Abstract
Background
Global longitudinal strain (GLS) has been used to identify patients at risk for cancer-therapy related cardiac dysfunction (CTRCD). However, there is limited data on the effectiveness of initiating cardioprotective therapy based on a strain-guided strategy in early stage HER2+ breast cancer patients. This randomized clinical trial assessed if treatment with carvedilol based on a strain-guided strategy can prevent development of CTRCD in HER2+ breast cancer patients on non-anthracycline based regimens.
Methods
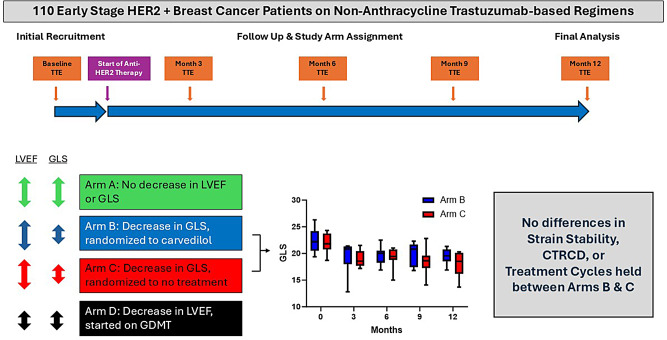
Study participants were prospectively assigned to one of four arms. Patients with normal LVEF and GLS remained in Arm A. Patients whose GLS decreased by > 15% from baseline or to < -15% during follow up were randomized 1:1 to prophylactic carvedilol (Arm B) or no therapy (Arm C). Patients who developed CTRCD were assigned to Arm D. The primary endpoint was GLS stability. The secondary endpoints were development of CTRCD and rate of anti-HER2 treatment interruption.
Results
Among 110 patients who completed follow up, 84 were assigned to Arm A, 10 each were randomized to Arms B or C, and 6 were assigned to Arm D. At the end of the study period, there were no significant differences in GLS stability, development of CTRCD, or number of cancer therapy cycles completed between patients who did and did not receive cardioprotective therapy.
Conclusions
In this prospective randomized GLS-guided study of prophylactic carvedilol in early stage HER2+ breast cancer patients on non-anthracycline regimens, there were no significant difference between groups in GLS stability, CTRCD or trastuzumab cycles held. These findings may identify a low-risk group of patients who may be considered for less intensive cardiac surveillance.
Trial registration
https://clinicaltrials.gov/study/NCT02993198. Start date: 4/2015. This trial included patients who were retrospectively registered.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40959-024-00291-5.
Keywords: Global longitudinal strain, Cardioprotective therapy, HER2+ breast cancer, CTRCD
Background
Cardiotoxicity due to trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2), remains the most common reason for trastuzumab interruption during breast cancer treatment with both cardiotoxicity and treatment interruption associated with reduced overall survival [1, 2]. Early trastuzumab interruption is also associated with lower breast cancer recurrence-free survival [3]. Thus, early identification of patients at risk for cardiotoxicity who may benefit from cardioprotective therapy (CPT) is vital to clinical care. Surveillance for cancer therapy-related cardiac dysfunction (CTRCD) has traditionally focused on left ventricular ejection fraction (LVEF) but there is now a wealth of evidence demonstrating that changes in global longitudinal strain (GLS) pre-date changes in LVEF and are more sensitive in identifying early cardiotoxicity [4–8]. Additionally, the SUCCOUR study showed that in comparison with an LVEF-guided strategy for implementing cardioprotective therapy, a GLS-guided strategy resulted in greater recognition of cardiotoxicity and greater uptake of CPT, arguing for a greater focus on GLS-guided surveillance in monitoring for CTRCD (although of note, there were no significant differences between strategies in terms of detection of CTRCD at 1 and 3 years) [9, 10]. However, these data are mainly derived from patients receiving anthracycline chemotherapy with sequential trastuzumab which is known to have higher rates of cardiotoxicity [11, 12]. In recent years, non-anthracycline regimens involving anti-HER2 agents have been increasingly used as first-line therapy for HER2-positive breast cancer due to similar efficacy and lower toxicity compared to anthracycline based regimens [13, 14]. In patients receiving these regimens there is limited data on strategies to identify patients at higher risk for CTRCD using GLS surveillance and a GLS-guided strategy to implement CPT has not been prospectively investigated in a randomized fashion in this population. Additionally, while there has been promising research examining the response to CPT with beta-blocker therapy in patients receiving anthracycline based regimens, there is limited data on the use of beta-blocker therapy in breast cancer patients receiving non-anthracycline based regimens [15–17]. Accordingly, we performed a prospective, single-blinded randomized study using a GLS-guided strategy to implement CPT with beta-blocker therapy in HER2-positive early-stage breast cancer patients receiving primarily non-anthracycline-based regimens to identify those at risk for CTRCD, to preserve GLS, and prevent cancer directed treatment interruptions.
Methods
Trial design and oversight
PROTECT HER2 was a single-blind, randomized clinical trial. The study was approved by the institutional review board of Northwestern University and prospectively registered (www.clinicaltrials.gov: NCT02993198). All participants provided written informed consent. The trial functions were coordinated by the Bluhm Cardiovascular Institute Clinical Trials Unit at Northwestern Memorial Hospital.
Participants
Female patients aged > 18 years of age with a new diagnosis of HER2-positive early stage (stages I-III) breast cancer were recruited at Northwestern Memorial Hospital between June 2015 to February 2020. Inclusion criteria were: LVEF ≥ 53% at baseline echocardiogram, New York Heart Association class I-II and scheduled to receive trastuzumab and/or pertuzumab-based regimens. Exclusion criteria were baseline LVEF < 53%, moderate to severe coronary artery disease, moderate to severe valvular heart disease, constrictive/restrictive cardiomyopathy, metastatic breast cancer, high degree atrioventricular block or sick sinus syndrome, heart rate < 50 bpm, systolic blood pressure < 85 mmHg, Child-Pugh class B and C liver disease, moderate to severe asthma, prior hypersensitivity to beta-blockers, pregnant or lactating women or unwilling to consent to study participation. Patients who had ever taken beta-blocker, angiotensin converting enzyme-inhibitor (ACEi) and angiotensin receptor blocker (ARB) therapy were excluded but other cardiac medications were allowed.
Echocardiography
Baseline echocardiography was performed before the start of chemotherapy and at 3-month intervals through 12 months. Transthoracic echocardiography was performed with a standardized protocol using a commercially available Vivid E9 ultrasound system (GE Medical Systems, Milwaukee, WI). Measurements were performed according to the American Society of Echocardiography guidelines [18]. Cine loops of standard apical 4-chamber, apical 2-chamber and apical 3-chamber views were acquired at frame rates between 50 and 70 frames per second. Offline speckle-tracking analysis was performed using EchoPAC (GE Medical Systems, Milwaukee, WI) to obtain GLS as the average peak systolic strain in these views. Two-dimensional (2D) LVEF was performed using the Simpson’s biplane method. Normal LVEF was defined as LVEF ≥ 53% throughout the study period. A significant decrease in GLS was defined as > 15% decrease from baseline GLS or absolute GLS < 15% strain units. CTRCD was defined as > 10% reduction from baseline in LVEF to < 53% according to the American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines which were standard in the timeframe that this study was undertaken [19]. Echocardiographic measurements were made by level III echocardiographers throughout the course of the study who were blinded to the treatment regimens, study arms, and clinical outcome.
Study randomization and interventions
Patients were grouped into 4 arms based on LVEF and GLS on echocardiography (Fig. 1). Arm A consisted of patients with normal LVEF and normal GLS throughout the study period. Arm B consisted of patients with normal LVEF and significant decrease in GLS during the study period who were then randomized to receive carvedilol. Arm C consisted of patients with normal LVEF and significant decrease in GLS during the study period who were then randomized to no beta-blocker therapy. Arm D consisted of patients who developed CTRCD by LVEF criteria during the study period and were initiated on standard of care heart failure therapy after diagnosis. Patients in Arms B and C were randomized 1:1 during the follow-up period when there was a significant decrease in GLS with normal LVEF to receive either prophylactic CPT with carvedilol or standard of care monitoring. Patients in Arms A, B, or C who developed CTRCD later in follow up subsequently crossed over into Arm D (Supplemental Fig. 1). Patients receiving prophylactic carvedilol were monitored during outpatient trastuzumab infusion visits every 3 weeks by a research coordinator who was blinded to echocardiographic findings. At each visit, the research coordinator made note of vitals and any side-effects from carvedilol. Based on the vitals and symptoms, the primary investigator recommended dose titration every 3 weeks. Carvedilol was started at 3.125 mg twice daily and up titrated to 25 mg twice daily. If the patient complained of dizziness, heart rate decreased to < 50 bpm or systolic blood pressure decreased to < 100 mmHg then titration was stopped and dose was reduced to the last increased increment. Patients in Arm B continued prophylactic carvedilol for duration of anti-HER2 therapy, up to 12 months.
Fig. 1.
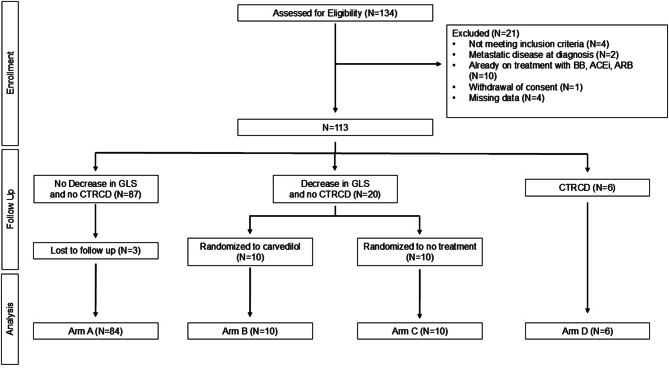
Design of the PROTECT HER2 trial. CONSORT diagram showing the design of the PROTECT HER2 trial from enrollment through analysis
Study end points
The primary outcome was GLS stability which was evaluated in two ways: (1) as a change of < 15% relative GLS of end strain compared to baseline (2) as a change of < 15% relative GLS of end strain compared to randomization point. Secondary outcomes were (1) development of CTRCD defined as a drop in calculated 2D EF of ≥ 10% from baseline to a value less than 53% at any timepoint over the course of 12 months, (2) rate of anti-HER2 treatment interruptions. Other echocardiographic parameters were compared between the treatment arms across the study timeframe as exploratory analyses.
Statistical analysis
This pilot study was powered based on assumptions that as many as 30 patients with normal LVEF but abnormal GLS would be available for 1:1 randomization to Arms B and C, fewer than 20% would drop out, and that no more than 10% of Arm B participants will experience a reduction in strain > 3% compared to 65% of Arm C participants. Under these assumptions and one-sample exact binomial arguments, a sample of 30 participants (randomized to Arms B and C) was estimated to provide power in excess of 80% to detect a difference between Arms B and C at two-sided 5% significance level.
Descriptive statistics were used to summarize baseline characteristics. Mean and standard deviations were used for continuous and normally distributed variables. Median and interquartile ranges were used for continuous and non-normally distributed variables. Frequency and percent were used for categorical variables. Baseline clinical characteristics and maintenance of anti-HER2 therapies were compared between study arms (A vs. B + C vs. D) using ANOVA, Kruskal-Wallis or Chi-squared test. Development of CTRCD and stability of GLS were compared between arms B and C using Wilcoxon signed-rank and chi-squared test.
We evaluated differences in echo parameters at randomization between study arms (B + C vs. D) using ANCOVA models with adjustments for baseline values. The change in echo parameters from randomization to end of study was compared between study arms (B vs. C) through ANOVA tests. Analyses were performed using SAS (Base 9.4) with p-values < 0.05 considered statistically significant. In instances where data were missing, a complete case analysis was performed.
Results
Patient characteristics
Of 134 patients with early stage HER2+ breast cancer enrolled in the study, 110 patients completed 12-month follow-up. 84 patients did not develop a decrease in either LVEF or GLS and were assigned to Arm A, 6 patients developed decreased LVEF and were assigned to Arm D, while 20 patients developed decreased GLS without decreased LVEF and were randomized to Arms B or C (Fig. 1, Supplemental Fig. 1). 24 patients were excluded due to not meeting enrolment criteria (n = 4), loss to follow-up (n = 3), missing data (n = 4), metastatic disease (n = 2), treatment with CPT at the time of enrolment (n = 10), or withdrawal of consent (n = 1). Baseline clinical characteristics are presented in Table 1. Patients were aged 50.7 ± 11.3 years and all were women. There were 32 (29.1%) patients who identified as non-Caucasian. Cardiac co-morbidities did not significantly differ between arms. More patients in Arms B, C, and D reported cardiac symptoms when compared with Arm A. Tumor location and stage did not differ between arms (Table 1). Clinical characteristics and echocardiographic parameters for Arms B and C at the time of randomization are presented in Supplemental Table 1. There were no significant differences between Arms B and C at the time of randomization.
Table 1.
Baseline clinical characteristics of study patients
| Variable, No. (%) | N | Arm A (N = 84) |
Randomized Arms (B + C) (N = 20) |
Arm D (N = 6) |
P-value1 | |||
|---|---|---|---|---|---|---|---|---|
| Age, years, Mean ± SD | 110(84,20,6) | 50.3 | ± 12.06 | 54.6 | ± 6.71 | 43.8 | ± 8.64 | 0.096 |
| Race | 110(84,20,6) | 0.079 | ||||||
| Caucasian | 58 | (69.0%) | 16 | (80.0%) | 1 | (16.7%) | ||
| Black | 14 | (16.7%) | 3 | (15.0%) | 2 | (33.3%) | ||
| Asian | 7 | (8.3%) | 0 | (0%) | 2 | (33.3%) | ||
| Other | 3 | (3.6%) | 0 | (0%) | 1 | (16.7%) | ||
| Unknown | 2 | (2.4%) | 1 | (5.0%) | 0 | (0%) | ||
| Body Mass Index, Mean ± SD | 110(84,20,6) | 26.2 | ± 5.65 | 27.3 | ± 6.28 | 22.8 | ± 4.43 | 0.254 |
| Cardiovascular Risk Factors | ||||||||
| Hypertension | 110(84,20,6) | 10 | (11.9%) | 2 | (10.0%) | 0 | (0%) | 0.658 |
| Diabetes | 110(84,20,6) | 6 | (7.1%) | 0 | (0%) | 0 | (0%) | 0.375 |
| Hyperlipidemia | 110(84,20,6) | 8 | (9.5%) | 2 | (10.0%) | 0 | (0%) | 0.726 |
| Tobacco use history | 110(84,20,6) | 28 | (33.3%) | 8 | (40.0%) | 2 | (33.3%) | 0.851 |
| Cardiac Symptoms | ||||||||
| ≥ 1of chest pain, SOB, PND, orthopnea, LE edema, presyncope, syncope | 110(84,20,6) | 6 | (7.1%) | 8 | (40.0%) | 3 | (50.0%) | < 0.001 |
| Prior Chemotherapy | 6(4,1,1) | 0.472 | ||||||
| Anthracycline-based | 2 | (50.0%) | 1 | (100%) | 1 | (100%) | ||
| Non-anthracycline regimen | 2 | (50.0%) | 0 | (0%) | 0 | (0%) | ||
| Tumor Characteristics | ||||||||
| Tumor Location | 110(84,20,6) | 0.473 | ||||||
| Right | 40 | (47.6%) | 8 | (40.0%) | 2 | (33.3%) | ||
| Left | 42 | (50.0%) | 10 | (50.0%) | 4 | (66.7%) | ||
| Bilateral | 2 | (2.4%) | 2 | (10.0%) | 0 | (0%) | ||
| Tumor Stage | 109(84,20,5) | 0.608 | ||||||
| Stage 1 | 34 | (40.5%) | 9 | (45.0%) | 3 | (60.0%) | ||
| Stage 2 | 44 | (52.4%) | 8 | (40.0%) | 2 | (40.0%) | ||
| Stage 3 | 6 | (7.1%) | 3 | (15.0%) | 0 | (0%) | ||
| HER2+ by Test | 109(84,20,5) | 0.429 | ||||||
| IHC | 69 | (82.1%) | 19 | (95.0%) | 4 | (80.0%) | ||
| FISH | 8 | (9.5%) | 1 | (5.0%) | 0 | (0%) | ||
| Both | 7 | (8.3%) | 0 | (0%) | 1 | (20.0%) | ||
| Tumor ER/PR Positive | 106(82,18,6) | 0.393 | ||||||
| Neither | 32 | (39.0%) | 8 | (44.4%) | 1 | (16.7%) | ||
| ER Positive | 20 | (24.4%) | 6 | (33.3%) | 1 | (16.7%) | ||
| Both ER/PR Positive | 30 | (36.6%) | 4 | (22.2%) | 4 | (66.7%) | ||
1Based on ANOVA, Kruskal-Wallis, Chi-squared, or Fishers Exact Test
Abbreviations: SOB (shortness of breath), PND (paroxysmal nocturnal dyspnea), LE (lower extremity), IHC (immunohistochemistry), HER2 (human epidermal growth factor receptor 2), FISH (fluorescence in situ hybridization), ER/PR (estrogen receptor/progesterone receptor)
Cardiovascular and cancer directed therapies during the study period are presented in Table 2. The majority of patients (96.4%) were treated with non-anthracycline trastuzumab-based regimens. Of these, 51 (48.1%) patients were treated with dual trastuzumab and pertuzumab regimens. The majority of patients (70 (63.6%)), received radiation therapy and 34 (30.9%) had either left-sided or bilateral breast radiation. The mean radiation dose for the entire cohort was 64.8 ± 25.2 Gy. Over half of the patients received reconstructive surgery. The total number of anti-HER2 therapy cycles completed by study patients is summarized in Supplemental Table 2. Trastuzumab cycles completed were similar between arms.
Table 2.
Cardiovascular and cancer directed therapies during study period
| Variable, No. (%) | N | Arm A (N = 84) |
Randomized Arms (B + C) (N = 20) |
Arm D (N = 6) |
P-value1 | |||
|---|---|---|---|---|---|---|---|---|
| General Medication | ||||||||
| ACEi | 110(84,20,6) | 0 | (0%) | 0 | (0%) | 3 | (50.0%) | < 0.001 |
| ARB | 110(84,20,6) | 0 | (0%) | 0 | (0%) | 0 | (0%) | NA |
| Beta-blocker | 110(84,20,6) | 4 | (4.8%) | 10 | (50.0%) | 4 | (66.7%) | < 0.001 |
| Calcium channel blocker | 110(84,20,6) | 6 | (7.1%) | 1 | (5.0%) | 0 | (0%) | 0.757 |
| Thiazide diuretic | 110(84,20,6) | 3 | (3.6%) | 0 | (0%) | 0 | (0%) | 0.620 |
| Loop diuretic | 110(84,20,6) | 2 | (2.4%) | 0 | (0%) | 0 | (0%) | 0.730 |
| Aldosterone receptor antagonist | 110(84,20,6) | 2 | (2.4%) | 1 | (5.0%) | 0 | (0%) | 0.742 |
| Statin | 110(84,20,6) | 4 | (4.8%) | 3 | (15.0%) | 0 | (0%) | 0.195 |
| Low-dose daily aspirin | 110(84,20,6) | 0 | (0%) | 1 | (5.0%) | 0 | (0%) | 0.103 |
| Chemotherapy Treatment | ||||||||
| Anthracycline-based regimen | 110(84,20,6) | 2 | (2.4%) | 2 | (10.0%) | 0 | (0%) | 0.233 |
| Non-anthracycline regimen | 106(82,18,6) | 0.775 | ||||||
| TCHP | 38 | (46.3%) | 9 | (50.0%) | 3 | (50.0%) | ||
| THC | 16 | (19.5%) | 4 | (22.2%) | 2 | (33.3%) | ||
| TH weekly 12 weeks | 22 | (26.8%) | 5 | (27.8%) | 0 | (0%) | ||
| THP | 1 | (1.2%) | 0 | (0%) | 0 | (0%) | ||
| Other | 5 | (6.1%) | 0 | (0%) | 1 | (16.7%) | ||
| Breast Radiation Treatment | ||||||||
| Prior or current breast radiation treatment | 110(84,20,6) | 0.558 | ||||||
| None | 32 | (38.1%) | 7 | (35.0%) | 1 | (16.7%) | ||
| Right breast | 29 | (34.5%) | 6 | (30.0%) | 1 | (16.7%) | ||
| Left breast | 22 | (26.2%) | 7 | (35.0%) | 4 | (66.7%) | ||
| Both breasts | 1 | (1.2%) | 0 | (0%) | 0 | (0%) | ||
| Radiation Dose (current cycle), cGy, Mean ± SD | 65(49,11,5) | 6353.4 | ± 2304.5 | 7192.5 | ± 3534.2 | 6111.4 | ± 2265.2 | 0.582 |
| Breast Surgery | ||||||||
| Reconstructive surgery | 110(84,20,6) | 0.872 | ||||||
| None | 38 | (45.2%) | 9 | (45.0%) | 3 | (50.0%) | ||
| Right breast | 12 | (14.3%) | 1 | (5.0%) | 0 | (0%) | ||
| Left breast | 7 | (8.3%) | 1 | (5.0%) | 1 | (16.7%) | ||
| Both breasts | 27 | (32.1%) | 9 | (45.0%) | 2 | (33.3%) | ||
1Based on ANOVA, Kruskal-Wallis, Chi-squared, or Fishers Exact Test
Abbreviations: ACEi (Angiotensin Converting Enzyme-inhibitor), ARB (Angiotensin-receptor blocker), TCHP (Docetaxel, Carboplatin, Trastuzumab, Pertuzumab), THC (Docetaxel, Trastuzumab, Carboplatin), TH (Docetaxel, Trastuzumab), THP (Docetaxel, Trastuzumab, Pertuzumab)
Of the patients who developed CTRCD and were assigned to Arm D, 3 (50%) were symptomatic (self-reported exertional dyspnea). Of the 6 patients in Arm D who experienced significant reduction in LVEF during the study period, 4/6 (66.7%) exhibited recovery of LVEF by the end of the study period, 1/6 (16.7%) exhibited recovery of LVEF by 1 year following the end of the study period, and 1/6 (16.7%) did not exhibit LVEF recovery as of most recent follow up.
GLS stability, CTRCD, and interruption of cancer directed treatment
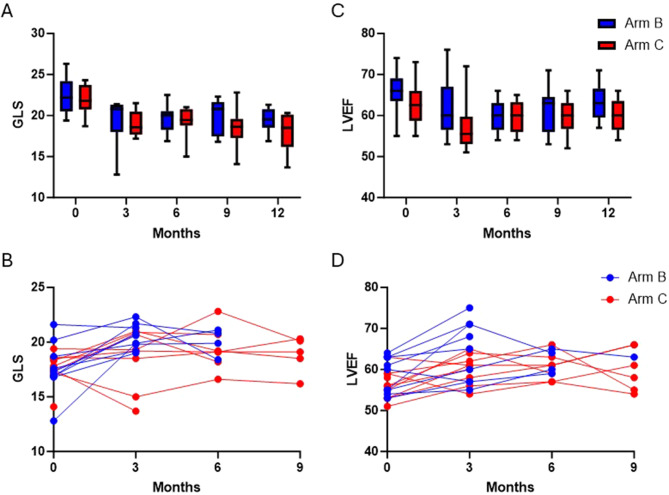
During the 1-year follow-up, 20 patients developed decreased GLS with normal LVEF and were randomized to Arms B or C. There was no significant difference in stability of GLS between Arms B and C when final GLS was compared either to baseline GLS at the start of the study or to GLS at the point of randomization (Table 3; Fig. 2A, B). No patients in either Arm B or Arm C developed CTRCD based on 2D LVEF criteria, and there were no significant differences in LVEF between these arms at any follow up timepoint (Table 3; Fig. 2C, D). One cycle of trastuzumab was held in one patient in Arm C and 10 total cycles were held between patients in Arm D (Table 3).
Table 3.
Comparison of GLS stability, CTRCD development, and anti-HER2 treatments between study arms
| Variable, No. (%) | N | Entire Cohort (N = 20) |
Group B (N = 10) |
Group C (N = 10) |
P-value1 | |||
|---|---|---|---|---|---|---|---|---|
| Stability of GLS compared to point of randomization2 | 20(10,10) | 19 | (95.0%) | 10 | (100%) | 9 | (90.0%) | 0.305 |
| Stability of GLS compared to baseline3 | 20(10,10) | 9 | (45.0%) | 6 | (60.0%) | 3 | (30.0%) | 0.178 |
| CTRCD4 | 20(10,10) | 0 | (0%) | 0 | (0%) | 0 | (0%) | NA |
| Number of Anti-HER2 Treatments, Mean ± SD | 20(10,10) | 19.4 | ± 3.80 | 20.2 | ± 4.13 | 18.5 | ± 3.44 | 0.331 |
1Based on two-sample t-test, Chi-squared, or Fishers Exact Test
2Stability of GLS (compared to randomized month) is defined as a change of less than 15% relative value by comparing patient’s end strain to strain at randomization month
3Stability of GLS (compared to baseline month) is defined as a change of less than 15% relative value by comparing patient’s end strain to strain at baseline month
4CTRCD was defined as a drop in 2D EF of > 10% from baseline and a value less than 53% at any timepoint, compared to baseline
Abbreviations: CTRCD (cancer therapy related cardiac dysfunction), HER2 (human epidermal growth factor receptor 2), GLS (global longitudinal strain)
Fig. 2.
LVEF and GLS did not significantly differ between patients in arms B and C either at baseline or at the end of follow up. (A) Box and whisker plots comparing GLS at baseline and at months 3, 6, 9, and 12 of follow up for patients randomized to arms B and C. (B) Time-series plots comparing GLS over the course of the study period for each patient randomized to arms B and C. (C) Box and whisker plots comparing LVEF at baseline and at months 3, 6, 9, and 12 of follow up for patients randomized to arms B and C. (D) Time-series plots comparing LVEF over the course of the study period for each patient randomized to arms B and C
Other echocardiographic parameters
There were no significant differences between all four arms in baseline echocardiographic parameters (Supplemental Table 3). For the entire cohort, baseline LVEF was 60.0% ± 10.0 and baseline GLS was 21.3% ± 2.30. There were no significant changes in echocardiographic parameters between Arms B and C from the point of randomization to the end of study (Table 4). In Arms B + C compared to Arm D we observed significantly higher 2D LVEF (58.0 ± 4.0% vs. 51.0 ± 3.0%, p = 0.001) and mitral inflow E velocity (0.73 m/s ± 0.14 vs. 0.57 m/s ± 0.18, p = 0.023) at the time of assignment (Supplemental Table 4). There was a significantly greater absolute change in 2D LVEF from baseline to assignment in Arm D compared to Arms B + C (-0.12 ± 0.03 vs. -0.07 ± 0.04, p = 0.006) and similarly in mitral inflow E velocity (-0.27 ± 0.24 vs. -0.05 ± 0.16, p = 0.019) (Supplemental Table 5). There were no differences in GLS, LVEDV and LVESV at the time of assignment between Arms B + C vs. D.
Table 4.
Comparison of echocardiographic parameters in study arms from assignment to end of study
| Change in Echo Parameters from End to Randomization, Mean ± SD | N | Arm B (N = 10) |
Arm C (N = 10) |
P-value1 |
|---|---|---|---|---|
| Averaged LVEDV (2 C&4 C), mL | (10,10) | 0.57 ± 19.64 | 9.99 ± 21.40 | 0.318 |
| Averaged LVESV (2 C&4 C), mL | (10,10) | -2.98 ± 7.38 | 1.01 ± 11.51 | 0.368 |
| 2D LVEF, % | (10,10) | 0.04 ± 0.04 | 0.04 ± 0.06 | 0.792 |
| RV Basal Diameter, mm | (9,10) | 0.09 ± 0.59 | 0.07 ± 0.45 | 0.926 |
| TAPSE, mm | (10,9) | 0.02 ± 0.31 | 0.10 ± 0.21 | 0.508 |
| RV S’, m/s | (9,10) | 0.01 ± 0.02 | -0.002 ± 0.02 | 0.474 |
| Mitral E, m/s | (10,10) | 0.05 ± 0.12 | 0.04 ± 0.16 | 0.850 |
| Mitral A, m/s | (10,10) | -0.05 ± 0.16 | 0.02 ± 0.17 | 0.369 |
| Mitral E/A | (10,10) | 0.19 ± 0.41 | 0.06 ± 0.45 | 0.526 |
| Mitral E/e’ (average) | (10,10) | -0.63 ± 2.22 | 0.03 ± 1.90 | 0.485 |
| 2D Circumferential Strain, % | (8,10) | -0.22 ± 4.11 | 3.20 ± 7.35 | 0.258 |
| 2D GLS, % | (10,10) | -1.84 ± 2.85 | -0.47 ± 1.92 | 0.224 |
1 Based on one-way ANOVA test
Abbreviations: LVEDV (left ventricular end-diastolic volume), LVESV (left ventricular end-systolic volume), 2D (two-dimensional), LVEF (left ventricular ejection fraction), RV (right ventricle), TAPSE (tricuspid annular plane systolic excursion), GLS (global longitudinal strain)
Discussion
This is the first prospective randomized strain-guided study of prophylactic CPT using carvedilol in early stage HER2+ breast cancer patients treated with largely non-anthracycline based regimens. We found that conventional non-anthracycline trastuzumab-based chemotherapies, including dual HER2 therapies, for breast cancer are associated with low rates of CTRCD. Furthermore, of the few patients who developed CTRCD, only 50% were symptomatic (dyspnea). Secondly, strain-guided selection of patients for CPT in this patient population did not result in significant differences in CTRCD or cycles of cancer directed therapy held. Thirdly, initiation of CPT did not result in significant changes in GLS stability compared to no treatment. From baseline to the randomization point there were changes observed in echocardiographic parameters alongside change in GLS for patients in Arms B + C, including decrease in LVEF, decrease in mitral E velocity, and increase in the average mitral E/e’ that require further study. The primary changes observed in echocardiographic parameters from baseline to randomization in Arms B + C vs. D were in LVEF and mitral E velocity. Interestingly, change in GLS from baseline to the randomization point was similar between Arms B + C vs. D.
A “one-size-fits-all” prophylactic strategy has not worked for all breast cancer patients on anthracycline and/or trastuzumab therapies [20–24]. Highest risk HER2+ breast cancer patients for development of cardiotoxicity are those who receive sequential administration of anthracycline followed by trastuzumab combination therapy [12, 14]. These patients have demonstrated benefit from prophylactic cardioprotective medications [23]. Historically, HER2+ breast cancer patients on anthracycline and trastuzumab combination sequential therapy have demonstrated subclinical LV dysfunction in GLS which predates overt changes in LVEF [6–8, 14, 25]. There is limited data, however, on HER2+ breast cancer patients who receive non-anthracycline anti-HER2-based therapies. Increasingly, these patients are the largest treatment group of HER2+ breast cancer patients. These patients generally have a lower risk for CTRCD [26]. Approximately 10% of patients without prior anthracycline will develop CTRCD at 1 year, as opposed to 25% of patients on sequential anthracycline and trastuzumab therapies [27, 28]. Non-anthracycline trastuzumab-based regimens are more commonly used as first line treatment in both early stage and metastatic HER2+ breast cancer [29, 30]. Prophylactic cardioprotective therapy for all-comer early stage HER2+ breast cancer patients who primarily received non-anthracycline HER2 regimens (78% of patients) was evaluated in the MANTICORE 101-Breast trial [22]. In this randomized double-blinded, placebo-controlled trial for prevention of cardiotoxicity, early-stage HER2+ breast cancer patients were randomized 1:1:1 to perindopril, bisoprolol or placebo and there was no difference between groups in the primary outcome of indexed LVEDVi by cardiac magnetic resonance (CMR) which represented an early change in cardiac remodeling [22]. Guglin et al. also evaluated a prophylactic cardioprotective strategy in early-stage breast cancer patients who received non-anthracycline HER2 regimens (60% of patients) in a multicenter, randomized, placebo-controlled trial [23]. Patients were randomized to lisinopril, carvedilol or placebo, and the primary outcome was LVEF ≥ 10% or LVEF ≥ 5% with absolute LVEF < 50% by echocardiogram. There was no between-group difference in patients who received the non-anthracycline regimens. Neither the MANTICORE 101-Breast trial or the Guglin et al. study risk stratified HER2+ breast cancer patients with imaging biomarkers (i.e. strain-guidance) before randomization to CPT [22, 23].
The SUCCOUR (Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes) trial compared imaging surveillance strategies in breast cancer patients who received anthracycline-based chemotherapy and had at least 1 heart failure risk factor [9, 10]. Patients were assigned to surveillance by three-dimensional (3D) LVEF vs. peak GLS. When patients met the threshold for CTRCD, guideline directed medical therapy (GDMT) was initiated. GDMT initiation was not randomized. There was no significant difference in the primary outcome of change in 3D LVEF at 1 year follow-up. The strain-guided group had a significantly greater use of CPT, and fewer patients met the criteria for CTRCD (5.8% vs. 13.7%, p = 0.02). Importantly, there was no control group who did not receive CPT in either the LVEF or GLS-guided strategies [9, 10].
In our study, we prospectively randomized patients who demonstrated subclinical LV dysfunction based on GLS during therapy to prophylactic beta-blockers. As higher risk HER2+ breast cancer patients on sequential regimens with anthracyclines followed by trastuzumab are already known to benefit from prophylactic CPT, our goal was to evaluate if GLS surveillance identified a higher risk group of HER2+ breast cancer patients receiving non-anthracycline anti-HER2 regimens who would likewise benefit from prophylactic CPT [23]. We found that prophylactic beta-blocker treatment administered at the randomization point based on GLS did not prevent more CTRCD or GLS instability, suggesting that this strategy may not be beneficial in this patient population.
Of note, strain is a load-dependent parameter which is influenced by many hemodynamic factors, including volume status, blood pressure and heart rate [31, 32]. We postulate that GLS changes in low-risk breast cancer patients do not always reflect a precursor of CTRCD, and needs to be interpreted cautiously in the context of all hemodynamic factors affecting strain values. The lack of difference in GLS between the randomization Arms (B + C) and CRTCD Arm (D), perhaps also reflects that use of one echocardiographic parameter cannot risk-stratify these patients. Combining echocardiographic measures of strain with 2D and Doppler measurements may increase our ability to detect CTRCD in HER2+ breast cancer patients [33]. Future work is needed investigating multiple imaging parameters for CTRCD risk stratification.
A major limitation of this study was that our randomization sample size was small which may not be sufficient to detect a difference in event rates and may limit our ability to generalize these results to a broader population. As a single center study which ended during the COVID-19 pandemic, there were challenges to patient enrollment which limited our ability to reach the target number of patients in the randomized Arms B and C. Furthermore, the event rate in this study was lower than initially predicted (this in and of itself is an interesting finding as noted above) which limited our ability to reach the target sample size. Another limitation of this study was an inability to perform a ‘wash-out period’ for patients who were already on beta-blockers or ACEi/ARB therapy due to the urgency of starting anti-cancer therapy. Patients were often on these medications for pre-existing hypertension or nephroprotection for diabetes mellitus. These excluded patients represented a higher risk cohort and likely affected the event rate, and therefore our ability to reach the target sample size. Additionally, the study was a single-blinded trial which did not include a placebo control for Arm C. However, all echocardiographers performing the analyses described above were fully blinded to the study arms and treatment regimens of each patient, which limited introducing bias into the analysis of follow up imaging. Patients were randomized to receive carvedilol primarily at 6, 9 and 12 months which may not have been long enough to incur benefit. Longer term follow-up or longer duration on cardioprotective therapies may reveal benefits not detected early. Therefore, our findings should be interpreted for this specific patient population. Importantly, there were no adverse effects of carvedilol in the randomization arm. There were no events that led to cessation of cancer therapy. This is reassuring for breast cancer patients who have a standard indication for this treatment. Overall, this study provides important data regarding the incidence rate of CTRCD and decreased GLS in low risk breast cancer patients as well as the utility of CPT for this patient population.
Conclusions
In this first prospective randomized strain-guided study of prophylactic carvedilol in early stage HER2+ breast cancer patients on non-anthracycline trastuzumab-based regimens, there was no significant difference in CTRCD, stability of GLS, or trastuzumab cycles held in patients who received prophylactic carvedilol therapy compared to standard of care. This patient group did not benefit from GLS surveillance or prophylactic CPT. These findings may identify a low-risk group of patients who may be considered for less intensive cardiac surveillance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ACEi
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- CPT
Cardioprotective therapy
- CTRCD
Cancer therapy-related cardiac dysfunction
- HER2
Human epidermal growth factor receptor 2
- GDMT
Guideline directed medical therapy
- GLS
Global longitudinal strain
- LVEF
Left ventricular ejection fraction
Author contributions
NA designed the study. HR consented and randomized participants. FFG, WBV, VR, SR, KC, LJ, NPP, IV, and MM collected clinical and echocardiographic data. FFG, EG, MZ, ACA, and ASB performed statistical analyses and created the figures and tables. All authors helped interpret the data and write the manuscript.
Funding
Michael Reese Faculty Foundation Grant and Abbott Laboratories Investigator Initiated Grant.
Data availability
Data is provided within the manuscript of supplementary information files. Any additional datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Northwestern University Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
SR is a consultant for Abbott Laboratories. GM is a full-time employee and shareholder of Abbott Laboratories. NA received research funding from Abbott Laboratories. The other authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gong I, Verma S, Yan AT. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat. 2016;157(3):535–44. [DOI] [PubMed] [Google Scholar]
- 2.Sardesai S, Sukumar S, Kassem M, et al. Clinical impact of interruption in adjuvant trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardiooncology. 2020;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copeland-Halperin R, Al-Sadawi M, Patil S, et al. Early trastuzumab interruption and recurrence-free survival in ERBB2-positive breast cancer. JAMA Oncol. 2020;6(12):1971–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonomou E, Kokkinidis DG, Kampaktsis PN, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4(10):1007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thavendiranathan P, Poulin F, Lim K, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25):2751–68. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26(5):493–8. [DOI] [PubMed] [Google Scholar]
- 9.Thavendiranathan P, Negishi T, Somerset E, et al. Strain-guided management of potentially cardiotoxic Cancer therapy. J Am Coll Cardiol. 2021;77(4):392–401. [DOI] [PubMed] [Google Scholar]
- 10.Negishi T, Thavendiranathan P, Penicka M, et al. Cardioprotection using strain-guided management of potentially cardiotoxic Cancer therapy: 3-Year results of the SUCCOUR Trial. JACC Cardiovasc Imaging. 2023;16(3):269–78. [DOI] [PubMed] [Google Scholar]
- 11.Thavendiranathan P, Abdel-Qadar H, Fischer HD, et al. Breast Cancer therapy-related Cardiac Dysfunction in Adult women treated in routine clinical practice: a Population-based Cohort Study. J Clin Oncol. 2016;34(19):2239–46. [DOI] [PubMed] [Google Scholar]
- 12.Narayan H, French B, Khan AM, et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain Predict Cancer therapeutics-related Cardiac Dysfunction. JACC Cardiovasc Imaging. 2016;9(10):1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slamon D, Eiermann NR, Pienkowski T, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilli M, Cipolla CM, Dent S, Minotti G, Cardinale DM. Anthracycline Cardiotoxicity in Adult Cancer patients: JACC: CardioOncology State-of-the-art review. JACC CardioOncol. 2024;6(5):655–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging. 2014;15(3). [DOI] [PubMed]
- 16.Elitok A, Fahrettin O, Cizgici AY, et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J. 2014;21(5):509–15. [DOI] [PubMed] [Google Scholar]
- 17.El-Shitany N, Tolba OA, El-Shanshory MR, et al. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail. 2012;18(8):607–13. [DOI] [PubMed] [Google Scholar]
- 18.Lang R, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. [DOI] [PubMed] [Google Scholar]
- 19.Plana J, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. [DOI] [PubMed] [Google Scholar]
- 20.Avila M, Ayub-Ferreira SM, de Barros Wanderley MR, et al. Carvedilol for Prevention of Chemotherapy-related cardiotoxicity: the CECCY Trial. J Am Coll Cardiol. 2018;71(20):2281–90. [DOI] [PubMed] [Google Scholar]
- 21.Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary Approach to Novel therapies in Cardio-Oncology Research (MANTICORE 101-Breast): a Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol. 2017;35(8):870–7. [DOI] [PubMed] [Google Scholar]
- 23.Guglin M, Kirscher J, Tamura R, et al. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in patients with breast Cancer. J Am Coll Cardiol. 2019;73(22):2859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hundley W, D’Agostino R, Crotts T et al. Statins and left ventricular ejection Fraction following Doxorubicin Treatment. NEJM Evid. 2022;1(9). [DOI] [PMC free article] [PubMed]
- 25.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 26.Armenian S, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 27.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Fernandez T, Lyon AR, Herrmann J. 2022 ESC guidelines on cardio-oncology: how can we improve the cardiovascular health of patients with cancer and cancer survivors? Eur Heart J Cardiovasc Pharmacother. 2022;9(1):4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gradishar W, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. [DOI] [PubMed] [Google Scholar]
- 30.Dent S, Moore H, Raval P, Alder L, Guha A. How to manage and monitor Cardiac Dysfunction in patients with metastatic HER2-Positive breast Cancer. JACC CardioOncol. 2022;4(3):404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voigt J, Cvijic M. 2- and 3-Dimensional myocardial strain in Cardiac Health and Disease. JACC Cardiovasc Imaging. 2019;12(9):1849–63. [DOI] [PubMed] [Google Scholar]
- 32.Ughreja A, Chow KY, Gong FF, et al. The Association between blood pressure control and Global Longitudinal Strain in patients with HER2-Positive breast Cancer on Trastuzumab-based regimens. J Am Soc Echocardiogr. 2023;36(10):1118–9. [DOI] [PubMed] [Google Scholar]
- 33.Esmaeilzadeh M, Urzua Fresno CM, Somerset E, et al. A combined Echocardiography Approach for the diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Women with early-stage breast Cancer. JAMA Cardiol. 2022;7(3):330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript of supplementary information files. Any additional datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.