Abstract
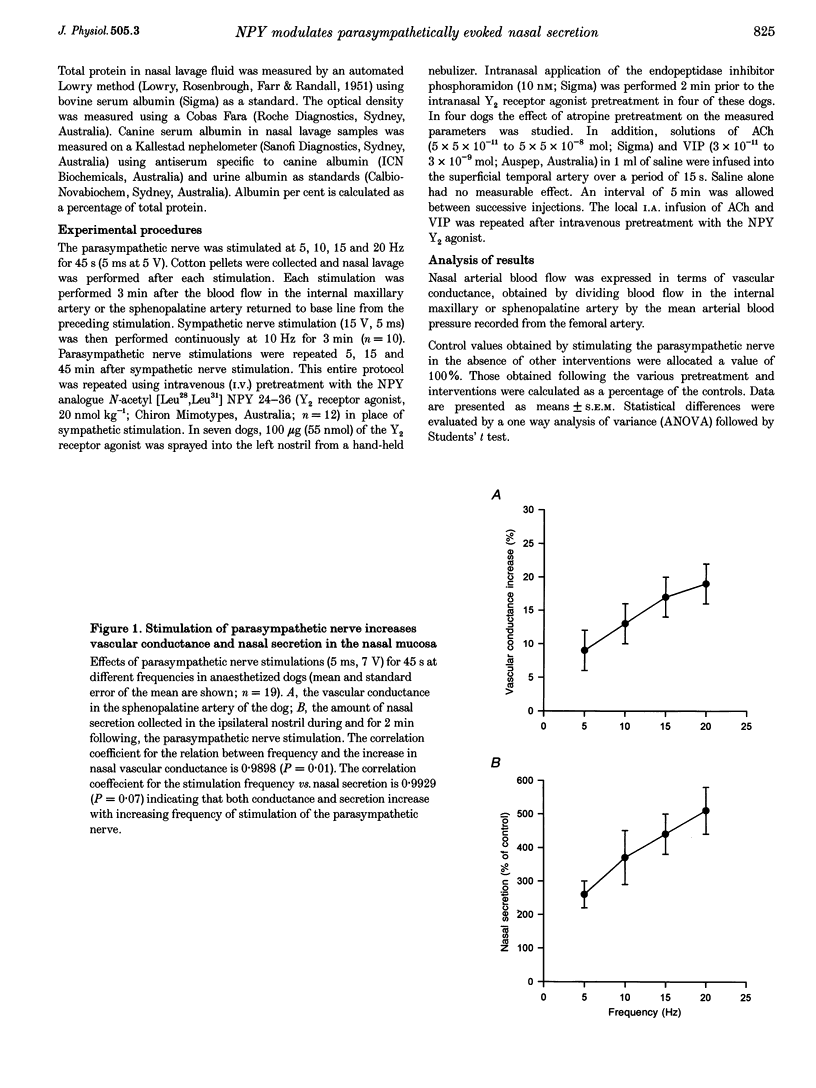
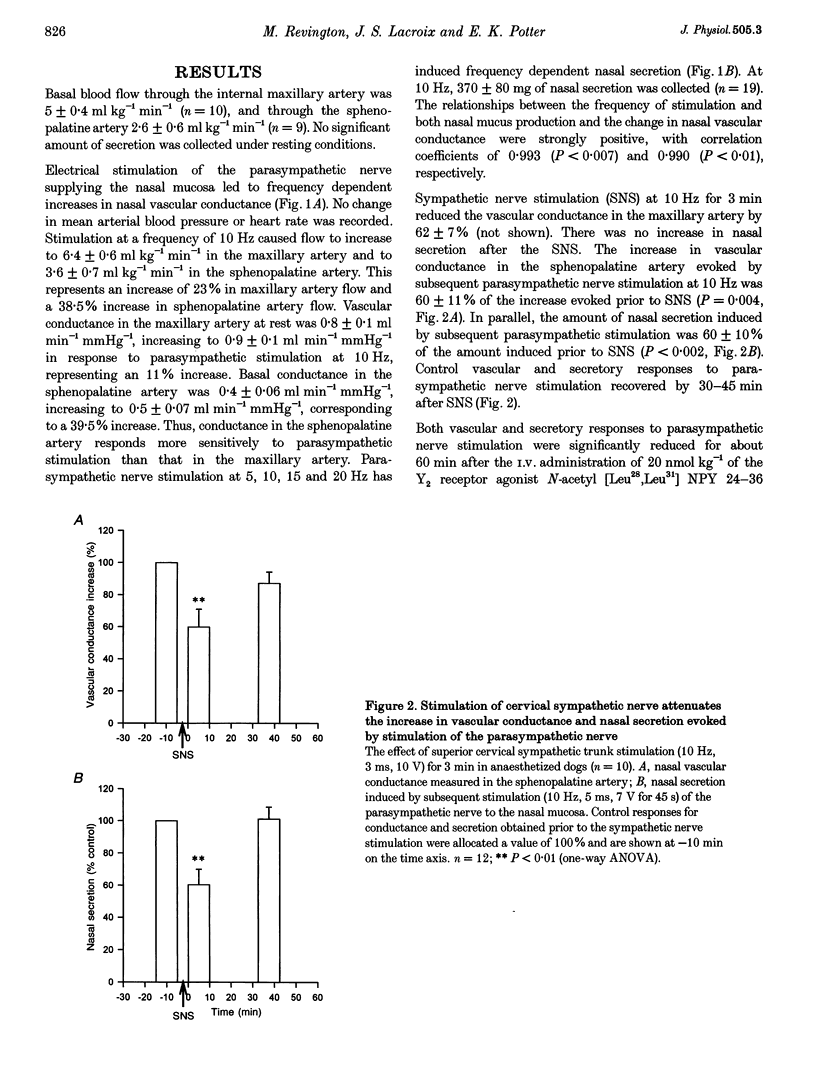
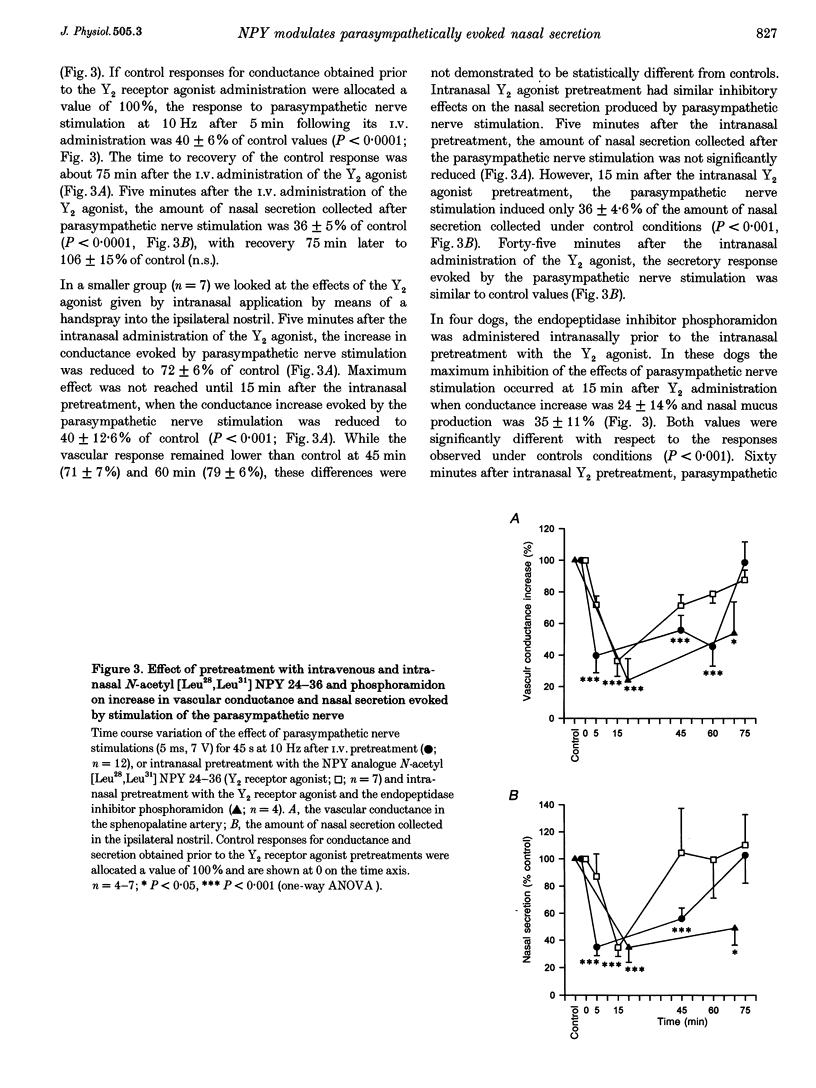
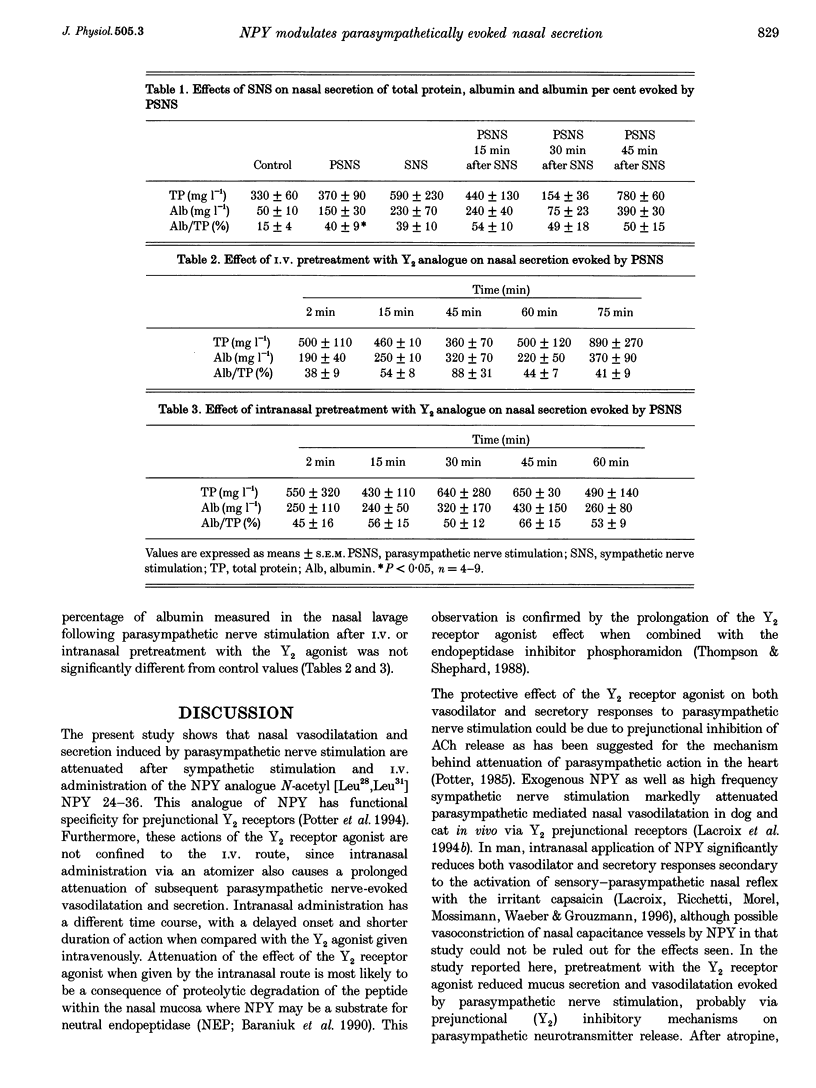
1. In dogs anaesthetized with pentobarbitone, electrical stimulation of the parasympathetic nerve fibres to the nasal mucosa evoked frequency dependent increases in both nasal arterial blood flow and nasal secretion. Blood flow was measured using a transonic flow probe placed around the artery. 2. Sympathetic nerve stimulation for 3 min at 10 Hz evoked significant and prolonged (> 30 min) attenuation of the vasodilator and secretory responses to subsequent parasympathetic stimulation. 3. Intravenous and intranasal administration of the neuropeptide Y (NPY) analogue N-acetyl [Leu28,Leu31] NPY 24-36, a selective NPY Y2 receptor agonist (20 nmol kg-1), significantly attenuated both vasodilator and secretory effects of subsequent parasympathetic nerve stimulation. When given intravenously, the inhibitory effect of this Y2 receptor agonist on vascular and secretory effects of parasympathetic nerve stimulation was rapid in onset (5 min) and lasted for more than 60 min. The modulatory effect of the Y2 receptor agonist was also seen with intranasal administration, but was slower in onset (15 min), and lasted less than 45 min. The effects of the intranasal pretreatment with the Y2 receptor agonist were significantly prolonged in the presence of the endopeptidase inhibitor phosphoramidon (10 nM). 4. Atropine pretreatment did not significantly reduce the change in vascular conductance evoked by parasympathetic nerve stimulation. Subsequent pretreatment with the NPY Y2 receptor agonist N-acetyl [Leu28,Leu31] NPY 24-36 reduced the stimulation induced increase in conductance by 30%. Nasal secretion was reduced by 70% following pretreatment with atropine and a further 30% by pretreatment with the NPY Y2 receptor agonist. Dose dependent vasodilator and secretory effects of local intra-arterial infusion of acetylcholine and vasoactive intestinal peptide were not modified by the NPY Y2 agonist. 5. Total protein and albumin concentration were measured in nasal lavage fluid collected after nerve stimulation. Atropine pretreatment increased the percentage of the total protein that was albumin in nasal lavage fluid. Neither sympathetic nerve stimulation nor Y2 receptor agonist pretreatment further modified the albumin exudation (a marker of vascular permeability) in nasal fluid lavage collected after parasympathetic nerve stimulation. 6. We propose that sympathetic nerve stimulation releases NPY, which acts on Y2 receptors, probably located on parasympathetic nerve endings, to attenuate both vasodilatation and nasal secretion evoked by subsequent parasympathetic nerve stimulation. This effect is also observed after pretreatment with the Y2-selective NPY analogue N-acetyl [Leu28,Leu31] NPY 24-36.
Full text
PDF

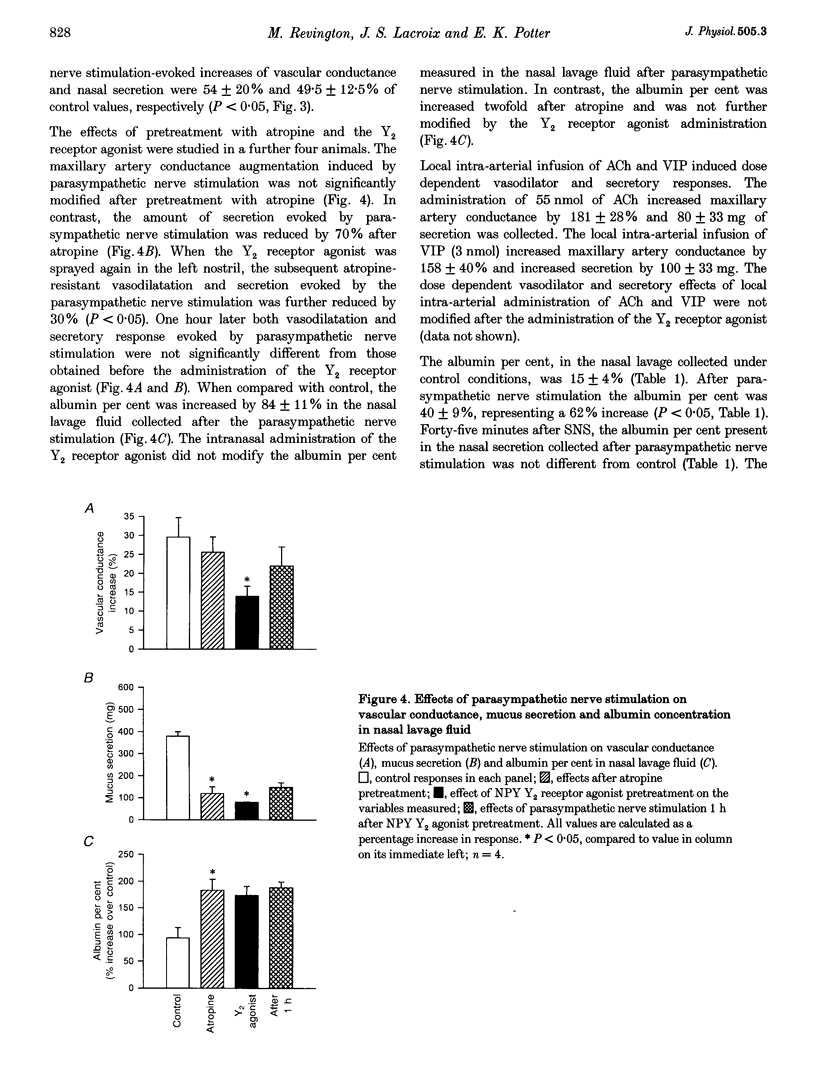


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anggård A. The effects of parasympathetic nerve stimulation on the microcirculation and secretion in the nasal mucosa of the cat. Acta Otolaryngol. 1974 Jul-Aug;78(1-2):98–105. [PubMed] [Google Scholar]
- BOJSEN-MOLLER F. TOPOGRAPHY OF THE NASAL GLANDS IN RATS AND SOME OTHER MAMMALS. Anat Rec. 1964 Sep;150:11–24. doi: 10.1002/ar.1091500103. [DOI] [PubMed] [Google Scholar]
- Baraniuk J. N., Castellino S., Lundgren J. D., Goff J., Mullol J., Merida M., Shelhamer J. H., Kaliner M. A. Neuropeptide Y (NPY) in human nasal mucosa. Am J Respir Cell Mol Biol. 1990 Aug;3(2):165–173. doi: 10.1165/ajrcmb/3.2.165. [DOI] [PubMed] [Google Scholar]
- Eccles R., Wilson H. The autonomic innervation of the nasal blood vessels of the cat. J Physiol. 1974 May;238(3):549–560. doi: 10.1113/jphysiol.1974.sp010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R., Wilson H. The parasympathetic secretory nerves of the nose of the cat. J Physiol. 1973 Apr;230(1):213–223. doi: 10.1113/jphysiol.1973.sp010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadlage R., Behnke E. E., Jackson R. T. Is the vidian nerve cholinergic? Arch Otolaryngol. 1975 Jul;101(7):422–425. doi: 10.1001/archotol.1975.00780360022005. [DOI] [PubMed] [Google Scholar]
- Jackson R. T., Burson J. H. Effect of inflammatory mediators on nasal mucosa. Arch Otolaryngol. 1977 Aug;103(8):441–444. doi: 10.1001/archotol.1977.00780250035001. [DOI] [PubMed] [Google Scholar]
- Klaassen A. B., van Megen Y. J., Kuijpers W., van den Broek P. Autonomic innervation of the nasal mucosa. ORL J Otorhinolaryngol Relat Spec. 1988;50(1):32–41. doi: 10.1159/000275967. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroix J. S., Anggård A., Hökfelt T., O'Hare M. M., Fahrenkrug J., Lundberg J. M. Neuropeptide Y: presence in sympathetic and parasympathetic innervation of the nasal mucosa. Cell Tissue Res. 1990 Jan;259(1):119–128. doi: 10.1007/BF00571436. [DOI] [PubMed] [Google Scholar]
- Lacroix J. S., Ricchetti A. P., Morel D., Mossimann B., Waeber B., Grouzmann E. Intranasal administration of neuropeptide Y in man: systemic absorption and functional effects. Br J Pharmacol. 1996 Aug;118(8):2079–2084. doi: 10.1111/j.1476-5381.1996.tb15647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J. S., Stjärne P., Anggärd A., Lundberg J. M. Sympathetic vascular control of the pig nasal mucosa (III): Co-release of noradrenaline and neuropeptide Y. Acta Physiol Scand. 1989 Jan;135(1):17–28. doi: 10.1111/j.1748-1716.1989.tb08546.x. [DOI] [PubMed] [Google Scholar]
- Lacroix J. S., Stjärne P., Anggård A., Lundberg J. M. Sympathetic vascular control of the pig nasal mucosa: (I). Increased resistance and capacitance vessel responses upon stimulation with irregular bursts compared to continuous impulses. Acta Physiol Scand. 1988 Jan;132(1):83–90. doi: 10.1111/j.1748-1716.1988.tb08301.x. [DOI] [PubMed] [Google Scholar]
- Lacroix J. S., Ulman L. G., Potter E. K. Modulation by neuropeptide Y of parasympathetic nerve-evoked nasal vasodilatation via Y2 prejunctional receptor. Br J Pharmacol. 1994 Oct;113(2):479–484. doi: 10.1111/j.1476-5381.1994.tb17014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J. S., Ulman L. G., Potter E. K. Sympathetic and parasympathetic interaction in vascular control of the nasal mucosa in anaesthetized cats. J Physiol. 1994 Oct 15;480(Pt 2):325–331. doi: 10.1113/jphysiol.1994.sp020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Emson P., Fahrenkrug J., Hökfelt T. Vasoactive intestinal polypeptide and cholinergic mechanisms in cat nasal mucosa: studies on choline acetyltransferase and release of vasoactive intestinal polypeptide. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5255–5259. doi: 10.1073/pnas.78.8.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- Lundblad L., Anggard A., Saria A., Lundberg J. M. Neuropeptide Y and non-adrenergic sympathetic vascular control of the cat nasal mucosa. J Auton Nerv Syst. 1987 Oct;20(3):189–197. doi: 10.1016/0165-1838(87)90148-2. [DOI] [PubMed] [Google Scholar]
- Mygind N., Borum P. Effect of a cholino-ceptor antagonist in the nose. Eur J Respir Dis Suppl. 1983;128(Pt 1):167–174. [PubMed] [Google Scholar]
- Potter E. K., Barden J. A., McCloskey M. J., Selbie L. A., Tseng A., Herzog H., Shine J. A novel neuropeptide Y analog, N-acetyl [Leu28,Leu31]neuropeptide Y-(24-36), with functional specificity for the presynaptic (Y2) receptor. Eur J Pharmacol. 1994 May 17;267(3):253–262. doi: 10.1016/0922-4106(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Potter E. K. Prolonged non-adrenergic inhibition of cardiac vagal action following sympathetic stimulation: neuromodulation by neuropeptide Y? Neurosci Lett. 1985 Mar 15;54(2-3):117–121. doi: 10.1016/s0304-3940(85)80065-3. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Druce H. M., Baraniuk J. N., Kaliner M. A. Pathophysiology of rhinitis. 1. Assessment of the sources of protein in methacholine-induced nasal secretions. Am Rev Respir Dis. 1988 Aug;138(2):413–420. doi: 10.1164/ajrccm/138.2.413. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Meredith S. D., Baraniuk J. N., Druce H. M., Banks S. M., Kaliner M. A. The pathophysiology of rhinitis. II. Assessment of the sources of protein in histamine-induced nasal secretions. Am Rev Respir Dis. 1989 Mar;139(3):791–800. doi: 10.1164/ajrccm/139.3.791. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Schade A. L., Butler W. T., Kasel J. A. The proteins in nasal secretion: a longitudinal study of the gammaA-globulin, gammaG-globulin, albumin, siderophilin, and total protein concentrations in nasal washings from adult male volunteers. J Clin Invest. 1966 May;45(5):768–776. doi: 10.1172/JCI105391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Sheppard D. Phosphoramidon potentiates the increase in lung resistance mediated by tachykinins in guinea pigs. Am Rev Respir Dis. 1988 Feb;137(2):337–340. doi: 10.1164/ajrccm/137.2.337. [DOI] [PubMed] [Google Scholar]
- Wells U., Widdicombe J. G. Lateral nasal gland secretion in the anaesthetized dog. J Physiol. 1986 May;374:359–374. doi: 10.1113/jphysiol.1986.sp016084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. G. The physiology of the nose. Clin Chest Med. 1986 Jun;7(2):159–170. [PubMed] [Google Scholar]


