations of thalamocortical cells. Neuroreport. 1992 Jan;3(1):65–68. doi: 10.1097/00001756-199201000-00017. [DOI] [PubMed] [Google Scholar]
- Uchimura N., Cherubini E., North R. A. Inward rectification in rat nucleus accumbens neurons. J Neurophysiol. 1989 Dec;62(6):1280–1286. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P. P., Goethals M., Simoens C. Use- and frequency-dependent blockade by UL-FS 49 of the if pacemaker current in sheep cardiac Purkinje fibres. Eur J Pharmacol. 1990 Oct 9;187(2):241–256. doi: 10.1016/0014-2999(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Turner J. P., Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB, receptor-mediated hyperpolarization. Neuroscience. 1995 May;66(1):133–141. doi: 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Tóth T. I., Turner J. P., Hughes S. W., Crunelli V. The 'window' component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol. 1997 Dec 15;505(Pt 3):689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble M. D., Moises H. C. Hyperpolarization-activated currents in neurons of the rat basolateral amygdala. J Neurophysiol. 1993 Nov;70(5):2056–2065. doi: 10.1152/jn.1993.70.5.2056. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Nakajima Y., Nakajima S., Stanfield P. R. Modulation of inwardly rectifying channels by substance P in cholinergic neurones from rat brain in culture. J Physiol. 1990 Jul;426:499–520. doi: 10.1113/jphysiol.1990.sp018151. [DOI] [PMC free article] [PubMed] [Google Scholar]


