Abstract
Background
Glutathione S-transferases (GSTs) are a crucial class of plant enzymes, playing pivotal roles in plant growth, development, and stress responses. However, studies on the functions and regulatory mechanisms of GSTs in plants remain relatively limited.
Results
This study aimed to comprehensively identify and analyze GST proteins in rye. A total of 171 rye GST genes were identified and classified into four subfamilies, Tau, Phi, Theta, and Zeta, based on their sequence similarity and structural features. Notably, genes classified under the Tau subfamily were the most abundant at 118, while only one gene was under the Theta subfamily. Subsequent phylogenetic and collinearity analysis revealed 29 tandem duplications and 6 segmental duplication events. There were 13 collinear genes between rye and wheat, maize, and rice, demonstrating the expansion and evolution of the GST gene family. An analysis of the expression profiles of 20 representative ScGST genes in different tissues and under various environmental stresses was performed to further understand the functions and expression patterns of ScGST genes. The results showed that these genes exhibited the highest expression levels in stems, followed by fruits and leaves.
Conclusions
This study provides a comprehensive identity, classification, and analysis of rye GST genes, which offer valuable insights into the functionality and regulatory mechanisms of the GST gene family in rye. Especially, ScGST39 was identified as a candidate gene because it was significantly upregulated under multiple stress conditions, indicating its potential crucial role in plant stress tolerance mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-11080-w.
Keywords: GST gene family, Rye, Evolution, ScGST39
Background
Glutathione S-transferases (GSTs) comprise an essential enzyme family widely distributed across plants, animals, fungi, and bacteria. They are involved in detoxifying exogenous substances, transporting secondary metabolites, and enhancing plant stress resistance. GSTs bind to harmful substances, reducing their toxicity and participating in plant detoxification [1–3]. Moreover, they are crucial carriers of anthocyanins, participating in transporting secondary metabolite [4]. GSTs protect plant cells from damage caused by biotic and abiotic stresses, such as pathogen attacks, heavy metal toxins, and ultraviolet radiation [5]. Lallement et al. classified plant GSTs into 14 families: phi (F), tau (U), theta (T), zeta (Z), lambda (L), hemerythrin (H), iota (I), ure2p, glutathionyl-hydroquinone reductase (GHR), elongation factor 1B gamma (EF1Bg), DHAR, tetrachlorohydroquinone dehalogenase (TCHQD), metaxin, and microsomal prostaglandin E synthase type-2 (mPGES-2) [6]. Dixon et al. classified plant GSTs into four families: F (Phi), U (Tau), T (Theta), and Z (Zeta) [7]. Notably, F (Phi) and U (Tau) are plant-specific and most abundant subfamilies [8]. GSTs from Arabidopsis, soybean, and poplar have broad substrate spectra, similar to GSTs involved in drug metabolism in animals [9]. This phenomenon enables them to bind various exogenous harmful substances, thereby protecting organisms from harm. Plant GSTs possess a dimeric structure formed by the non-covalent association of two subunits. Typical GSTs consist of an N-terminal and a C-terminal domain. The N-terminal domain, known as the glutathione (GSH) binding site (G-site), is highly conserved with a crucial Ser residue. It primarily catalyzes the conjugation reaction between glutathione and toxic compounds, thereby facilitating toxin degradation. The C-terminal domain constitutes the substrate-binding site (H-site) and is composed of amino acid residues from the carboxyl terminus, capable of binding various substrate [10–12].
Glutathione S-transferases (GSTs) have been extensively studied in various plants, including A. thaliana [13], O. sativa [14], Z. mays [15], G. max [15], M.pumila Mill [16]. , S. lycopersicum [17], M. sativa L [18], T. aestivum [19], C. melo L [20]. , M. nana Lour [21]. and S. italica [22]. The discovery of GSTs in animals dates back to the 1960s when they were identified to play crucial roles in the metabolism and detoxification of drugs [23]. In 1970, GSTs were first identified in maize as catalysts that aided the formation of the covalent bond between chloro-S-triazine atrazine and glutathione (GSH) [24]. This reaction contributes to the protection of maize crops against chloro-S-triazine atrazine herbicides. GSTs are expressed in various growth stages and tissues in plants [25, 26]. This report affirms that GST genes participate in detoxification responses to herbicides and play significant roles in biotic and abiotic stress responses. For instance, introducing the GmGSTL1 gene from soybean into Arabidopsis significantly enhances salt stress tolerance in Arabidopsis [27]. In the same line, expression of GsGSTU14, GsGSTU19, and GsGSTU13 genes in transgenic alfalfa positively regulates salt and alkali stress tolerance [28]. Shi et al. reported that the expression activity of the PpGST2 gene was higher than that of PpGST1 during the ripening and senescence of pear fruits. Moreover, the contents of the PpGST2 gene remained high for 140 days after fruit ripening, suggesting its involvement in pear fruit ripening. Studies postulate that salicylic acid (SA) and indole-3-acetic acid (IAA) induce the expression of PpGST1 and PpGST2 in pear fruits, which suggests that SA and IAA signals regulate these two genes during fruit development [29]. Overexpression of cotton Gst-cr1 gene and salt marsh SbGSTU gene in transgenic tobacco plants enhances oxidative and salt stress tolerance, respectively [30]. Similarly, overexpression of rice tau-class GST gene OsGSTU4 in transgenic Arabidopsis reduces sensitivity to hormones while increasing tolerance to salt and oxidative stress [31, 32]. Moreover, expression of Phi and Tau class GST genes is induced when plants are subjected to biotic or abiotic stress. The expression level of GST genes has thus become an important indicator of plant stress responses [33].
Rye is an important cereal crop cultivated extensively worldwide. It possesses robust stress adaptation capabilities, which enhances its growth in adverse environments [34]. Investigating the stress adaptation mechanisms of rye, especially those associated with the GST gene family, can aid in understanding its responses to adversities, such as drought, high temperature, low temperature, and salinity, consequently providing insights for breeding stress-tolerant varieties [1, 2]. In this study, 171 GST genes were identified in rye [35]. The genes were classified into four classes based on their homology with Arabidopsis. Their conserved motifs, gene structures, and gene duplication characteristics were subsequently analyzed. The study also revealed the phylogenetic relationships and collinearity of the GST genes between rye and Arabidopsis, soybean, wheat, maize, and rice. The expression data of 20 representative genes were further analyzed under different developmental stages, tissues, and stress conditions. In summary, this study comprehensively identified GST gene families in rye and revealed their potential roles in regulating rye development and responsing to biotic and abiotic stresses by analyzing their gene expression data. These findings provided a reference for further research on the functions and mechanisms of GST gene families.
Results
Identification of rye GST proteins and analysis of their phylogenetic relationship
Homology comparisons were conducted with Arabidopsis to identify glutathione S-transferase (GST) proteins in rye. The candidate proteins were further confirmed using the Pfam database [36] and SMART website [37]. A total of 171 rye GST genes were identified and were named ScGST1 to ScGST171 based on their chromosomal positions. This study is the first systematic investigation of the GST gene family in rye.
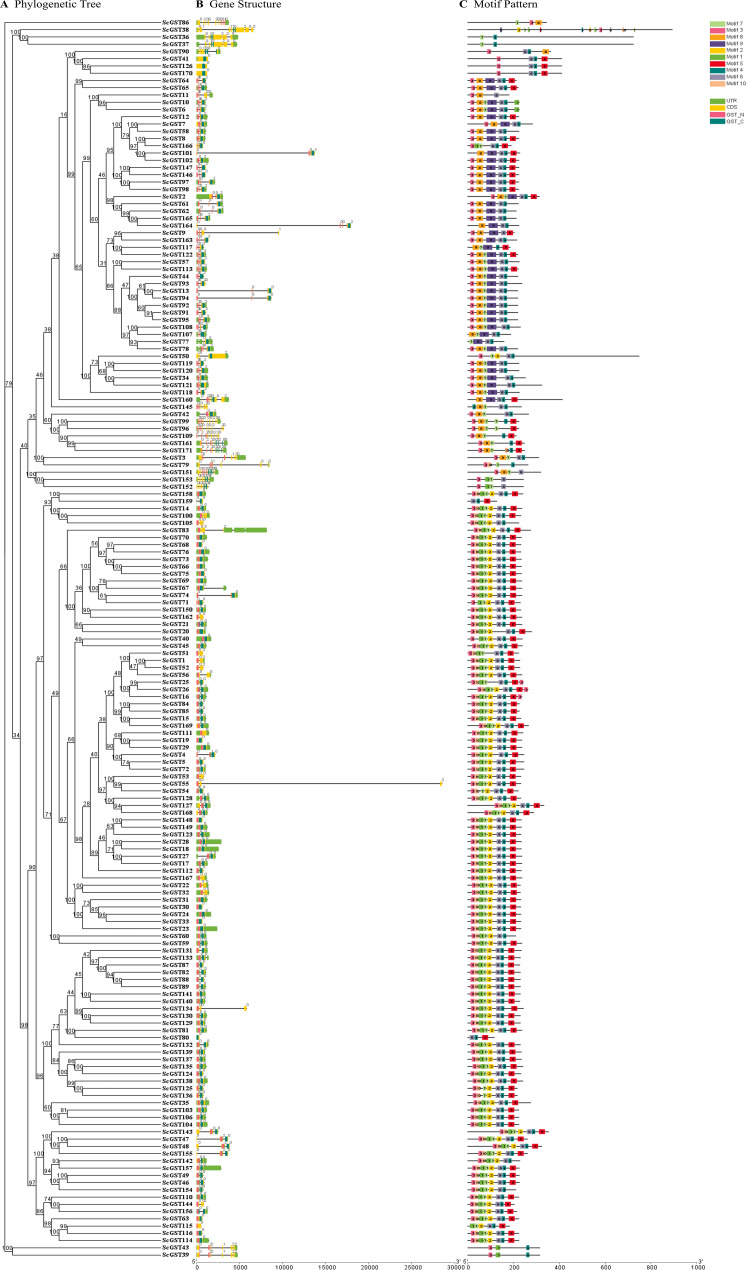
Phylogenetic analysis of the 171 ScGST proteins and subsequent construction of an NJ evolutionary tree using Mega 7.0 software was done by comparing them with 28 Arabidopsis GST proteins to gain insights into their classification and evolutionary relationships. The phylogeny results revealed that the rye GST gene family could be categorized into four subfamilies: Tau, Phi, Theta, and Zeta. Notably, most ScGST proteins were categorized into Tau and Phi subfamilies, comprising 118 and 45 proteins, respectively. Five proteins were categorized in the Zeta subfamily, while only one was in the Theta subfamily (Fig. 1; Additional files 1: Fig. S1).
Fig. 1.
An unrooted phylogenetic tree of GST genes in rye and Arabidopsis thaliana. Rye GST proteins were classified into four subfamilies based on conserved domains of the GST gene and subfamily classification in Arabidopsis. Arabidopsis GST proteins are depicted in black, while rye GST proteins are depicted in red
Further analysis of the sequence characteristics of the 171 ScGST proteins revealed that their sequence lengths ranged between 118 (ScGST80) and 891 (ScGST38) amino acid residues, with most gene lengths being within 1000 bp. The molecular weights of these proteins ranged between 13.26 and 99.36 kDa, while their isoelectric points (pI) ranged between 4.8 and 9.35, with an average pI value of 6.04 (Additional file 2: Table S1).
Conserved motifs and structural analysis of ScGST genes
The Gene Structure Display Server was used to align the cDNA and genomic DNA sequences of the ScGST genes and construct an evolutionary tree to predict the structure of the ScGST genes [38]. Notably, all genes contained the GST_N or GST_C domain, confirming their classification within the GST gene family. The GST gene family was divided into four subfamilies based on previous classifications (Fig. 2A). Herein, a comparative analysis indicated that different subfamilies exhibited distinct intron-exon structures. In the Phi subfamily, gene intron numbers ranged between 0 and 3. Genes in the Theta subfamily contained 4 introns, while those in the Zeta subfamily contained 8 introns. Notably, two genes (ScGST86 and ScGST160) were not classified into these four subfamilies but exhibited rich structural complexity. ScGST86 possessed the highest number of introns, totaling 11, whereas ScGST160 contained 7 introns. In the same line, 27 genes lacked intron structures (Fig. 2B).
Fig. 2.
Phylogenetic analysis based on conserved motifs and structures of 171 ScGST proteins. (A) Phylogenetic tree derived from the alignment of ScGST proteins. (B) Schematic representation of the ScGST gene structure, with exons depicted in yellow and labeled 0, 1, and 2, and introns depicted in gray. (C) Prediction and distribution of 10 conserved motifs within the GST protein. Each motif is represented by a distinct color
The MEME program was employed to analyze the conserved motifs to further predict the structure of ScGST proteins [39]. The analysis was set with an amino acid size range of 15 to 50. Analysis of 10 putative conserved motifs revealed that all proteins contained motifs 3 and 4, which suggested that the two motifs were the most conserved domains within the ScGST gene family. The Tau subfamily constituted the largest member count, with most proteins containing motifs 1, 2, 3, 4, 5, 6, 7, and 10. In contrast, genes in the Phi subfamily exhibited typical motifs, such as motifs 8 and 9. All genes within the Zeta subfamily contained motif 7. Notably, genes ScGST96 and ScGST99 possessed duplicated motif 7, which elicited special interest (Fig. 2C; Additional file 3: Table S2).
Chromosomal spread and gene duplication in ScGST genes
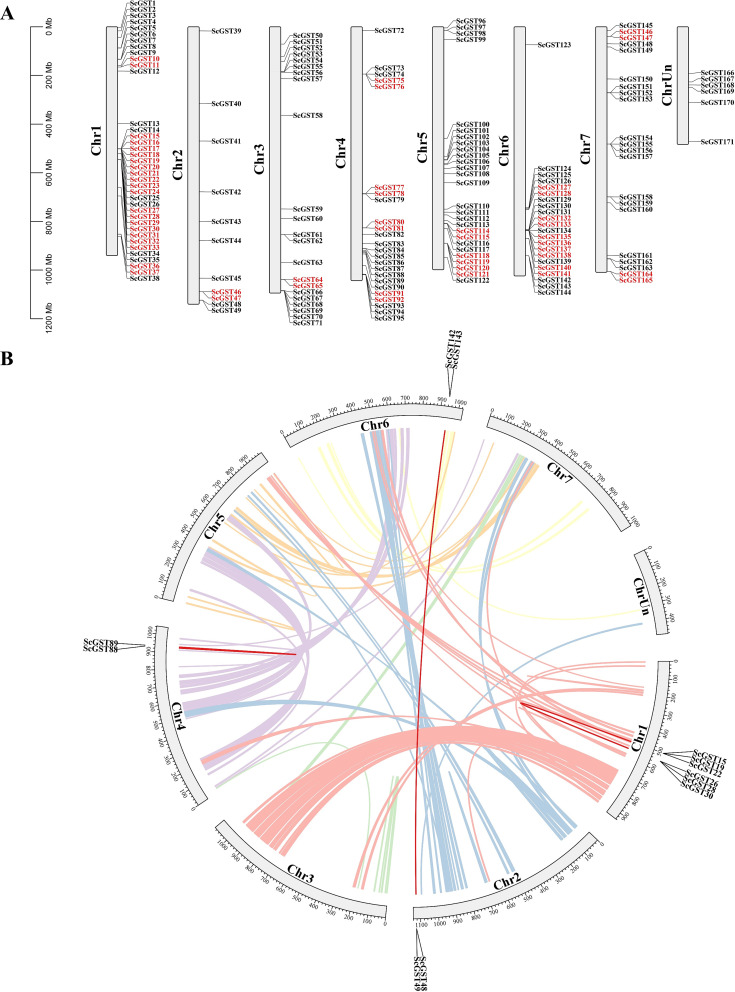
Visualization analysis of the chromosomal distribution of the 171 ScGST genes was conducted using the TBtools software [40]. Only 6 ScGST genes were not located on the seven chromosomes of rye. The remaining 165 ScGST genes were unevenly distributed across seven chromosomes. Chromosome 1 had the highest number of genes, with a total of 38 genes, while chromosome 2 harbored the fewest genes, with only 11 genes. Notably, ScGST genes on chromosome 6 were mainly concentrated at one end of the chromosome. There was no apparent pattern in distribution of ScGST genes on the other chromosomes (Fig. 3A). Furthermore, genes from different ScGST subfamilies did not exhibit specific distribution patterns on chromosomes. ScGST145 from the Theta subfamily was located on chromosome 7, while genes from other subfamilies were randomly distributed.
Fig. 3.
Analysis of chromosome distribution and repetitive events of GST genes in rye. (A) Chromosomal distribution of 171 ScGST genes across the rye genome. Each gray bar represents a different chromosome, with the number of genes indicated on the side of each bar. Genes highlighted in red denote tandem repeat genes. (B) Interchromosomal segmental repeats of GST genes in the rye genome, depicted by colored lines representing synthetic blocks within the rye genome. Red lines specifically indicate duplicate pairs among the 171 ScGST genes
Tandem repeats and segmental duplications are common repetitive sequences in the genome, playing crucial roles in expansion of gene families and genome evolution [41]. Herein, 29 pairs of tandem repeat events unevenly distributed across the seven chromosomes of rye were identified. Specifically, chromosome 1 exhibited the highest number of tandem repeat events, with a total of 12 pairs, while chromosomes 2 and 3 had the fewest tandem repeat events, with only 1 pair each (Fig. 3A; Additional file 4: Table S3). This finding suggests that chromosome 1 played the most significant role in expansion of the GST family. Moreover, the tandem repeat events predominantly occurred in the Tau and Phi subfamilies, with 21 and 8 pairs occurring in the Tau and Phi subfamilies, respectively. This finding indicated that tandem repeat events contribute more significantly to expansion of the Phi and Tau families. In the same line, there were 6 pairs of duplicated fragments in the rye GST gene family (Fig. 3B; Additional file 5: Table S4). The duplicated fragments were distributed across four chromosomes of rye. Chromosome 1 had the highest number of fragment duplication events, involving three pairs of duplicated fragments. Notably, ScGST49/ScGST142 and ScGST48/ScGST143 were fragment duplication events located on different chromosomes.
Evolutionary analysis of ScGSTgenes and GST genes from several different species
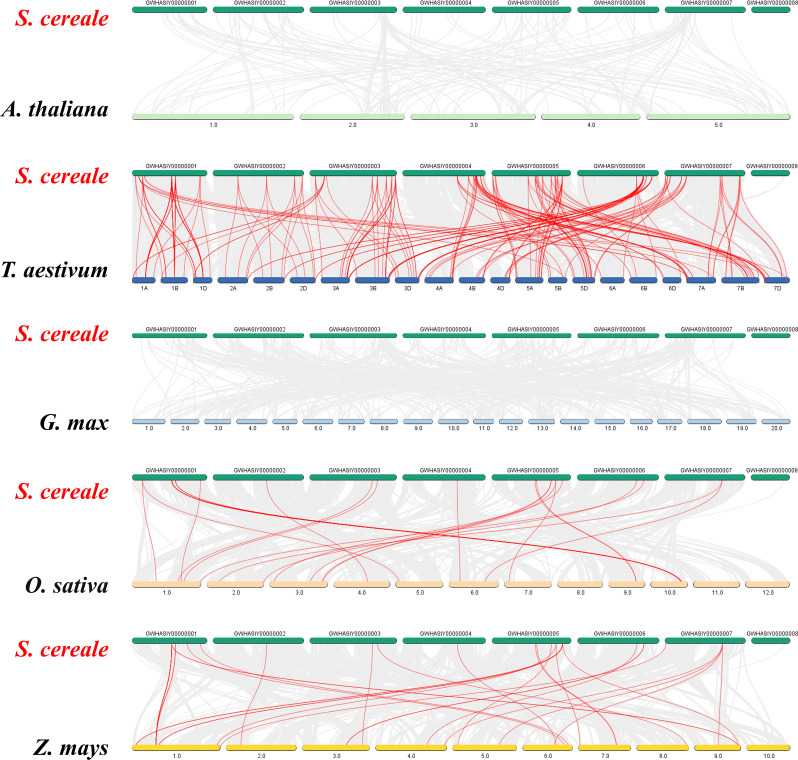
To study the evolutionary relationships between rye and wheat, maize, rice, soybean, and Arabidopsis, we constructed NJ phylogenetic trees using MEGA 7.0 software. Of note, most ScGST genes in rye were closer to GST genes in wheat, indicating a closer genetic affinity between them. Further analysis of conserved motifs revealed a relatively higher abundance of Phi and Tau subfamilies, with nearly all genes initiating with motif 4 and terminating with motif 6. The Tau subfamily exhibited unique motifs 1 and 2, while the Phi subfamily contained unique motif 9 (Fig. 4; Additional file 3: Table S2). Generally, GST proteins in different species displayed similar motif compositions. These findings formed a strong basis for studying the evolutionary relationships and gene functions among these species.
Fig. 4.
Phylogenetic analysis, gene structure, and motif distribution of GST proteins across six species: Secale cereale, Arabidopsis thaliana, Oryza sativa, Zea mays, Triticum aestivum, and Glycine max. (A) Phylogenetic evolutionary tree illustrating the relationships among GST proteins in the six species. (B) Distribution of GST protein sequence motifs, represented by colored boxes along the black line indicating protein length. Ten motifs are depicted with distinct colors
To assess the linear relationships and evolutionary history among genes in the genome, we selected two representative dicot species, including Arabidopsis thaliana and Glycine max, and three monocot species, including Zea mays, Triticum aestivum, and Oryza sativa, for inter-species collinearity analysis with rye (Secale cereale) [42, 43]. Of note, no collinear genes were identified in dicot species (Arabidopsis and soybean) (Fig. 5; Additional file 6: Table S5). In monocot species, rye exhibited the highest number of collinear genes with wheat, totaling 220 genes. Conversely, the collinear gene count with rice was the lowest, comprising 29 genes. Notably, there was a significant overlap of collinear genes between rye and wheat, maize, and rice, including ScGST14, ScGST15, ScGST22, ScGST26, ScGST38, ScGST60, ScGST61, ScGST103, ScGST106, ScGST110, ScGST114, ScGST126, and ScGST160. This finding suggested a closer genomic affinity among the four crops. Rye exhibited the highest number of collinear genes with wheat, which suggested a potential closer evolutionary relationship between them.
Fig. 5.
Synteny analysis of GST genes between rye and five representative plant species. Gray lines depict all genes, while red lines indicate collinear gene pairs
Expression patterns of ScGST genes in different plant organs
The expression patterns of 20 selected ScGST genes across different tissues and developmental stages were determined using the qRT-PCR assay to predict their roles in growth and development [44]. Generally, there were diverse expression patterns of ScGST genes across tissues. A majority of genes were upregulated, while only four genes were downregulated based on the expression levels in root tissue (Additional file 8: Table S7). ScGST118 exhibited the highest expression level, highlighting its significance in growth and development(P < 0.05). Noteworthy, six genes exhibited the highest expression levels in stems, whereas only two genes exhibited the highest expression levels in leaves. Flowers, fruits, and roots showed the highest expression levels for three, four, and five genes, respectively.
The expression patterns of ScGST genes in rye seeds at different developmental stages (7, 14, 21, 28, and 35 days) were further examined to ascertain their role at different stages. Previous studies postulate that GSTs play a crucial role in fruit development [45]. Herein, ScGST82 exhibited the highest expression level, increasing gradually with fruit development. Conversely, the expression level of ScGST72 decreased as fruit development progressed. ScGST genes displayed distinct expression patterns across different developmental stages: ScGST118 showed the most significant expression at 14 days, ScGST33, ScGST41, ScGST42, ScGST63, ScGST105, and ScGST109 exhibited the highest expression at 21 days, ScGST12, ScGST92, ScGST106, and ScGST113 peaked at 28 days, while ScGST3, ScGST39, ScGST82, and ScGST150 peaked at 35 days (P < 0.05) (Fig. 6).
Fig. 6.
Tissue-specific and fruit stage-specific gene expression patterns of 20 ScGST genes and correlations between ScGST gene expression profiles. (A) Quantitative real-time PCR (qRT-PCR) analysis depicting the expression patterns of 20 ScGST genes across various tissues including flower, leaf, root, stem, and fruit. Error bars represent standard error (n = 3). Lowercase letters denote significant differences (α = 0.05, LSD) among treatments. (B) Correlation coefficients indicating the relationships between the expression patterns of ScGST genes. Positive numbers denote positive correlations, while negative numbers indicate negative correlations. Red numbers represent statistically significant (α = 0.05) correlations. (C) qRT-PCR analysis illustrating the expression patterns of 20 ScGST genes at different developmental stages of rye fruit (7 DPA, 14 DPA, 21 DPA, 28 DPA, and 35 DPA). Error bars represent standard error (n = 3). Lowercase letters indicate significant differences (α = 0.05, LSD) among treatments. (D) Correlation analysis of gene expression profiles across different fruit developmental stages, with positive and negative correlations depicted. Red numbers indicate significant correlations (α = 0.05)
Expression patterns of ScGST genes under various treatments
The expression data of rye under NaCl, drought, ultraviolet (UV) radiation, waterlogging, high temperature, and low-temperature treatments was collated to investigate the regulation of expression of ScGST genes under various environmental stresses. Generally, most of the ScGST genes exhibited significant induction or repression under specific stress conditions. Based on the GST gene expression levels in roots as a control, most genes were upregulated under NaCl, drought, high-temperature, and low-temperature stresses but downregulated under UV and waterlogging stress (Additional file 8: Table S7).
Some genes, including ScGST3, ScGST33, ScGST82, ScGST145, ScGST150, and ScGST155, exhibited similar expression patterns in both cold and heat stress. However, ScGST12 and ScGST42 exhibited contrasting expression patterns. Under cold stress, ScGST172 exhibited the highest expression in roots but exhibited the highest expression in stems under heat stress. The majority of genes were downregulated under UV and waterlogging stress, highlighting the potential importance of roots under these stress conditions. Conversely, ScGST39, ScGST41, and ScGST42 exhibited upregulation under UV and waterlogging stress. ScGST39 and ScGST72 exhibited the highest expression under PEG and NaCl treatment, respectively (P < 0.05). In summary, these findings reveal differential expression regulation of ScGST genes under various environmental stresses. Some genes exhibited specific expression patterns under particular stress conditions (Fig. 7).
Fig. 7.
Expression analysis of 20 ScGST genes under various abiotic stresses including NaCl, PEG, UV radiation, flooding, heat, and cold stress. (A) Quantitative real-time PCR (qRT-PCR) was employed to assess the expression patterns of 20 ScGST genes under different stress conditions. Error bars represent standard errors (n = 3). Lowercase letters above the bars denote significant differences (α = 0.05, LSD) among treatments. (B) Correlation coefficients illustrating the relationships between the expression patterns of the 20 ScGST genes under stress conditions. Positive numbers indicate positive correlations, while negative numbers indicate negative correlations. Red numbers represent statistically significant correlations (α = 0.05)
Discussion
Characteristics of ScGST genes
Glutathione S-transferases (GSTs) constitute a crucial enzyme family that plays pivotal roles in plant growth, development, and response to environmental stresses. GSTs participate in various physiological and biological processes, including cellular detoxification, antioxidant reactions, hormone metabolism, and stress responses [46, 47]. To date, the GST gene family has been extensively studied and reported in various plants, such as A.thaliana, G.max, Z.mays, T. aestivum and O.sativa. However, there is a lack of comprehensive and systematic information regarding the GST gene family of rye despite it being an important economic crop.
Herein, 171 GST genes in rye, classified into four subfamilies, Tau, Phi, Theta, and Zeta, were identified following homology-based studies with Arabidopsis. Tau and Phi subfamilies harbor the largest number of GST genes unique to plants. The sequence lengths of these GST proteins ranged between 118 and 891 amino acid residues, reflecting their diversity in function and catalytic activity. Of note, approximately 92.4% of GST genes were localized in chloroplasts. Chloroplasts are crucial sites for photosynthesis. GSTs play essential roles in maintaining chloroplast function, detoxification, and combating damage from stress factors. Phylogenetic tree construction revealed the presence of a single A. thaliana GST gene in each subfamily, suggesting that ScGST genes in these subfamilies have not been lost during evolution. Interestingly, two genes (ScGST86 and ScGST120) were not classified amongst these four subfamilies, suggesting that they may represent newly evolved genes with novel functions and characteristics. The N-terminal domain of GSTs contain conserved motif 3, containing leucine and tyrosine residues, which are crucial for substrate binding and transfer reactions. Conserved motifs include the G-site and H-site, which are involved in glutathione binding and substrate transfer reactions, respectively. The C-terminal domain contains a conserved motif 4, involved in subcellular localization of GSTs and protein-protein interactions.
Herein, there was an uneven distribution of GST genes across the seven chromosomes, with the highest density of GST genes located on chromosome 1. Further analysis revealed distinct tandem repeat events among the subfamilies of the GST gene family. Specifically, 21 and 8 tandem repeat events occurred within the Tau and Phi subfamilies, respectively. This finding suggested that GST genes may have undergone gene family amplification, segmental recombination, and variation events, resulting in changes in the number and sequence of members within the subfamilies. Noteworthy, all six segmental duplication events occurred within the Tau subfamily, further indicating its significant contribution to the expansion of the GST gene family. Collinearity analysis further revealed that the majority of the 13 collinear genes shared between rye and maize, rice, and wheat were found within the Tau subfamily, which reaffirmed the importance of the Tau subfamily in genome. These collinear genes, including ScGST14, ScGST15, ScGST22, ScGST26, ScGST38, ScGST60, ScGST103, ScGST106, ScGST110, ScGST114, and ScGST126, potentially resulted from the shared evolutionary history and conserved genome structure among the monocotyledonous plants. These findings provided valuable insights for further exploring the phylogenetic relationships and gene evolution within the Poaceae family.
ScGST expression patterns and functional prediction
GST genes exert significant influence over numerous aspects of plant growth and development [46]. For instance, transgenic Arabidopsis plants expressing sense (GST-S6), antisense (GST-A4), and double-stranded (GST-DS1) BjGSTF2 RNA were generated by isolating a Phi-class mustard gene, BjGSTF2, thereby impacting plant growth and development internally. Transgenic GST-S6 plants exhibited advancement in flowering time by 2 days and displayed relatively stronger tolerance to HgCl2 and glyphosate stress. In contrast, transgenic GST-DS1 plants exhibited the poorest stress resistance, delaying flowering by 1 week [47]. Herein, ScGST150 and ScGST12, belonging to the Phi subfamily, are two genes of interest. ScGST150 exhibited the highest expression levels in stems and fruits, especially during the late ripening stage of fruit development, while ScGST12 exhibited the highest expression in flowers. Kumar et al. demonstrated the involvement of rice GSTs (OsGSTL2) in early plant growth and development by generating transgenic Arabidopsis expressing OsGSTL2 [48]. In the study, the transgenic plants cultured in MS media exhibited enhanced seedling growth, doubled germination rates, and improved cotyledon development. AtGSTU17 potentially controls the elongation of hypocotyls through the positive regulation of certain components in light signal transduction and the negative regulation of auxin-responsive genes. Moreover, AtGSTU17 negatively affects root development by downregulating an auxin transporter in the presence of ABA. AtGSTU17 is thus potentially involved in light signal transduction and regulation of various aspects of Arabidopsis development by coordinating with phyA and plant hormones, and by influencing the glutathione pool [49, 50]. ScGST63, ScGST86, and ScGST92, belonging to the Tau subfamily, exhibited relatively high expression levels in roots, implying that they are potentially involved in root regulation. Conversely, ScGST3, ScGST39, and ScGST105 exhibited higher expression levels in fruits, suggesting their involvement in fruit development. Notably, most GST genes exhibited peak expression at day 21 of plant development, implying their potential roles in regulating the maturation stage of rye.
GST genes play significant roles in response to abiotic stressors. Although there are numerous relevant GST data on Arabidopsis and rice, research on other plants remains relatively scarce. Herein, 20 ScGST genes with significant clustering differences based on the phylogenetic tree were selected to investigate their responses to six abiotic stressors across different tissues. The results revealed significant differential expression of most genes under various stress conditions. For instance, 2 ScGST genes were upregulated in the roots, 6 in the stems, and 7 in the leaves in salt-stressed rye. Similarly, 5 ScGST genes were upregulated in the roots, 6 in the stems, and 7 in the leaves in PEG-stressed rye. These findings indicated tissue-specific stress responses of ScGST genes, further highlighting their involvement in a complex regulatory network.
Tau and Phi subfamilies of GST genes have been extensively studied. In Arabidopsis, transgenic lines overexpressing AtGSTU19 exhibited higher GST activity, significantly longer average root lengths, and increased salt tolerance compared to the wild type [51]. Similarly, significant upregulation of ScGST39, ScGST45, ScGST92, ScGST105, ScGST106, ScGST109, and ScGST118, belonging to the same subfamily, might enhance salt tolerance in rye. Transgenic rice plants overexpressing OsGSTU30 exhibited improved tolerance to osmotic stress compared to the wild type. OsGSTU30 exhibited both GST and glutathione peroxidase-like activities, underscoring its role in signal transduction and catalytic activity in abiotic stress responses, which further enhanced plant resilience [52]. Herein, the Phi subfamily gene ScGST72 exhibited significant upregulation under PEG stress, suggesting its potential involvement in drought stress response. Transgenic rice plants containing endogenous Zeta subfamily GST genes exhibit higher GST and glutathione peroxidase activities, which significantly increased the germination and growth rates of seedlings under cold soaking conditions [53]. In the same line, ScGST41, a homologous gene to ScGST72, exhibited significant upregulation in roots of rye under low temperature, PEG, and NaCl stress conditions. Similarly, ScGST41 was upregulated in the leaves of UV-stressed. These findings suggested the existence of tissue-specific response mechanisms of ScGST41 under different stress conditions. Of note, ScGST39 was significantly upregulated under various stress conditions, implying its crucial role in plants and potential association with multiple stress response mechanisms. However, further studies were required to elucidate whether ScGST39 possesses unique functions within the ScGST gene family.
Conclusion
Herein, 171 ScGST genes were identified through a comprehensive analysis of glutathione S-transferase (GST) proteins in rye. These genes were designated ScGST1 to ScGST171 and classified based on their chromosomal positions. Analysis of the sequence features, structure, and conserved motifs of these genes confirmed their membership in the GST gene family, further categorizing them into four subfamilies: Tau, Phi, Theta, and Zeta. Notably, the ScGST genes exhibited diverse expression patterns in different tissues, developmental stages, and under various stress conditions. These findings provided valuable insights into the functionality and regulatory mechanisms of the GST gene family in rye, paving the way for further studies on their roles in rye physiology and stress response.
Materials & methods
Rye plant materials, growth conditions, and abiotic stress treatments
Weining rye served as the experimental material in this study. It was provided by Dr. Yu Fan from Guizhou University. It was grown for 21 days in an incubator under the following conditions: 16 h of daylight at 25 °C and 8 h of nighttime at 20 °C, with a relative humidity of 75%. Uniformly grown plants were selected for treatment after 21 days. The experiment included six different stress treatments: salt (5% NaCl), drought (30% PEG6000), ultraviolet (UV), waterlogging, high temperature (40 °C), and low temperature (4 °C) stresses. Samples were collected for analysis at 0, 2, and 12 h after each stress treatment. Moreover, samples were collected every week at different fruit development stages for 5 weeks. Samples from different tissues, including roots, stems, leaves, flowers, and fruits, were selected during the mid-term collection. Sample collection and stress treatment were conducted in uniformly grown plants in 5 replicates and 3 technical replications to ensure the reliability of the results.
Total RNA extraction, cDNA reverse transcription, and qRT‑PCR analysis
RNA was isolated from the samples using Vazyme’s RNA extraction kit according to the manufacturer’s guidelines. The extracted RNA was then reverse transcribed into cDNA using Vazyme’s reverse transcription kit for use in the subsequent qRT-PCR analysis [54]. The Beacon Designer 7 software was employed for rational primer sequence design based on the desired qRT-PCR primer characteristics (Additional file 7: Table S6). The actin gene (ScGAPDH gene) was used as the reference gene for relative quantification analysis. The expression levels of the target genes were analyzed using the delta-delta Ct (2−ΔΔCt) method [55].
Genome‑wide identification of ScGSTs in rye
Two BLAST methods [56, 57] were employed to screen the GST genes in rye. Initially, the entire genome of rye was compared to that of Arabidopsis to identify the candidate genes. Pfam [36]and HMMER 3.0 [58] software (with a threshold of 0.01) were subsequently used to eliminate the redundant genes. The CD-Search tool [59](https://www.ncbi.nlm.nih.gov/structure/bwrpsb) and SMART tool [60](http://smart.embl-heidelberg.de/) were employed to ensure the presence of GST_N and GST_C conserved domains and confirm the accuracy of the candidate genes. The identified ScGST genes were further validated using CD-Search and SMART tools. A total of 171 ScGST genes were identified following the aforementioned screening and confirmation steps. The characteristics of these genes were further evaluated by analyzing their lengths, molecular weight (MW), isoelectric point (pI), and subcellular localization of their proteins using prediction tools.
Phylogenetic analysis, classification, chromosomal distribution, and gene duplication of the ScGST gene family
GeneDoc and Mega7.0 software [61, 62] (with a bootstrap value set to 1000) were employed to construct phylogenetic trees for Arabidopsis and rye. Gene sequence data for Arabidopsis and rye were collated, followed by sequence alignment and analysis using GeneDoc software. Phylogenetic analysis and construction of phylogenetic trees for Arabidopsis and rye were subsequently conducted using Mega7.0 software. Similar methods were applied to construct phylogenetic trees for rye compared to wheat, maize, rice, soybean, and Arabidopsis to further understand the phylogenetic relationship between rye and other related species.
The exon-intron structure of the gene was analyzed using the Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn) tool [38]. The lengths and positions of exons and introns of different genes were visualized and compared using GSDS, aiding in understanding the structural characteristics of genes. The MEME online [39] program was subsequently used to analyze motif sequences within the genes. Ten motifs, with an optimal width ranging between 6 and 200 residues, were identified. The frequently occurring motif sequences in ScGST genes were also identified, aiding in the exploration of gene function and regulatory mechanisms.
The Circos program [63] was employed to map different genes to different chromosomes of rye and visualize the genomic position information of genes. The Multiple Collinearity Scan Toolkit (MCScanX) [64, 65] was subsequently used to study gene duplication events of ScGST genes. MCScanX was used to analyze the collinearity of ScGST genes in rye genome, identify gene duplication events, and explore the evolutionary history and functional conservation of gene families.
Statistical analysis
In this study, analysis of variance (ANOVA) was conducted using JMP 6.0 software [66] to assess the significance of mean differences between different treatment groups. The significance level was set at p < 0.05. The Least Significant Difference (LSD) test was subsequently employed upon detection of significant differences to further compare the data at significance levels of 0.05 and 0.01. Origin version 8.0 software was employed for scientific data analysis and visualization to facilitate statistical analysis, chart plotting, and the generation of visual representations [67]. Notably, Origin version 8.0 software aided in the interpretation and presentation of experimental data, enhancing the comprehension of the research findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all our colleagues for providing useful discussions and technical assistance. We are very grateful to the editor and reviewers for critically evaluating the manuscript and providing constructive comments for its improvement.
Abbreviations
- GSTs
Glutathione S-transferases
- ScGST
Secale cereale GST
- qRT-PCR
Quantitative real-time polymerase chain reaction
- LSD
Lowercase letters denote
- HMM
Hidden Markov Model
- pI
Isoelectric point
Author contributions
HLS: Conceptualization, Data curation, Formal analysis, Investigation. KW: Methodology, Writing-original draft. BD: Methodology, Software. YYR: Investigation, Writing-review & editing. LHH: Methodology, Validation. CZ: Investigation, Validation. SNY: Methodology. ZLL: Conceptualization, Supervision. HG: Writing-review & editing. JKD: Funding acquisition, Conceptualization, Supervision.
Funding
This research was supported by the Doctoral Program of Shaanxi Academy of Sciences (No. 2020 K-30), the Natural Science Basic Research Program of Shaanxi (No. 2023-JC-QN-0275) and Science and Technology Program of Xi’an [No.22NYGG0002].
Data availability
Data is provided within the manuscript or supplementary information files. This article does not contain any studies involving human participants or animals performed by the authors. The whole genome sequence information of was obtained from the Ensembl genome website (http://ensemblgenomes.org/). In the experiment, the rye material used was provided by Yu Fan from Guizhou University. The datasets supporting the conclusions of this study are included in the article and its additional files.
Declarations
Ethics approval and consent to participate
This article does not contain any studies involving human participants or animals performed by the authors. These methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Bio-Agriculture Institute of Shaanxi.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dixon DP, Lapthorn A, Edwards R. Plant glutathione transferases. Genome Biol. 2002;3(3):Reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Zhang D, Chen L, Wang J, Zhang WH. Genome-wide analysis of the glutathione S-Transferase family in wild Medicago ruthenica and drought-tolerant breeding application of MruGSTU39 gene in cultivated alfalfa. TAG Theoretical Appl Genet Theoretische und Angewandte Genetik. 2022;135(3):853–64. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Zhang X, Duan R, Han C, Yang J, Wang L, Wang S, Su Y, Wang L, Dong Y et al. Genomic analysis of the glutathione S-Transferase family in Pear (Pyrus communis) and functional identification of PcGST57 in Anthocyanin Accumulation. Int J Mol Sci 2022, 23(2). [DOI] [PMC free article] [PubMed]
- 4.Liu Y, Jiang H, Zhao Y, Li X, Dai X, Zhuang J, Zhu M, Jiang X, Wang P, Gao L, et al. Three Camellia sinensis glutathione S-transferases are involved in the storage of anthocyanins, flavonols, and proanthocyanidins. Planta. 2019;250(4):1163–75. [DOI] [PubMed] [Google Scholar]
- 5.Mo Z, Huang Y, Pu T, Duan L, Pi K, Luo J, Long B, Lu A, Liu R. Genome-wide identification and characterization of glutathione S-Transferases (GSTs) and their expression profile under abiotic stresses in tobacco (Nicotiana tabacum L). BMC Genomics. 2023;24(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallement PA, Meux E, Gualberto JM, Prosper P, Didierjean C, Saul F, Haouz A, Rouhier N, Hecker A. Structural and enzymatic insights into Lambda glutathione transferases from Populus trichocarpa, monomeric enzymes constituting an early divergent class specific to terrestrial plants. Biochem J. 2014;462(1):39–52. [DOI] [PubMed] [Google Scholar]
- 7.Dixon DP, Cummins L, Cole DJ, Edwards R. Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol. 1998;1(3):258–66. [DOI] [PubMed] [Google Scholar]
- 8.Dixon DP, Davis BG, Edwards R. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J Biol Chem. 2002;277(34):30859–69. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Pang Y, Zhong Y, Cai Z, Ma Q, Wen K, Nian H. GmGSTU23 encoding a tau class glutathione S-Transferase protein enhances the salt tolerance of soybean (Glycine max L). Int J Mol Sci 2023, 24(6). [DOI] [PMC free article] [PubMed]
- 10.Board PG. The omega-class glutathione transferases: structure, function, and genetics. Drug Metab Rev. 2011;43(2):226–35. [DOI] [PubMed] [Google Scholar]
- 11.Salinas AE, Wong MG. Glutathione S-transferases–a review. Curr Med Chem. 1999;6(4):279–309. [PubMed] [Google Scholar]
- 12.Labrou NE, Papageorgiou AC, Pavli O, Flemetakis E. Plant GSTome: structure and functional role in xenome network and plant stress response. Curr Opin Biotechnol. 2015;32:186–94. [DOI] [PubMed] [Google Scholar]
- 13.Edwards R, Dixon DP. Plant glutathione transferases. Methods Enzymol. 2005;401:169–86. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Dubey RS, Trivedi PK. Differential expression of Rice Lambda Class GST Gene Family members during Plant Growth, Development, and in response to stress conditions. Plant Mol Biology Report. 2013;31(3):569–80. [Google Scholar]
- 15.McGonigle B, Keeler SJ, Lau SM, Koeppe MK, O’Keefe DP. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 2000;124(3):1105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao YW, Wang CK, Huang XY, Hu DG. Genome-wide analysis of the glutathione S-Transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of Anthocyanin Accumulation in Apple. Genes 2021, 12(11). [DOI] [PMC free article] [PubMed]
- 17.Islam S, Rahman IA, Islam T, Ghosh A. Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: gaining an insight to their physiological and stress-specific roles. PLoS ONE. 2017;12(11):e0187504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han XM, Yang ZL, Liu YJ, Yang HL, Zeng QY. Genome-wide profiling of expression and biochemical functions of the Medicago glutathione S-transferase gene family. Plant Physiol Biochemistry: PPB. 2018;126:126–33. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Ma J, Zhang Q, Wu C, Zhao H, Wu Y, Yang G, He G. Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L). BMC Genomics. 2019;20(1):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Zhang Z, Wu J, Han X, Wang-Pruski G, Zhang Z. Genome-wide identification, characterization, and expression analysis related to autotoxicity of the GST gene family in Cucumis melo L. Plant Physiol Biochemistry: PPB. 2020;155:59–69. [DOI] [PubMed] [Google Scholar]
- 21.Vaish S, Parveen R, Gupta D, Basantani MK. Genome-wide identification and characterization of glutathione S-transferase gene family in Musa acuminata L. AAA group and gaining an insight to their role in banana fruit development. J Appl Genet. 2022;63(4):609–31. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Fu H, Zhao J, Wang J, Dong S, Yuan X, Li X, Chen M. Genome-wide identification and expression profiling of glutathione S-Transferase Gene Family in Foxtail Millet (Setaria italica L). Plants (Basel Switzerland) 2023, 12(5). [DOI] [PMC free article] [PubMed]
- 23.Wilce MC, Parker MW. Structure and function of glutathione S-transferases. Biochim Biophys Acta. 1994;1205(1):1–18. [DOI] [PubMed] [Google Scholar]
- 24.Shimabukuro RH, Swanson HR, Walsh WC. Glutathione conjugation: atrazine detoxication mechanism in corn. Plant Physiol. 1970;46(1):103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallé Á, Czékus Z, Bela K, Horváth E, Ördög A, Csiszár J, Poór P. Plant glutathione transferases and light. Front Plant Sci 2018, 9:1944. [DOI] [PMC free article] [PubMed]
- 26.Vaish S, Gupta D, Mehrotra R, Mehrotra S, Basantani MK. Glutathione S-transferase: a versatile protein family. 3 Biotech. 2020;10(7):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan C, Lam HM. A putative lambda class glutathione S-transferase enhances plant survival under salinity stress. Plant Cell Physiol. 2014;55(3):570–9. [DOI] [PubMed] [Google Scholar]
- 28.Jia B, Sun M, Sun X, Li R, Wang Z, Wu J, Wei Z, DuanMu H, Xiao J, Zhu Y. Overexpression of GsGSTU13 and SCMRP in Medicago sativa confers increased salt-alkaline tolerance and methionine content. Physiol Plant. 2016;156(2):176–89. [DOI] [PubMed] [Google Scholar]
- 29.Shi HY, Li ZH, Zhang YX, Chen L, Xiang DY, Zhang YF. Two pear glutathione S-transferases genes are regulated during fruit development and involved in response to salicylic acid, auxin, and glucose signaling. PLoS ONE. 2014;9(2):e89926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha B, Sharma A, Mishra A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol Biol Rep. 2011;38(7):4823–32. [DOI] [PubMed] [Google Scholar]
- 31.Moons A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal- and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003;553(3):427–32. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Sahoo A, Devendran R, Jain M. Over-expression of a rice tau class glutathione s-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS ONE. 2014;9(3):e92900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon DP, Edwards R. Glutathione transferases. arabidopsis book. 2010;8:e0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur P, Singh Sandhu K, Singh Purewal S, Kaur M, Kumar Singh S. Rye: a wonder crop with industrially important macromolecules and health benefits. Food Res Int (Ottawa Ont). 2021;150(Pt A):110769. [DOI] [PubMed] [Google Scholar]
- 35.Rabanus-Wallace MT, Hackauf B, Mascher M, Lux T, Wicker T, Gundlach H, Baez M, Houben A, Mayer KFX, Guo L, et al. Chromosome-scale genome assembly provides insights into rye biology, evolution and agronomic potential. Nat Genet. 2021;53(4):564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren Y, Ma R, Fan Y, Zhao B, Cheng P, Fan Y, Wang B. Genome-wide identification and expression analysis of the SPL transcription factor family and its response to abiotic stress in Quinoa (Chenopodium quinoa). BMC Genomics. 2022;23(1):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo AY, Zhu QH, Chen X, Luo JC. [GSDS: a gene structure display server]. Yi Chuan = Hereditas. 2007;29(8):1023–6. [PubMed] [Google Scholar]
- 39.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–208. [DOI] [PMC free article] [PubMed]
- 40.Schlegel R. 100 years of chromosome research in Rye, Secale L. Plants (Basel Switzerland) 2022, 11(13). [DOI] [PMC free article] [PubMed]
- 41.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer E, Schmutzer T, Barilar I, Mascher M, Gundlach H, Martis MM, Twardziok SO, Hackauf B, Gordillo A, Wilde P, et al. Towards a whole-genome sequence for rye (Secale cereale L). Plant Journal: Cell Mol Biology. 2017;89(5):853–69. [DOI] [PubMed] [Google Scholar]
- 43.Martis MM, Zhou R, Haseneyer G, Schmutzer T, Vrána J, Kubaláková M, König S, Kugler KG, Scholz U, Hackauf B, et al. Reticulate evolution of the rye genome. Plant Cell. 2013;25(10):3685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jozefczuk J, Adjaye J. Quantitative real-time PCR-based analysis of gene expression. Methods Enzymol. 2011;500:99–109. [DOI] [PubMed] [Google Scholar]
- 45.Cao Q, Lv W, Jiang H, Chen X, Wang X, Wang Y. Genome-wide identification of glutathione S-transferase gene family members in tea plant (Camellia sinensis) and their response to environmental stress. Int J Biol Macromol. 2022;205:749–60. [DOI] [PubMed] [Google Scholar]
- 46.Gong H, Jiao Y, Hu WW, Pua EC. Expression of glutathione-S-transferase and its role in plant growth and development in vivo and shoot morphogenesis in vitro. Plant Mol Biol. 2005;57(1):53–66. [DOI] [PubMed] [Google Scholar]
- 47.Moons A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitam Horm. 2005;72:155–202. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Dubey RS, Trivedi PK. Expression of a rice Lambda class of glutathione S-transferase, OsGSTL2, in Arabidopsis provides tolerance to heavy metal and other abiotic stresses. J Hazard Mater. 2013;248–249:228–37. [DOI] [PubMed] [Google Scholar]
- 49.Jiang HW, Liu MJ, Chen IC, Huang CH, Chao LY, Hsieh HL. A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010;154(4):1646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012;158(1):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Tian YS, Xing XJ, Peng RH, Zhu B, Gao JJ, Yao QH. Over-expression of AtGSTU19 provides tolerance to salt, drought and methyl viologen stresses in Arabidopsis. Physiol Plant. 2016;156(2):164–75. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava D, Verma G, Chauhan AS, Pande V, Chakrabarty D. Rice (Oryza sativa L.) tau class glutathione S-transferase (OsGSTU30) overexpression in Arabidopsis thaliana modulates a regulatory network leading to heavy metal and drought stress tolerance. Metallomics: Integr Biometal Sci. 2019;11(2):375–89. [DOI] [PubMed] [Google Scholar]
- 53.Zhao FY, Wang XY, Zhao YX, Zhang H. [Transferring the Suaeda salsa glutathione S-transferase and catalase genes enhances low temperature stress resistance in transgenic rice seedlings]. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue bao = J Plant Physiol Mol Biology. 2006;32(2):231–8. [PubMed] [Google Scholar]
- 54.Ren Y, Ma R, Xie M, Fan Y, Feng L, Chen L, Yang H, Wei X, Wang X, Liu K, et al. Genome-wide identification, phylogenetic and expression pattern analysis of HSF family genes in the Rye (Secale cereale L). BMC Plant Biol. 2023;23(1):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif). 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 56.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu M, Ma Z, Wang A, Zheng T, Huang L, Sun W, Zhang Y, Jin W, Zhan J, Cai Y et al. Genome-wide investigation of the Auxin Response Factor Gene Family in Tartary Buckwheat (Fagopyrum tataricum). Int J Mol Sci 2018, 19(11). [DOI] [PMC free article] [PubMed]
- 58.Sun H, Ren M, Zhang J. Genome-wide identification and expression analysis of fibrillin (FBN) gene family in tomato (Solanum lycopersicum L). PeerJ. 2022;10:e13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–331. [DOI] [PMC free article] [PubMed]
- 60.Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46(D1):D493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, Hou R, Zhang MF, Shang JX. First report of Fusarium commune causing root rot of blueberry in Guizhou Province, China. Plant disease 2022.
- 62.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martínez-García JF, Bilbao-Castro JR, Robertson DL. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, Poplar, rice, moss, and algae. Plant Physiol. 2010;153(3):1398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Li J, Paterson AH. MCScanX-transposed: detecting transposed gene duplications based on multiple colinearity scans. Bioinf (Oxford England). 2013;29(11):1458–60. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan Y, Wei X, Lai D, Yang H, Feng L, Li L, Niu K, Chen L, Xiang D, Ruan J, et al. Genome-wide investigation of the GRAS transcription factor family in foxtail millet (Setaria italica L). BMC Plant Biol. 2021;21(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan Y, Yang H, Lai D, He A, Xue G, Feng L, Chen L, Cheng XB, Ruan J, Yan J, et al. Genome-wide identification and expression analysis of the bHLH transcription factor family and its response to abiotic stress in sorghum [Sorghum bicolor (L.) Moench]. BMC Genomics. 2021;22(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files. This article does not contain any studies involving human participants or animals performed by the authors. The whole genome sequence information of was obtained from the Ensembl genome website (http://ensemblgenomes.org/). In the experiment, the rye material used was provided by Yu Fan from Guizhou University. The datasets supporting the conclusions of this study are included in the article and its additional files.