Abstract
Background
With the continuous development of minimally invasive thoracic surgery, thoracoscopic thymectomy has become a routine operation. This method, now widely recognized, better protects lung function, reduces intraoperative blood loss and pain, and shortens postoperative hospital stay. We now introduce a standardized right thoracic incision for thoracoscopic thymic tumor resection, which has achieved favorable clinical outcomes.
Methods
This retrospective study involves 63 patients who underwent standardized right thoracic incision for thoracoscopic thymic tumor resection from October 2022 to January 2024. We analyzed the relevant clinical data, including postoperative pathological diagnosis, operation time, intraoperative blood loss, chest tube drainage duration, postoperative hospital stay, and follow-up data.
Results
All 63 patients underwent thoracoscopic surgery. Thoracoscopic surgery excised thymic tumors and mediastinal fat in 62 cases, while 1 case of thymic carcinoma underwent thoracoscopic biopsy due to invasion of the superior vena cava and innominate vein. Postoperative pathological diagnosis revealed thymoma in 35 cases (33 cases of thymoma and 2 cases of thymic adenocarcinoma). R0 resection was achieved in 33 cases and R1 resection in 1 case. Additionally, 2 patients diagnosed with thymic adenocarcinoma were advised to undergo postoperative radiotherapy. Postoperative pathological diagnosis of the other 28 cases showed thymus hyperplasia in 13 cases and thymus cysts in 15 cases. The mean operation time was 57.3 ± 14.2 min, and intraoperative blood loss was 22.5 ± 14.7 ml. The mean chest tube drainage duration was 2.7 ± 1.3 days, and the postoperative hospital stay was 3.7 ± 1.4 days. No secondary operations were required, and no severe complications or mortality were observed during the perioperative period. All patients were discharged smoothly after surgery. Ten patients were not followed up due to recent surgery. The remaining 53 patients were followed up for 6 to 12 months, with no recurrence or distant metastasis observed.
Conclusion
The standardized right thoracic incision for thoracoscopic thymic tumor resection simplifies the procedure of thymectomy. The operation’s risk points are relatively fixed, making it safe and feasible. It is easy for the operator to master, and the thymic tumor resection rate is high. It possesses characteristics of being minimally invasive, enabling rapid recovery, having fewer complications, and requiring simple perioperative management.
Keywords: Standardization right thoracic incision, Thoracoscopy, Thymic tumors
Background
Over the past few decades, traditional surgical methods for thymic tumors have included median sternal incision, posterolateral incision, and anterolateral incision. These approaches are particularly challenging due to the thymic tumor’s typical location in front of the aorta and heart, which significantly increases the operation’s risk. Surgeons often face poor exposure during these procedures, resulting in large wounds, slow postoperative recovery, and significant incision pain, among other complications [1–4].
In recent years, the continuous advancement of minimally invasive thoracic surgery has led to the widespread adoption of thoracoscopic thymectomy as a routine procedure. Minimally invasive surgery is now widely recognized for its ability to better preserve lung function, reduce intraoperative blood loss and pain, and shorten postoperative hospital stays [2–4]. Thymic tumors are classified according to the recommendations of the World Health Organization (WHO) into thymomas, which are further divided into low-grade (A, AB) and high-grade (B1, B2, B3) thymic tumors with neuroendocrine features (MNT), and thymic carcinomas (TC) [2, 5].
Currently, there are multiple incision options for thoracoscopic resection of thymic tumors, each with its own advantages and disadvantages. We have adopted a standardized right thoracic incision for thoracoscopic thymic tumor resection, which has proven to be simple, effective, and practical. From October 2022 to January 2024, we performed thoracoscopic resection of thymic tumors using the standardized right thoracic approach in 63 cases, achieving excellent results.
Clinical data and methods
General data
There were 63 cases in this group, including 33 males and 30 females. The age ranged from 23 to 74 years old, with a mean of 45.8 ± 9.0 years. Forty-five cases had no obvious symptoms and were found during physical examination, 15 cases were occasionally accompanied by chest tightness and other discomfort, and 3 cases had ocular myasthenia gravis. According to preoperative CT or MRI examination, the diameter of thymic tumors was less than 20 mm in 27 cases and 20–50 mm in 36 cases. There was no definite diagnosis before operation, no history of radiotherapy and chemotherapy, no obvious abnormalities in preoperative examination, and no obvious contraindications before operation. The patients’ characteristics are listed in Table 1.
Table 1.
Patients’ characteristics (N = 63)
| Variables | |
|---|---|
|
Age (years) Sex (M/F) |
45.8 ± 9.0 33/30 |
| Tumor sizes, n | |
|
<20 mm 20 ~ 50 mm No obvious symptoms Chest tightness and other discomfort Ocular myasthenia gravis |
27 36 45 15 3 |
Case selection criteria [2, 6]: CT or MRI revealed anterior mediastinal thymic lesions, clinical diagnosis of thymic tumors; chest CT or MRI revealed solid tumors ≤ 5 cm in diameter, smooth edges, clear boundaries, and no infiltration or adhesion with surrounding tissues and organs; CT or MRI suggested cystic lesions.
Methods
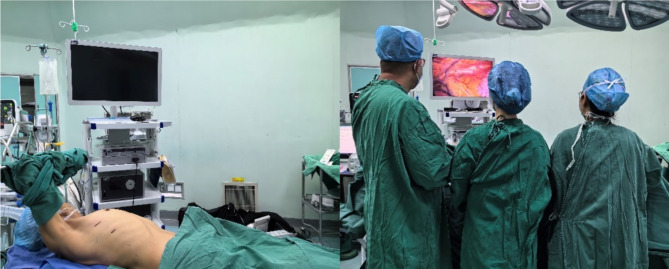
Three patients with thymic tumors complicated with myasthenia gravis needed to take an appropriate amount of brompistimine before the operation [3]. After the symptoms were relieved, the other 60 patients with no obvious surgical contraindications were treated with routine operations. Double-lumen endotracheal intubation, general anesthesia, right chest elevated 30°, right upper limb fixed to the anesthesia frame (Fig. 1A), and right lung collapse were performed. The thoracoscopic display was positioned on the left side of the patient, with the operator and assistant positioned on the right side (Fig. 1B). A 1 cm observation incision was made at the sixth intercostal space of the anterior axillary line, and a trocar and 30° video-assisted thoracoscopy were placed. Under direct vision, a 2 cm operating incision was made between the third intercostal space of the anterior axillary line (for females, the breast should be pushed to prevent damage), and the incision protective cover was placed (Fig. 1A). Electric hook, harmonic ACE, separation forceps, attractor, and other instruments were used to complete total thymectomy and mediastinal fat resection.
Fig. 1.
Imaging of surgical posture details and surgical incision selection. A Right chest pad 30°high, right upper limb fixed to anesthesia frame, the operating incision and the observation incision are located in the third intercostal space of the anterior and sixth intercostal space of the anterior axillary line, respectively. B The thoracoscopic display is positioned on the left side of the patient, and the operator and an assistant are positioned on the right side of the patient
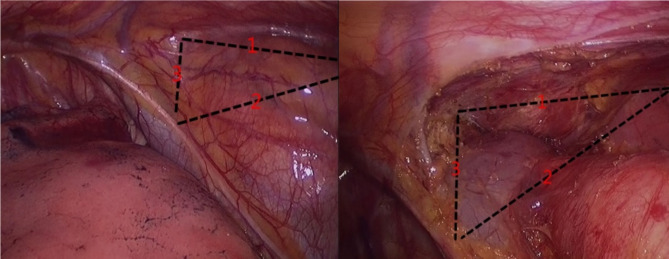
Before the operation, the surgical field was divided into three lines (Fig. 2A), and the procedure was carried out sequentially. The first step is to use an electric hook and harmonic ACE to separate the space between the sternum and the thymic tumor and fat, expose the left internal mammary vein, and separate to the contralateral pleura to maintain the integrity of the left pleura. The second step is to separate the thymic tumor and fat from the space outside the pericardium and excise the adipose tissue adjacent to the right phrenic nerve. The third step is to lift the thymic tissue to separate the thymus along the innominate vein. The thymus vein can be cut off with a harmonic ACE after clamping with titanium or vascular clips. Continue until the upper pole of the thymus; the tumor is completely removed, the mediastinal fat is cleared, and the bleeding is completely stopped. Hemostatic gauze packing can be used to manage a small amount of bleeding, and the closed thoracic drainage tube is routinely retained at the end of the operation, completing the standardized procedure. After the operation, the thymus and mediastinal fat were completely resected, and the anatomical structure of the anterior mediastinum could be clearly seen (Fig. 2B).
Fig. 2.
Imaging of preoperative and postoperative operation area. A The space between retrosternal and thymic tumor and fat (Line 1), the space between the thymic tumor and fat outside the pericardium (Line 2), and lift the thymus tissue to separate the thymus along the direction of the innominate vein (Line 3). B Angle of view after parasternal thymic tumor and fat resection (Line 1), angle of view after resection of the thymic tumor and fat outside the pericardium (Line 2), and angle of view after resection of the thymic tumor and fat near the innominate vein (Line 3)
Observation indicators
We observed the postoperative pathological diagnosis, operation time, intraoperative blood loss, chest tube drainage duration, postoperative hospital stay, and follow-up data to evaluate the safety and feasibility of this method.
Statistical analysis
All statistical analyses were carried out with SPSS 19.0 software. The parameters of each sample were tested for normality, and the results showed that they conformed to a normal distribution and are described as x̄ ± s.
Results
All 63 patients underwent thoracoscopic surgery. Among them, thoracoscopic surgery excised thymic tumors and mediastinal fat in 62 cases, while 1 case of thymic carcinoma underwent thoracoscopic biopsy due to invasion of the superior vena cava and innominate vein.
The mean operation time was 57.3 ± 14.2 min, and intraoperative blood loss was 22.5 ± 14.7 ml. The mean chest tube drainage duration was 2.7 ± 1.3 days, and the postoperative hospital stay was 3.7 ± 1.4 days. No secondary operations were required, and no severe complications or mortality were observed during the perioperative period. All patients were discharged smoothly after surgery.
Postoperative pathological diagnosis included thymoma in 33 cases: 12 cases of type A, 7 cases of type AB, 3 cases of type B1, 6 cases of type B2, and 5 cases of type B3. Thymic hyperplasia was observed in 13 cases and thymic cysts in 15 cases. Among the thymoma cases (33 cases) and thymic adenocarcinoma cases (2 cases), R0 resection was performed in 33 thymoma cases and R1 resection in 1 thymoma case. Two patients diagnosed with thymic adenocarcinoma were advised to undergo postoperative radiotherapy.
Ten patients were not followed up due to recent surgery. The remaining 53 patients were followed up for 6 to 12 months, with no recurrence or distant metastasis observed. The 53 patients were followed up for 6–12 months by hospital visits (45 cases, 84.9%) and telephone (8 cases, 15.1%). The mean duration of follow-up was 8.9 ± 1.8 months. There were no recurrences, metastases, or deaths during the follow-up period. The clinical characteristics of the 63 patients are shown in Table 2.
Table 2.
Clinical characteristics of patients (N = 63)
| Variables | |
|---|---|
| Surgical mode, n | |
|
Total thymectomy and mediastinal fat dissection Thoracoscope biopsy Operation time, mean ± SD (min) Intraoperative blood loss, mean ± SD (ml) Chest tube drainage duration, mean ± SD (days) Postoperative hospital stay, mean ± SD (days) Secondary operation and severe complications, n |
62 1 57.3 ± 14.2 22.5 ± 14.7 2.7 ± 1.3 3.7 ± 1.4 0 |
| Postoperative pathological diagnosis, n | |
|
Thymic hyperplasia Thymic cyst Thymic adenocarcinoma(type C) Thymoma(type A/type AB/type B1/type B2/type B3) |
13 15 2 33(12/7/3/6/5) |
| Thymoma Masaoka clinical stages, n | |
|
I IIa IIb IIIa IIIb |
15 10 8 1 1 |
| Resection method of thymoma, n | |
|
R0 resection R1 resection Thoracoscopic biopsy Postoperative radiotherapy cases, n Follow-up cases, n Hospital visits Telephone The mean duration of follow-up, (mths) |
33 1 1 2 53 45 (84.9%) 8 (15.1%) 8.9 ± 1.8 |
Discussion
Thymomas and thymic adenocarcinoma are the most common primary anterior mediastinal masses, while thymic adenocarcinoma is far rarer but much more likely to spread [7]. Patients with early thymic tumors often have no obvious clinical symptoms. With the continuous growth of thymic tumors, patients may develop symptoms such as chest tightness, chest pain, and even superior vena cava syndrome. These symptoms are generally caused by tumor infiltration or compression, making it difficult to operate at this stage and challenging to achieve complete removal [2, 8]. With the development of the economy and society, more and more healthcare facilities have included low-dose spiral CT in routine physical examinations. Many patients with early thymic tumors have been identified for the first time during these examinations [9], indicating that chest CT plays an irreplaceable role in screening and diagnosis [10, 11]. When a thymic tumor is first detected, we suggest performing an enhanced CT to evaluate its relationship with the innominate vein, pericardium, aorta, and branches, and to assess the feasibility of tumor resection. When the diagnosis is difficult, enhanced MRI [11, 12] can be performed to distinguish solid tumors or cysts. Thymic tumors are the most common mediastinal tumors. They are often located in the anterior superior mediastinum, with the sternum in front, innominate veins, pericardium, aorta, and branch vessels behind, and bilateral pleura and lung tissue on both sides [8]. Because of its special anatomical location, thymic tumor resection is often considered high-risk surgery [8]. Since Landreneau [13] et al. first reported video-assisted thoracoscopic resection of mediastinal tumors in 1992, video-assisted thoracoscopy has played an important role in the diagnosis and treatment of thymic tumors [3, 14, 15]. However, there is no article that uses a standardized approach to surgery as we do, so there is an urgent need for a standardized surgical method to simplify the operation.
All 62 patients (98.4%) underwent standardization right thoracic incision for thoracoscopic thymic tumors and mediastinal fat resection. Only 1 patient (1.6%) underwent thoracoscopic biopsy because of tumor infiltration into superior vena cava and innominate vein. There were no postoperative severe pulmonary infections, pulmonary embolism, or death; these results were satisfactory. A meta-analysis [14] showed that the operation time was 65–249.8 min, intraoperative blood loss was 92.3-137.5 ml, and postoperative hospital stay was 2.9–10.6 days for minimally invasive thymic operations. Our methods showed that the mean operation time was 57.3 ± 14.2 min, intraoperative blood loss was 22.5 ± 14.7 ml, and postoperative hospital stay was 3.7 ± 1.4 days. No secondary operations were required, and no severe complications or mortality were observed during the perioperative period. We have better performance in the above data, demonstrating that the standardized operation improves the efficiency of the procedure.
Fifty-three patients were followed up for 6 to 12 months, with no recurrence, metastasis, or death during the follow-up period. Two patients with thymic adenocarcinoma were advised to undergo postoperative radiotherapy, which is considered to have an ideal curative effect and survival rate [16, 17]. With the continuous progress of medical technology, new treatments for thymic adenocarcinoma, such as targeted therapy [18–20] and immunotherapy [19, 21], may become important treatments in the future [20–22].
Thymectomy, the surgical removal of the thymus gland, can be performed using various techniques, each with distinct advantages and disadvantages. The primary methods include median sternotomy, transcervical approach, video-assisted thoracoscopic surgery (VATS), and robotic-assisted thoracic surgery (RATS). Median sternotomy offers excellent exposure of the anterior mediastinum, facilitating thorough resection crucial for advanced thymomas and thymic carcinomas [23]. However, it is highly invasive, associated with significant morbidity, prolonged recovery times, and increased postoperative pain [23]. The transcervical approach, although minimally invasive and avoiding sternal complications, is limited by restricted exposure, making it less suitable for larger or invasive tumors and presenting a steep learning curve for surgeons [24]. VATS, on the other hand, is less invasive, with reduced postoperative pain, faster recovery, and better cosmetic outcomes [25]. Despite these benefits, it has a limited field of vision compared to open surgery and requires significant expertise, posing challenges for larger or more invasive tumors [25]. RATS enhances precision and dexterity with robotic instruments, providing superior visualization through 3D imaging, but it is costly, less widely available, and demands a steep learning curve with longer setup and operative times [26]. In addition to these methods, some recent studies have introduced hybrid techniques that combine the benefits of different approaches to overcome individual limitations. For example, combining VATS with robotic assistance can leverage the minimally invasive nature of VATS while utilizing the precision and enhanced visualization of robotic systems [26]. This hybrid approach can potentially address the challenges of limited field of vision and improve the resection of complex or larger tumors. Another emerging technique is single-port VATS, which aims to minimize trauma further by using a single incision, thereby enhancing cosmetic outcomes and reducing recovery time [27]. However, these newer techniques require extensive training and have their own set of challenges, including limited instrument maneuverability and increased technical difficulty.
Our standardized right thoracic incision for thoracoscopic thymic tumor resection presents several distinct advantages over traditional methods. The right thoracic approach offers superior exposure of the anterior mediastinum, facilitating thorough resection of thymic tumors and mediastinal fat by avoiding obstruction from the aortic arch and its branches, thereby enhancing operative visibility and precision. This technique is less invasive than median sternotomy, resulting in reduced postoperative pain, shorter hospital stays, and faster recovery with favorable cosmetic outcomes due to smaller incisions. The standardized approach simplifies the procedure, making it easier for surgeons to master. Our data demonstrate that the mean operation time, intraoperative blood loss, and postoperative hospital stay are significantly reduced compared to traditional methods, underscoring the operational efficiency of this technique. Additionally, this method allows for complete removal of thymic tumors and mediastinal fat, which is essential for preventing recurrence and metastasis. The comprehensive nature of this resection is vital for long-term patient outcomes, minimizing the risk of recurrence and ensuring thorough tumor removal. By combining the benefits of minimally invasive surgery with improved exposure and operational efficiency, this approach stands out as a superior alternative to traditional thymectomy techniques. Future studies and long-term follow-up will further validate the efficacy and safety of this technique, potentially establishing it as the gold standard for thymic tumor resection.
To achieve the above results, we used the following management strategies: (1) Consuming more than 300 g of beef per day starting on the first day after the operation can provide a large number of nutrients to promote wound healing and help reduce wound exudation. (2) Patients were instructed to practice coughing and expectoration before the operation, and medical staff and family members helped patients to cough and expectorate after the operation to actively prevent pulmonary complications. (3) Getting out of bed early after the operation: some patients can move their lower limbs on their own in bed for 6 h after the operation, alternating the movement of both lower limbs and strengthening the exercise of getting out of bed on the second day after the operation. For the elderly, they can be accompanied by medical staff and family members. This method can better prevent thrombotic diseases [28]. According to the standardized method for the prevention of postoperative complications, satisfactory results were obtained. We summarized it as “eat well, cough well, exercise well, everyone is satisfied” and referred to it as “three well and one satisfaction” for minimally invasive thoracic surgery and non-esophageal surgery in the perioperative period. We adopted a standardized scheme for operation and perioperative management, optimized the operation process, shortened the operation time, chest tube extraction time, and postoperative hospital stay, and further improved postoperative rehabilitation training. Satisfactory results were achieved.
Conclusions
The standardized right thoracic incision for thoracoscopic thymic tumor resection simplifies the procedure of thymectomy. The operation’s risk points are relatively fixed, making it safe and feasible. It is easy for the operator to master, and the thymic tumor resection rate is high. It possesses characteristics of being minimally invasive, enabling rapid recovery, having fewer complications, and requiring simple perioperative management.
Acknowledgements
Not applicable.
Author contributions
JQ designed the work. JZ, EY, JY, XL and YM performed the surgery. SX and DG performed data extraction and statistical analysis and wrote the article. All authors read and approved the final version of the manuscript.
Funding
This research is supported by the Academic and Technical Leader Reserve Project for Middle-aged and Young People in Yunnan Province (202405AC350006), the Yunnan Province Science and Technology Department Science and Technology Program (202401AY070001-249), the Academic and Technical Leader Reserve Project for Middle-aged and Young People in Dehong (2021RC003), and the Scientific Research Fund Project of Dehong People’s Hospital (2022DY002).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board and Ethics Committee of the Dehong People’s Hospital, Affiliated Dehong Hospital of Kunming Medical University (2022DY002).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shihao Xu, Dongzhao Gao and Xinming Li contributed equally to this work.
References
- 1.Zhang Y, Lin D, Aramini B et al. Thymoma and Thymic Carcinoma: Surgical Resection and Multidisciplinary Treatment. Cancers (Basel) 2023;15. [DOI] [PMC free article] [PubMed]
- 2.Ruffini E, Filosso PL, Guerrera F, Lausi P, Lyberis P, Oliaro A. Optimal surgical approach to thymic malignancies: new trends challenging old dogmas. Lung Cancer. 2018;118:161–70. [DOI] [PubMed] [Google Scholar]
- 3.Comacchio GM, Marulli G, Mammana M, Natale G, Schiavon M, Rea F. Surgical decision making: Thymoma and Myasthenia Gravis. Thorac Surg Clin. 2019;29:203–13. [DOI] [PubMed] [Google Scholar]
- 4.Manolache V, Gonzalez-Rivas D, Bosinceanu ML, Gallego-Poveda J, Garcia-Perez A, Motas N. Uniportal robotic-assisted thoracic surgery for mediastinal tumors. Ann Cardiothorac Surg. 2023;12:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: continuity and changes. J Thorac Oncol. 2015;10:1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg. 2010;37:824–6. [DOI] [PubMed] [Google Scholar]
- 7.Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332–50. [DOI] [PubMed] [Google Scholar]
- 8.Zieliński M. Surgical approaches to Myasthenia Gravis: perspective of anatomy and radicality in surgery. Thorac Surg Clin. 2019;29:159–64. [DOI] [PubMed] [Google Scholar]
- 9.Tuan PA, Vien MV, Dong HV, Sibell D, Giang BV. The value of CT and MRI for determining Thymoma in patients with Myasthenia Gravis. Cancer Control. 2019;26:1073274819865281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JW, Lee KS, Shin MH, et al. Thymic epithelial tumors: prognostic determinants among clinical, histopathologic, and computed tomography findings. Ann Thorac Surg. 2015;99:462–70. [DOI] [PubMed] [Google Scholar]
- 11.Priola AM, Priola SM, Gned D, Giraudo MT, Fornari A, Veltri A. Comparison of CT and chemical-shift MRI for differentiating thymoma from non-thymomatous conditions in myasthenia gravis: value of qualitative and quantitative assessment. Clin Radiol. 2016;71:e157–69. [DOI] [PubMed] [Google Scholar]
- 12.Ottlakan A, Borda B, Morvay Z, Maraz A, Furak J. The Effect of Diagnostic Imaging on Surgical Treatment Planning in diseases of the Thymus. Contrast Media Mol Imaging. 2017;2017:9307292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landreneau RJ, Dowling RD, Ferson PF. Thoracoscopic resection of a posterior mediastinal neurogenic tumor. Chest. 1992;102:1288–90. [DOI] [PubMed] [Google Scholar]
- 14.Friedant AJ, Handorf EA, Su S, Scott WJ. Minimally invasive versus Open Thymectomy for Thymic malignancies: systematic review and Meta-analysis. J Thorac Oncol. 2016;11:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matilla JR, Klepetko W, Moser B. Thymic minimally invasive surgery: state of the art across the world-Europe. J Vis Surg. 2017;3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim YJ, Kim E, Kim HJ, et al. Survival impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV thymomas: a systematic review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94:1129–36. [DOI] [PubMed] [Google Scholar]
- 17.Fu H, Gu ZT, Fang WT, et al. Long-term Survival after Surgical Treatment of Thymic Carcinoma: a retrospective analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol. 2016;23:619–25. [DOI] [PubMed] [Google Scholar]
- 18.Kelly RJ. Systemic treatment of advanced thymic malignancies. Am Soc Clin Oncol Educ Book 2014:e367–73. [DOI] [PubMed]
- 19.Dapergola A, Gomatou G, Trontzas I, et al. Emerging therapies in thymic epithelial tumors (review). Oncol Lett. 2023;25:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan M, Ganti AK. The role of targeted therapy in thymic carcinoma. J Oncol Pharm Pract. 2019;25:1712–8. [DOI] [PubMed] [Google Scholar]
- 21.Perrino M, Cordua N, De Vincenzo F et al. Thymic epithelial tumor and Immune System: the role of Immunotherapy. Cancers (Basel) 2023;15. [DOI] [PMC free article] [PubMed]
- 22.Ao YQ, Gao J, Wang S, et al. Immunotherapy of thymic epithelial tumors: molecular understandings and clinical perspectives. Mol Cancer. 2023;22:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L, He T, Hu J. Minimally invasive thoracic surgery: robot-assisted versus video-assisted thoracoscopic surgery. Wideochir Inne Tech Maloinwazyjne. 2023;18:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He G, Yao T, Zhao L et al. A proof-of-concept study: advantages of the subxiphoid over the lateral intercostal approach. Interdiscip Cardiovasc Thorac Surg 2024;38. [DOI] [PMC free article] [PubMed]
- 25.Kumar A, Asaf BB, Pulle MV, Puri HV, Bishnoi S, Gopinath SK. Minimal Access surgery for Thymoma. Indian J Surg Oncol. 2020;11:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comacchio GM, Schiavon M, Zirafa CC et al. Robotic thymectomy in thymic tumours: a multicentre, nation-wide study. Eur J Cardiothorac Surg 2024;65. [DOI] [PubMed]
- 27.Song N, Li Q, Aramini B, et al. Double sternal elevation subxiphoid versus uniportal thoracoscopic thymectomy associated with superior clearance for stage I-II thymic epithelial tumors: Subxiphoid thymectomy compared with VATS. Surgery. 2022;172:371–8. [DOI] [PubMed] [Google Scholar]
- 28.Mazzolai L, Ageno W, Alatri A, et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022;29:1248–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.