Abstract
Pathogenic species of the genus Yersinia evade the bactericidal functions of phagocytes. This evasion is mediated through their virulence effectors, Yops, which act within target cells. In this study we investigated the effect of Yersinia pseudotuberculosis on Ca2+ signaling in polymorphonuclear neutrophils. The intracellular free calcium concentration in single adherent human neutrophils was monitored during bacterial infection and, in parallel, the encounter between the bacteria and cells was observed. When a plasmid-cured strain was used for infection, adherence of a single bacterium to the cellular surface induced a β1 integrin-dependent transient increase in the intracellular concentration of free calcium. This was, however, not seen with Yop-expressing wild-type bacteria, which adhered to the cell surface without generating any Ca2+ signal. Importantly, the overall Ca2+ homeostasis was not affected by the wild-type strain; the Ca2+ signal mediated by the G-protein-coupled formyl-methionyl-leucyl-phenylalanine receptor was still functioning. Hence, the blocking effect was restricted to certain receptors and their signaling pathways. The use of different Yop mutant strains revealed that the protein tyrosine phosphatase YopH was responsible for the inhibition. This virulence determinant has previously been implicated in very rapid Yersinia-mediated effects on target cells as the key effector in the blockage of phagocytic uptake. The present finding, that Y. pseudotuberculosis, via YopH, specifically inhibits a self-induced immediate-early Ca2+ signal in neutrophils, offers more-detailed information concerning the effectiveness of this virulence effector and implies an effect on Ca2+-dependent, downstream signals.
Polymorphonuclear neutrophils are active in the front line of defense against infections caused by microorganisms. Binding of foreign particles or soluble substances to neutrophil surface receptors activates signal transduction cascades leading to phagocytosis, production of reactive oxygen metabolites, and secretion of inflammatory mediators. Involved in these signaling cascades are the activation of phospholipases and protein kinases and the transient elevation of intracellular free calcium ([Ca2+]i) (for reviews, see references 2 and 53).
The three virulent species of the genus Yersinia, the bubonic plague bacteria Yersinia pestis and the enteropathogenic Y. enterocolitica and Y. pseudotuberculosis, are able to evade the bactericidal functions of phagocytes by distinct inhibition of phagocytosis, cytokine release, and oxidative burst (5, 14, 45, 46, 52, 65). This property allows these pathogens to survive and multiply in lymphatic tissues (58). The precise mechanisms behind the evasion of phagocytes are yet to be understood, but involved in these actions are an ensemble of virulence-associated proteins, Yersinia outer proteins (Yops) (for reviews, see references 12 and 15). The Yops are encoded on a 70-kb plasmid that is common for the virulent species of Yersinia. Upon intimate contact with target cells, some of these Yop effectors are translocated from the bacteria into the interacting cell. The translocation is polarized and occurs by way of a type III secretion system; no Yops are secreted into the surrounding media (16, 40, 42, 48, 51).
The Yop effector responsible for blockage of phagocytic engulfment is YopH (14, 45). This effector is homologous to eukaryotic protein tyrosine phosphatases (PTPases) (13, 18, 62) and is by far the most active of all known PTPases (66). The molecular mechanism by which YopH hinders the bacteria from being ingested by host cells was recently suggested to involve specific disruption of β1 integrin-containing focal complex structures associated with dephosphorylation of focal adhesion kinase and p130Cas (20, 38, 39). YopE is another Yop effector implicated in phagocytic blockage, although the contribution of YopE is considerably less than that of YopH (14, 46, 47). YopE is a contact-dependent cytotoxin that mediates disruption of F actin in target cells (46–48). The molecular mechanism behind the action of YopE is, however, unclear.
Nonopsonized Yersinia binds with high affinity to a subset of β1 integrins on target cells via its surface determinant, invasin (26, 63). In the absence of YopH, β1 integrins also mediate the actual ingestion of the bacteria. Members of the integrin family are expressed on most mammalian cells and are involved in cell-cell adhesion, cell-matrix interactions, cell signaling, and inflammation (24, 55). When bound to extracellular ligands, such as fibronectin, laminin, and collagen, integrins cluster and their intracellular domains associate with a diverse set of proteins forming focal adhesion complexes (29, 36). A variety of signaling events are generated in association with this formation: tyrosine phosphorylation, serine-threonine phosphorylation, changes in [Ca2+]i and pH, and lipid metabolism (for a review, see reference 11). The Yersinia surface protein invasin has, compared to the natural ligand fibronectin, approximately 100-fold-higher affinity for the β1 integrin receptor. It is believed that this very high affinity allows the pathogen to compete efficiently for integrin binding on attached cells and also promotes internalization of the bacterium (63). The internalization occurring in the absence of YopH involves focal complex formation and subsequent signaling to the cytoskeleton (38, 39).
Yersinia spp. abrogate, through YopH, very early infection-induced events within macrophages and neutrophils. This includes the inhibition of phagocytosis and the associated phosphotyrosine signaling (1, 45, 65). These events occur almost immediately upon binding of a bacterium to the cell surface. Since β1 integrin activation, in addition to stimulating phosphotyrosine signaling, also stimulates immediate increases in [Ca2+]i, we wanted to investigate whether Y. pseudotuberculosis has any effects on this early signal. For this purpose, we monitored [Ca2+]i by detection of Fura-2 fluorescence in single adherent human neutrophils during bacterial infection and concurrently monitored the encounter between the neutrophils and bacteria on a video screen. We were thereby able to detect the immediate neutrophil response to bacterial attachment and to correlate the induced Ca2+ signal to the site of bacterial attachment. By using this experimental setup, we could demonstrate that attachment of a plasmid-cured Yersinia bacterium to the neutrophil surface mediates a rapid rise in [Ca2+]i. This rise was dependent on the interaction between invasin and β1 integrins. The Yersinia sp.-induced Ca2+ signal was, however, abrogated in the presence of the Yersinia virulence factor YopH, showing an immediate and local inhibitory effect of YopH close to the site of bacterial attachment.
MATERIALS AND METHODS
Chemicals.
The chemicals and their sources were as follows: brain-heart infusion broth (Becton Dickinson, Meylan, France), dextran and Ficoll-Hypaque (Pharmacia, Uppsala, Sweden), EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid] and MgCl2 (Fluka, BioChemica, Switzerland), formyl-methionyl-leucyl-phenylalanine (fMLP), paraformaldehyde, and Triton X-100 (Sigma Chemical Co., St. Louis, Mo.), Fura-2/AM (Molecular Probe, Inc., Eugene, Oreg.), genistein (Research Biochemicals International, Natick, Mass.), mouse anti-human integrin β1 (adhesion blocking monoclonal antibody [MAb] clone 6S6; Chemicon, Temecula, Calif.), mouse anti-human leukocyte antigen (HLA) class I antigen (MAb clone W6/32; Dako A/S, Glostrup, Denmark), swine anti-rabbit immunoglobulin conjugates (tetramethyl rhodamine isothiocyanate [TRITC] and fluorescein isothiocyanate [FITC]; Dakopatts, Glostrup, Denmark). Dimethyl sulfoxide (Sigma) was used to dilute fMLP to 10 mM and the Fura-2/AM to 1 mM. All further dilutions were made in Krebs-Ringer phosphate buffer (KRG; pH 7.3), containing glucose (10 mM), Mg2+ (1.2 mM), and Ca2+ (1 mM). The 10-kDa cutoff filters were from Millipore (Bedford, Mass.).
Preparation of neutrophils and bacteria.
Heparinized blood was collected from healthy blood donors and separated according to the method of Böyum (10). The neutrophils were isolated by dextran sedimentation, followed by a brief hypotonic lysis of the remaining erythrocytes and centrifugation on a Ficoll-Hypaque gradient. The neutrophils were finally washed twice and resuspended in KRG lacking Ca2+ to 107/ml. The Y. pseudotuberculosis strains used in this study are listed in Table 1. For maximal expression of Yop proteins, the bacteria were cultured in brain heart infusion broth supplemented with 5 mM EGTA and 20 mM MgCl2 overnight at 26°C on a rotary shaker. The following day the cultures were diluted to 108 bacteria/ml (optical density at 550 nm of 0.1), further incubated at 26°C for 1 h, and then incubated at 37°C for an additional 2 h.
TABLE 1.
Y. pseudotuberculosis strains used in the present study
Loading cells with Fura-2/AM.
The fluorescent Ca2+ indicator Fura-2 was used to probe [Ca2+]i. Human neutrophils (5 × 105 cells/ml) were loaded by incubation with Fura-2/AM (4 μM) at 37°C for 30 min. The cells were then washed two times in KRG lacking Ca2+ and finally resuspended in KRG to 107/ml. The neutrophils were then allowed to adhere for 5 min to serum-coated coverslips (42 mm in diameter) held in a coverslip holder (Bachofer Laboratory Equipment, Reutling, Germany). To monitor the [Ca2+]i in adherent cells, the coverslip holder was incubated at 37°C in a sealed chamber on an inverted fluorescence microscope (as described below). The intracellular calcium measurements were done immediately and at 2-s intervals. The bacteria were added after 10 initial measurements. Microscopic analysis revealed that Fura-2 remained homogenous within the cytosol throughout the experiments.
Measurements of intracellular free calcium.
[Ca2+]i was measured by using an imaging microspectrofluorometry technique. A Zeiss (Oberkochen, Germany) Axiovert 35 inverted microscope setup was used for Fura-2 measurements and a 75-W stabilized xenon lamp was used as a UV source. The microscope was equipped with a computer-controlled filter wheel (Sutter Instrument Co., Novato, Calif.) furnished with 10-nm bandpass and 340- and 380-nm UV excitation filters. A glycerol-immersion, ×100 Zeiss Neofluar objective was used for all measurements. A 510-nm bandpass filter was used in the emission path. An image intensifier (Hamamatsu night viewer C2101) was used to enhance the fluorescent signal. A second Newicon video camera (Hamamatsu C2400) was employed to monitor cell morphology and bacterium-cell interactions, which were displayed on a separate video monitor.
The fluorescent video signal from the camera was digitized with a frame grabber (Innovativ Vision AB, Linköping, Sweden) mounted in an HP Vectra PC. The central part (128 by 128 pixels) of the digitized images was used for measurements (19). Every image frame was calculated as an average of 16 frames acquired within 1.6 s. Neutrophils were observed for a maximum of 15 min. The images were stored in sequence for later evaluation. Locally developed software was utilized to control the shutters and filter wheel. In order to produce traces of [Ca2+]i, all pixel values above a selective threshold (a pixel value of 25 in the 340-nm image) were added together. The ratio of the fluorescence (R = F340/F380) was used to calculate [Ca2+]i for each image by using the formula: [Ca2+]i = Kd · β · (R − Rmin)/(Rmax − R) (17).
Determination of phagocytosis.
Neutrophils were allowed to adhere to serum-coated glass coverslips for 15 min at 37°C in a humid chamber. A bacterial suspension of plasmid-cured Y. pseudotuberculosis was washed and resuspended to 2 × 107 bacteria per ml in KRG. Neutrophils were then incubated with the bacteria at a calculated bacterium/cell ratio of 50:1 for another 30 min at 37°C. Thereafter, nonadherent bacteria were washed away with KRG, and the cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.3) for 30 min at 4°C and then washed for 30 min in PBS (pH 7.6). The intra- and extracellular locations of bacteria were distinguished as previously described (14). To stain extracellularly bound bacteria, the coverslips were covered with rabbit anti-Yersinia antiserum (diluted 1:500) for 30 min at 37°C. After four washes in PBS, the cells were covered with TRITC-conjugated swine anti-rabbit immunoglobulins (12 μg/ml), incubated for 20 min at 37°C, and then washed in PBS as described above. To stain all bacteria associated with the neutrophils, the cells were permeabilized with 0.5% Triton X-100 for 3 min, followed by four washes in PBS. The total amount of adherent bacteria (both extra- and intracellular) was labeled with rabbit anti-Yersinia antiserum for 30 min at 37°C, washed, and overlaid with FITC-conjugated swine anti-rabbit immunoglobulins (12 μg/ml) for 20 min of further incubation at 37°C. Finally, after being rinsed in PBS, the coverslips were mounted on a glass slide and examined under a fluorescence microscope (Zeiss Axioscope) equipped with a Plan-apochromate ×63/1.40 oil immersion objective. For each cell, the number of extracellularly bound bacteria was detected by excitation at 530 to 585 nm, and the total number of cell-associated bacteria was detected by using an excitation at 450 to 490 nm; in each experiment, this was done for 25 to 50 cells per coverslip in randomly selected fields.
Detection of bacterial attachment.
The total amount of cell-attached bacteria was calculated by using a method similar to the one for detecting phagocytosis described above. The difference in this procedure was that the anti-Yersinia serum and the subsequently fluorescent antibodies added to the cells before permeabilization in Triton X-100 were excluded.
RESULTS
Plasmid-cured Y. pseudotuberculosis stimulates a β1 integrin-dependent immediate-early Ca2+ signal in neutrophils.
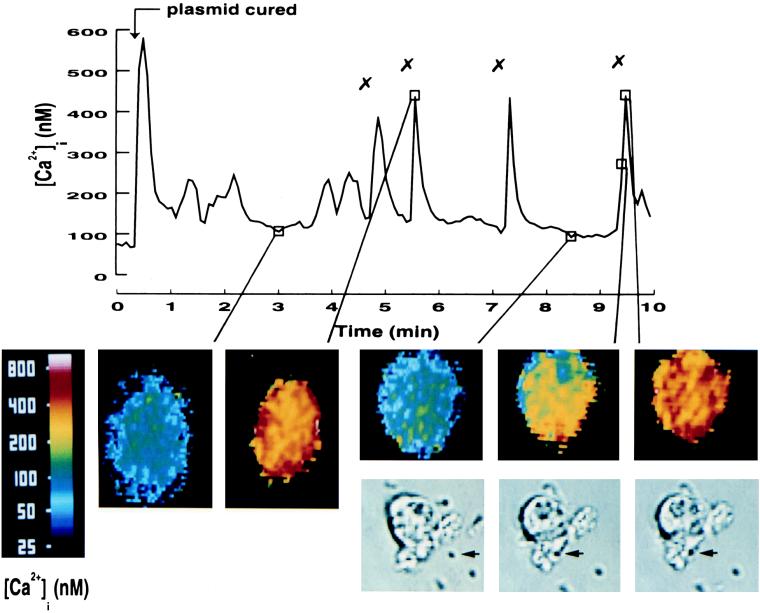
Initially, we investigated if binding of the Yersinia surface determinant invasin to neutrophil β1 integrins affects the intracellular free calcium concentration ([Ca2+]i) of these cells. Human neutrophils, prepared from fresh blood, were exposed to the plasmid-cured strain of Y. pseudotuberculosis (i.e., in absence of Yop effectors). Analysis of [Ca2+]i in single neutrophils during a 10-min infection period revealed that this type of encounter stimulated several transient elevations in [Ca2+]i in these cells (Fig. 1 and Table 2). Visual observation on the video screen revealed that the motile bacteria reached the cell and often grazed along the cell surface before adhering. Interestingly, the actual attachment of the bacterium correlated with an immediate elevation in [Ca2+]i, and subsequent binding of additional bacteria to the same cell resulted in new transient elevations of [Ca2+]i. The basal levels of [Ca2+]i in resting cells were 80 to 100 nM, while the bacterium-mediated calcium peaks reached 400 to 500 nM. Translation of the fluorescent signal into pseudocolor images showed that the [Ca2+]i transients started locally, at the site of bacterial attachment, and then spread rapidly throughout the entire cell (Fig. 1, insets; the bacterial attachment is indicated by arrows in the phase images, and the [Ca2+]i transients in the same cell are shown above). The initial rise in [Ca2+]i that occurred upon addition of the bacterial suspension was not caused by bacterial attachment; rather, it was due to low-molecular-weight bacterial products, as this response was detected upon addition of the bacterial supernatant ultrafiltered through a 10-kDa-cutoff filter but not with the KRG buffer alone (not shown). Occasionally, lower elevations in [Ca2+]i occurred when no bacterial attachment was observed, but these occurrences could be correlated to changes in cell morphology.
FIG. 1.
Bacterium-induced Ca2+ signaling in human neutrophils. Time course of [Ca2+]i in a single neutrophil upon infection with Y. pseudotuberculosis plasmid-cured bacteria. The plasmid-cured strain was added to adherent neutrophils at a calculated bacterium/cell ratio of 50:1 (arrow). The “✗” symbol indicates contact between a bacterium and the neutrophil. Attachment of bacteria was visually observed on a video screen, and this was correlated to the [Ca2+]i transients. A representative time course is presented (of 12 separate experiments), together with some selected images in pseudocolor (time points are indicated in the graph). The phase images show the attachment of a single bacterium (indicated by an arrow) to the same neutrophil shown in the pseudocolor image situated above. The mean number of [Ca2+]i transients is shown in Table 2.
TABLE 2.
Summary of observed [Ca2+]i transients
| Condition | Figurea | Mean no. of [Ca2+]i transients ± SEM (no. of expts)b |
|---|---|---|
| Plasmid-cured strain | 1 | 3.00 ± 0.28 (12) |
| Anti-β1 MAb blocking | 2A | 0.40 ± 0.16 (10) |
| Anti-HLA MAB blocking | 2B | 2.00 ± 0.41 (4) |
| Wild-type strain | 3 | 0.77 ± 0.26 (13) |
| yopH mutant strain | 4A | 3.91 ± 0.84 (11) |
| yopE mutant strain | 4B | 0.89 ± 0.45 (9) |
| MYMpyopHC/A strain | 4C | 3.36 ± 0.61 (11) |
| MYMpyopH strain | 4D | 0.50 ± 0.19 (12) |
Representative graphs are shown in the figures indicated.
The mean number of [Ca2+]i transients occurring during a 10-min period of observation is given. The [Ca2+]i transients included were those that increased at least threefold from the baseline level, and overall the magnitude ranged from 300 to 600 nM.
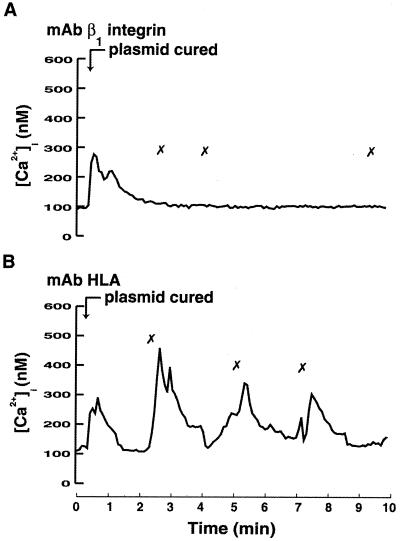
When an invasin mutant of Y. pseudotuberculosis was used, no attachments of bacteria to the cells were observed, and consequently no induction of [Ca2+]i transients was detected (results not shown). This correlated with earlier results showing that the expression of invasin is necessary for macrophage uptake of nonopsonized Yersinia spp. (14). To evaluate the involvement of β1 integrins in the Yersinia-induced induction of [Ca2+]i transients, MAbs directed against the β1 integrin receptor were used before the addition of plasmid-cured yersiniae to block interaction. These antibodies totally abolished the Ca2+ signaling (Fig. 2A), and no firm attachment of the bacteria to the cells was observed, although bacteria were seen grazing along the surface. No inhibition of bacterial attachment was observed when control MAbs directed against HLA class I were used; neither was the subsequent Ca2+ signaling affected (Fig. 2B). It is noteworthy that the initial [Ca2+]i elevation was still detected in the presence of anti-β1 integrin MAb (Fig. 2A), suggesting that this response was not due to β1 integrin engagement. These data show that Yersinia-induced Ca2+ signaling in human neutrophils depends on its binding to β1 integrins.
FIG. 2.
Involvement of β1 integrins in Yersinia-induced [Ca2+]i elevations in neutrophils. Time courses of [Ca2+]i in single neutrophils after receptor blockage and during infection with Y. pseudotuberculosis are shown. Adherent neutrophils were pretreated with blocking antibodies (10 μg/ml) directed against β1 integrins (A) or HLA (B) for 10 min. The Y. pseudotuberculosis plasmid-cured strain was then added at a calculated bacterium/cell ratio of 50:1 (arrow). The “✗” symbol indicates contact between a bacterium and the neutrophil. Attachment of bacteria was visually observed on a video screen, and this was correlated with the [Ca2+]i transients. Representative time courses are presented (of 4 to 10 separate experiments). The mean numbers of [Ca2+]i transients are shown in Table 2.
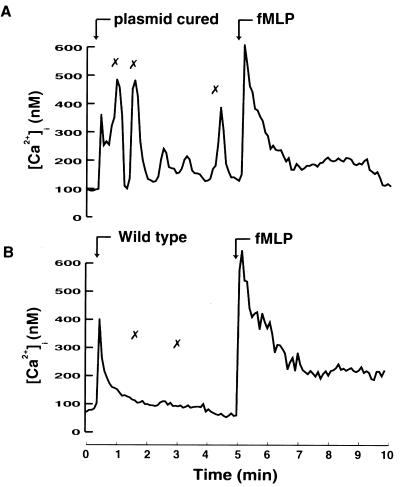
Wild-type Y. pseudotuberculosis inhibits the bacterium-induced Ca2+ signal and subsequent phagocytosis.
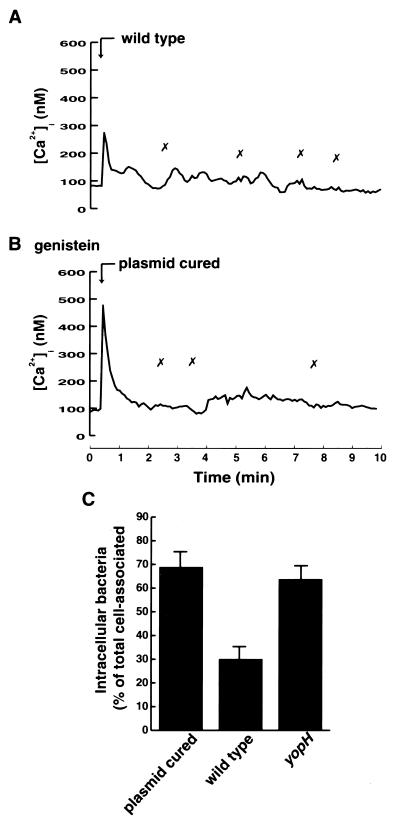
To study the effect of the virulent Y. pseudotuberculosis strain on the bacterium-induced immediate-early Ca2+ signaling in human neutrophils, we infected the cells with the wild-type strain, which is able to express and translocate Yops into target cells. The wild-type strain was observed to attach to cells; however, in contrast to that seen with the plasmid-cured strain, this attachment did not induce any rise in [Ca2+]i (Fig. 3A). Thus, wild-type Yersinia, through its Yop effectors, could inhibit the self-induced immediate-early Ca2+ signal in neutrophils. This implies that the underlying mechanism must be extremely rapid. Control experiments show that wild-type bacteria do attach to the neutrophils; 4.61 ± 0.81 wild-type bacteria bound per cell. The corresponding figures were 6.04 ± 2.01 for the plasmid-cured strain and 4.49 ± 1.15 for the yopH mutant strain.
FIG. 3.
Inhibition of bacterium-induced elevations in [Ca2+]i. (A and B) Time courses of [Ca2+]i in single neutrophils upon infection with Y. pseudotuberculosis wild-type strain (A) or plasmid-cured strain (B) at a calculated bacterium/cell ratio of 50:1 (arrow). In panel B, the neutrophils were preincubated at 37°C for 15 min with the tyrosine kinase inhibitor genistein before bacterial infection. The “✗” symbols indicate contact between bacteria and the neutrophil. Attachment of bacteria was visually observed on a video screen, and this was correlated with the [Ca2+]i transients. Representative time courses are presented (of 9 to 13 separate experiments). The mean number of [Ca2+]i transients is shown in Table 2. (C) Phagocytosis of Y. pseudotuberculosis strains. The plasmid-cured strain, the wild type, and the yopH mutant were exposed to neutrophils at a calculated bacterium/cell ratio of 50:1 for 30 min. Determinations of cellular association and discrimination between intra- and extracellularly associated bacteria were done as described in Materials and Methods. The data given represent means ± the standard errors of the means of five separate experiments and are expressed as percentages of neutrophil-associated bacteria that were located intracellularly.
It is known from earlier studies that wild-type Y. pseudotuberculosis inhibits phagocytosis in macrophages by a plasmid-encoded mechanism (14, 45, 46). Also, human neutrophils fail to phagocytose opsonized Y. enterocolitica (50, 65). To investigate the effect of YopH on neutrophils phagocytosing nonopsonized Y. pseudotuberculosis, the plasmid-cured, wild-type, and yopH mutant strains were presented to the cells for 30 min and thereafter the numbers of intra- and extracellularly associated bacteria were determined (Fig. 3C). The virulence plasmid-containing wild-type strain inhibited ingestion of the bacteria; only 30% of the cell-associated bacteria were ingested. For the plasmid-cured and yopH mutant strains, the corresponding percentages of ingestion were 69 and 64%, respectively. Thus, analogous to the effects on macrophage phagocytosis and on neutrophil opsonophagocytosis, YopH also mediated the inhibition of neutrophil phagocytosis of nonopsonized Y. pseudotuberculosis.
Phagocytosis of plasmid-cured Y. pseudotuberculosis by macrophages is inhibited in the presence of the tyrosine kinase inhibitor genistein (1). To test whether tyrosine kinase inhibition affected the bacterium-induced Ca2+ signaling, neutrophils were pretreated with 100 μM genistein for 15 min before the addition of bacteria. In these cells the attachment of plasmid-cured Y. pseudotuberculosis did not induce any Ca2+ signal (Fig. 3B). This suggests that the bacterium-mediated Ca2+ signaling depends on tyrosine kinase activity.
YopH is the virulence effector responsible for blocking the Yersinia-induced Ca2+ signal.
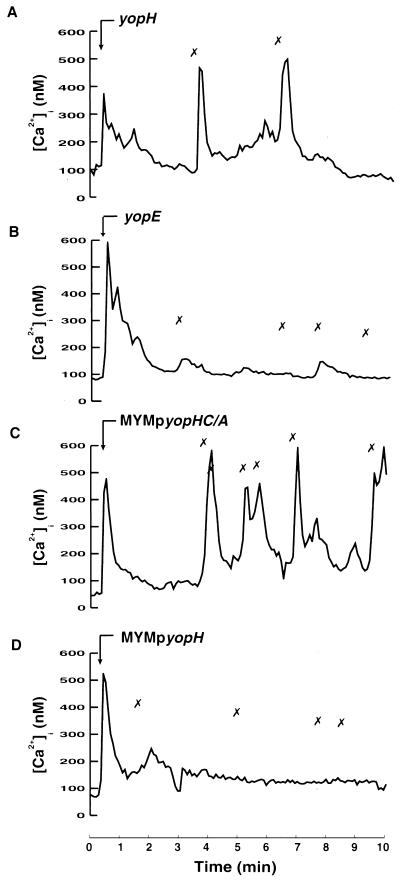
To identify the Yop effector responsible for the observed inhibition of β1 integrin-dependent Ca2+ signaling in neutrophils, Y. pseudotuberculosis strains mutated in various yop loci were used. Interestingly, neutrophils exposed to a yopH mutant strain responded with transient increases in [Ca2+]i that correlated with the attachment of single bacteria in a way similar to that seen with the plasmid-cured strain (Fig. 4A). In contrast, neutrophils exposed to a yopE mutant strain (expressing YopH) were inhibited in their calcium response towards bacterial attachment in a way similar to that seen with the wild-type strain (Fig. 4B). This implies that the PTPase YopH was responsible for the inhibition of neutrophil Ca2+ signaling. To investigate the role of the PTPase activity of YopH in this blockage, a Y. pseudotuberculosis multiple yop mutant strain (MYM) expressing active or inactive forms of YopH was used. This specifically engineered MYM strain does not express the virulence effectors YadA, YopH, YopE, YopM, YopK, or YpkA but is able to regulate, secrete, and translocate Yops (25). Introduction into this strain of a multicopy plasmid that encodes a given Yop allows studies of the behavior of that particular Yop within target cells without interference from the Yop effectors mentioned above. To study the effect of YopH, we used an isogenic pair of plasmid-bearing MYM strains, i.e., MYM expressing the wild-type YopH protein (MYMpyopH) and MYM expressing a catalytically inactive form of YopH (MYMpyopHC/A). The YopHC/A protein has, as indicated, a single amino acid substitution (Cys403→Ala), which totally abolishes the PTPase activity (18). Infection of neutrophils with the MYM strain (not shown) or with the MYM strain expressing the inactive YopH resulted in transient elevations in [Ca2+]i after bacterial attachment (Fig. 4C). In contrast, the MYM strain expressing the active YopH inhibited the [Ca2+]i transients to the same extent as did the wild-type strain (Fig. 4D). These data clearly demonstrate that the PTPase activity of YopH is required to block the Yersinia-induced immediate-early Ca2+ signal.
FIG. 4.
Involvement of YopH in inhibition of intracellular Ca2+ signaling. Time courses of [Ca2+]i in single neutrophils during attachment of different Y. pseudotuberculosis strains are shown. Adherent neutrophils were exposed to the Yersinia yopH mutant (A), yopE mutant (B), MYM transcomplemented with yopHC/A (C), or MYM transcomplemented with wild-type yopH (D) at a calculated bacterium/cell ratio of 50:1 (arrow). The “✗” symbols indicate contact between the bacteria and the neutrophil. Attachment of bacteria was visually observed on a video screen, and this was correlated with the [Ca2+]i transients. Representative time courses are presented (of 9 to 12 separate experiments). The mean numbers of [Ca2+]i transients are shown in Table 2.
YopH-mediated inhibition does not affect certain G-protein-coupled receptors.
To investigate the specificity of the YopH-mediated inhibition of neutrophil Ca2+ signaling, another type of surface receptor was tested on neutrophils that had been pretreated with wild-type Y. pseudotuberculosis. For this purpose we chose the G-protein-coupled fMLP receptor, since this receptor is expressed at high levels on these cells and also is known to mediate a rapid Ca2+ response (28). We found that neutrophils exposed for 5 min to the Yersinia plasmid-cured strain, as well as to the wild-type strain, responded to fMLP with an immediate rise in [Ca2+]i (Fig. 5A and B). This response was as rapid and reached approximately the same levels as the fMLP-mediated response of control cells, i.e., cells not pretreated with bacteria (not shown). Moreover, the fMLP-mediated response was not affected even if the cells were pretreated with YopH-expressing bacteria for 30 min (not shown). Hence, the blocking effect of Y. pseudotuberculosis on the β1 integrin-dependent Ca2+ signal did not affect the signaling properties of neutrophil fMLP receptors. This clearly demonstrates that YopH-mediated inhibition is specific to certain receptors and excludes the possibility that a general effect of YopH affects the overall cellular Ca2+ homeostasis.
FIG. 5.
Receptor specificity of the wild-type-mediated inhibition. Time courses of [Ca2+]i in single neutrophils during infection with Y. pseudotuberculosis and subsequent stimulation with fMLP are shown. Adherent neutrophils were exposed to Yersinia plasmid-cured strain (A) or to the wild-type strain (B) at a calculated bacterium/cell ratio of 50:1 for 5 min. The “✗” symbols indicate contact between bacteria and the neutrophil. Neutrophils were then stimulated with 10−8 M fMLP (arrow). Representative time courses are presented (of three separate experiments).
DISCUSSION
In the present investigation, we show that virulent Y. pseudotuberculosis has the ability to block an immediate-early phagocyte response. The pathogen inhibited the rapid Ca2+ signal in human neutrophils, which was initiated when the bacterium bound to β1 integrins on the cellular surface. We could also show that one of its virulence proteins, namely, the PTPase YopH, was responsible for this inhibition. Moreover, the YopH-mediated inhibiting effect had a certain specificity and did not affect general Ca2+ signaling within the target cell.
In the absence of YopH, the bacterium induced a transient Ca2+ signal, and this occurred immediately upon its binding to the cell surface. This indicates that the blocking effect that is seen in the presence of the YopH virulence effector is remarkably rapid. YopH has been shown to abrogate β1 integrin-mediated phosphotyrosine signals by rapid dephosphorylation of specific proteins in macrophages (1). Studies in HeLa cells and macrophages have shown that YopH dephosphorylates focal adhesion proteins and disrupts focal complex structures close to the site where the bacterium binds (4, 20, 39). The common molecular target protein of YopH in these cells is the focal adhesion protein p130Cas (4, 20, 39). Interestingly, it has recently been found that the YopH protein contains an inherent “focal complex targeting” sequence that is necessary for Yersinia to block phagocytosis by macrophages, and this sequence has been implicated in Yersinia virulence in mice (38). This implies that YopH acts where the phosphotyrosine signal is initially generated, which is in close proximity to the cytoplasmic part of β1 integrins clustered by the bacterium that generates the PTPase. We have shown here that the increase in [Ca2+]i seen in the absence of YopH also starts locally, close to where the bacterium binds. It is therefore likely that the Yersinia-induced Ca2+ signal is also initiated by the invasin-engaged β1 integrins and that YopH-expressing strains inhibit this signal by injecting YopH close to the clustered receptors. Hence, our findings suggest that focal-complex-like structures are in some way involved in mediating the Yersinia-induced Ca2+ signal.
There are several reports demonstrating that ligation of β1 integrins stimulates an increase in [Ca2+]i in various cell types (32, 54, 59, 64). Although β1 integrins are expressed on the neutrophil surface (6, 44), β2 integrins constitute the major subclass of neutrophil integrins, and consequently these are also the most-studied integrins in these cells. Engagement of neutrophil β2 integrins through receptor cross-linking, spreading on ligand-coated surfaces, or exposure to particles triggers local elevations in [Ca2+]i, and the signaling pathway involved in this is tyrosine phosphorylation of phospholipase Cγ2 (PLCγ2) with subsequent formation of IP3 (22, 28, 41, 43). The present finding of a local β1 integrin-dependent Ca2+ response in neutrophils, which can be inhibited by a locally delivered PTPase or by the tyrosine kinase inhibitor genistein, suggests that also this signal is regulated by tyrosine kinases. The players involved in mediating this Yersinia-induced β1 integrin-dependent Ca2+ signal are not known, but as the origin of the signal has been detected locally, at the site of receptor engagement, proteins within focal-complex-like structures would be possible actors. The fact that PLCγ has been found within such structures (30, 35) supports this hypothesis.
Single-cell analysis of neutrophils shows rapid and localized elevations of [Ca2+]i during phagocytosis of immunoglobulin G- or complement-opsonized particles. While the Fc receptor-mediated phagocytosis is Ca2+ dependent, phagocytosis by complement receptors is Ca2+ independent (33, 34). The complement receptor-induced [Ca2+]i changes detected after particle contact are not required for the actual ingestion step but are needed for translocation of granules to the phagosome (60, 61). Hence, [Ca2+]i elevations might not be necessary for phagocytic uptake but rather for other functions important for microbial clearance, such as granule release, phagolysosome fusion, and oxidative activation (3, 27, 37, 57, 60). It is therefore likely that the Yersinia-mediated blockage of Ca2+ signaling affects these antimicrobial functions. Yersinia is able to circumvent the respiratory burst in phagocytes (21, 50). However, this inhibition is not mediated by only YopH, nor is the oxidative activation an immediate response. Secretion of neutrophil granules, on the other hand, is an early neutrophil event activated by [Ca2+]i transients (37, 57). This secretion is an important feature of neutrophils during the early activation of host defense. It results in release of bactericidal enzymes and mobilization of chemotactic and phagocytic receptors to the cell surface (56). Another central feature of neutrophils involving integrin activation is the capability to migrate. Ca2+ signals have been suggested to be important for disrupting integrin clusters and for recycling the integrins to the front of the migrating cell (23, 31). Thus, Yersinia can, by blocking the self-induced fluctuations in [Ca2+]i, interfere with antibacterial responses such as granule release and migration. This could, in addition to inhibiting engulfment, profoundly impair the neutrophil clearance function. The specific effect of Yersinia-mediated inhibition of the Ca2+ response on neutrophil function, however, is yet to be understood.
The present findings present a very direct effect of the Yersinia virulence factor YopH inside the phagocytic cell; namely, the inhibition of local Ca2+ signaling. As this occurs in close proximity to the site to which the bacterium binds, it is likely that this inhibition hinders further antibacterial actions of the phagocyte versus the attached bacteria.
ACKNOWLEDGMENTS
We thank Vesa-Matti Loitto for advice and for help with the Ca2+ imaging equipment.
This work was supported by grants from the Swedish Medical Research Council (project numbers 5968, 6251, 7490, 11222, and 10618), the King Gustaf V 80 Year Foundation, the Magn Bergvall Foundation, the Swedish Foundation for Strategic Research, the Medical Faculty Research Foundation at Umeå University, the Swedish Society of Medicine, the Swedish Research Council for Engineering Sciences, the Lions Foundation, and the Swedish Natural Science Research Council (project number 4426-307).
REFERENCES
- 1.Andersson K, Carballeira N, Magnusson K-E, Persson C, Stendahl O, Wolf-Watz H, Fällman M. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 2.Aplin A, Howe A, Alahari S, Juliano R. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- 3.Bei L, Hu T, Qian Z, Shen X. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim Biophys Acta. 1998;1404:475–483. doi: 10.1016/s0167-4889(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 4.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliska J B, Black D S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect Immun. 1995;63:681–685. doi: 10.1128/iai.63.2.681-685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnsack J, Zhou X. Divalent cation substitution reveals CD18- and very late antigen-dependent pathways that mediate human neutrophil adherence to fibronectin. J Immunol. 1992;149:1340–1347. [PubMed] [Google Scholar]
- 7.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bölin I, Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988;2:237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 10.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 11.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 13.Denu J M, Stuckey J A, Saper M A, Dixon J E. Form and function in protein dephosphorylation. Cell. 1996;87:361–364. doi: 10.1016/s0092-8674(00)81356-2. [DOI] [PubMed] [Google Scholar]
- 14.Fällman M, Andersson K, Håkansson S, Magnusson K-E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fällman M, Persson C, Wolf-Watz H. Yersinia proteins that target host cell signaling pathways. J Clin Invest. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis M S, Wolf-Watz H. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol Microbiol. 1998;29:799–813. doi: 10.1046/j.1365-2958.1998.00973.x. [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 18.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson M, Magnusson K. A novel principle for quantitation of fast intracellular calcium changes using Fura-2 and a modified image processing system—applications in studies of neutrophil motility and phagocytosis. Cell Calcium. 1992;13:473–486. doi: 10.1016/0143-4160(92)90016-l. [DOI] [PubMed] [Google Scholar]
- 20.Hamid, N., A. Gustavsson, K. Andersson, K. McGee, C. Persson, C. Rudd, and M. Fällman. Unpublished data. [DOI] [PubMed]
- 21.Hartland E L, Green S P, Phillips W A, Robins-Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellberg C, Molony L, Zheng L, Andersson T. Ca2+ signalling mechanisms of the β2 integrin on neutrophils: involvement of phospholipase Cγ2 and Ins(1,4,5)P3. Biochem J. 1996;317:403–409. doi: 10.1042/bj3170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huttenlocher A, Palecek S, Lu Q, Zhang W, Mellgren R, Lauffenburger D, Ginsberg M, Horwitz A. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 24.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 25.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 26.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 27.Jaconi M, Lew D, Carpentier J, Magnusson K, Sjögren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaconi M, Theler J, Schlegel W, Appel R, Wright S, Lew P. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991;112:1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jockusch B M, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 30.Kanner S. Focal adhesion kinase-related fakB is regulated by the integrin LFA-1 and interacts with the SH3 domain of phospholipase C gamma 1. Cell Immunol. 1996;171:164–169. doi: 10.1006/cimm.1996.0188. [DOI] [PubMed] [Google Scholar]
- 31.Lawson M, Maxfield F. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 32.Leavesley D, Schwartz M, Rosenfeld M, Cheresh D. Integrin β1- and β3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lew D, Andersson T, Hed J, Di Virgilio F, Pozzan T, Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985;315:509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- 34.Marks P, Maxfield F. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium. 1990;11:181–190. doi: 10.1016/0143-4160(90)90069-7. [DOI] [PubMed] [Google Scholar]
- 35.McBride K, Rhee S, Jaken S. Immunocytochemical localization of phospholipase Cγ in rat embryo fibroblasts. Proc Natl Acad Sci USA. 1991;88:7111–7115. doi: 10.1073/pnas.88.16.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995a;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nüsse O, Serrander L, Lew D, Krause K. Ca2+-induced exocytosis in individual human neutrophils: high- and low-affinity granule populations and submaximal responses. EMBO J. 1998;17:1279–1288. doi: 10.1093/emboj/17.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson, C., K. Andersson, R. Nordfelth, Å. Forsberg, H. Wolf-Watz, and M. Fällman. Unpublished data. [DOI] [PubMed]
- 39.Persson C, Carballeira N, Wolf-Watz H, Fällman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 41.Petersen M, Williams J, Hallett M. Cross-linking of CD11b or CD18 signals release of localized Ca2+ from intracellular stores in neutrophils. Immunology. 1993;80:157–159. [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K-E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 43.Pettit E, Hallett M. Temporal and spatial resolution of Ca2+ release and influx in human neutrophils using a novel confocal laser scanning mode. Biochem Biophys Res Commun. 1996;229:109–113. doi: 10.1006/bbrc.1996.1765. [DOI] [PubMed] [Google Scholar]
- 44.Rieu P, Lesavre P, Halbwachs-Mecarelli L. Evidence for integrins other than β2 on polymorphonuclear neutrophils: expression of α6β1 heterodimer. J Leukoc Biol. 1993;53:576–582. doi: 10.1002/jlb.53.5.576. [DOI] [PubMed] [Google Scholar]
- 45.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 50.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmond G P, Reeves P J. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 52.Schesser K, Spiik A-K, Dukuzumuremyi J-M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eucaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz M. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993;53:1503–1506. [PubMed] [Google Scholar]
- 54.Schwartz M, Denninghoff K. αv integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994;269:11133–11137. [PubMed] [Google Scholar]
- 55.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 56.Sengeløv H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 57.Sengeløv H, Kjeldsen L, Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 58.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sjaastad M, Nelson W. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays. 1997;19:47–55. doi: 10.1002/bies.950190109. [DOI] [PubMed] [Google Scholar]
- 60.Stendahl O, Krause K, Krischer J, Jerstrom P, Theler J, Clark R, Carpentier J, Lew D. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. doi: 10.1126/science.8073285. [DOI] [PubMed] [Google Scholar]
- 61.Theler J, Lew D, Jacon I M, Krause K, Wollheim C, Schlegel W. Intracellular pattern of cytosolic Ca2+ changes during adhesion and multiple phagocytosis in human neutrophils. Dynamics of intracellular Ca2+ stores. Blood. 1995;85:2194–2201. [PubMed] [Google Scholar]
- 62.Tonks N K, Neel B G. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 63.Tran Van Nhieu G, Isberg R R. Bacterial internalization mediated by β1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993;12:1887–1895. doi: 10.1002/j.1460-2075.1993.tb05837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weismann M, Guse A, Sorokin L, Broker B, Frieser M, Hallmann R, Mayr G. Integrin-mediated intracellular Ca2+ signaling in Jurkat T lymphocytes. J Immunol. 1997;158:1618–1627. [PubMed] [Google Scholar]
- 65.Visser L G, Annema A, van Furth R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun. 1995;63:2570–2575. doi: 10.1128/iai.63.7.2570-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z Y, Clemens J C, Schubert H L, Stuckey J A, Fischer M W, Hume D M, Saper M A, Dixon J E. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J Biol Chem. 1992;267:23759–23766. [PubMed] [Google Scholar]