Abstract
1. The circadian pacemaker regulates the timing, structure and consolidation of human sleep. The extent to which this pacemaker affects electroencephalographic (EEG) activity during sleep remains unclear. 2. To investigate this, a total of 1.22 million power spectra were computed from EEGs recorded in seven men (total, 146 sleep episodes; 9 h 20 min each) who participated in a one-month-long protocol in which the sleep-wake cycle was desynchronized from the rhythm of plasma melatonin, which is driven by the circadian pacemaker. 3. In rapid eye movement (REM) sleep a small circadian variation in EEG activity was observed. The nadir of the circadian rhythm of alpha activity (8.25-10.5 Hz) coincided with the end of the interval during which plasma melatonin values were high, i.e. close to the crest of the REM sleep rhythm. 4. In non-REM sleep, variation in EEG activity between 0.25 and 11.5 Hz was primarily dependent on prior sleep time and only slightly affected by circadian phase, such that the lowest values coincided with the phase of melatonin secretion. 5. In the frequency range of sleep spindles, high-amplitude circadian rhythms with opposite phase positions relative to the melatonin rhythm were observed. Low-frequency sleep spindle activity (12.25-13.0 Hz) reached its crest and high-frequency sleep spindle activity (14.25-15.5 Hz) reached its nadir when sleep coincided with the phase of melatonin secretion. 6. These data indicate that the circadian pacemaker induces changes in EEG activity during REM and non-REM sleep. The changes in non-REM sleep EEG spectra are dissimilar from the spectral changes induced by sleep deprivation and exhibit a close temporal association with the melatonin rhythm and the endogenous circadian phase of sleep consolidation.
Full text
PDF


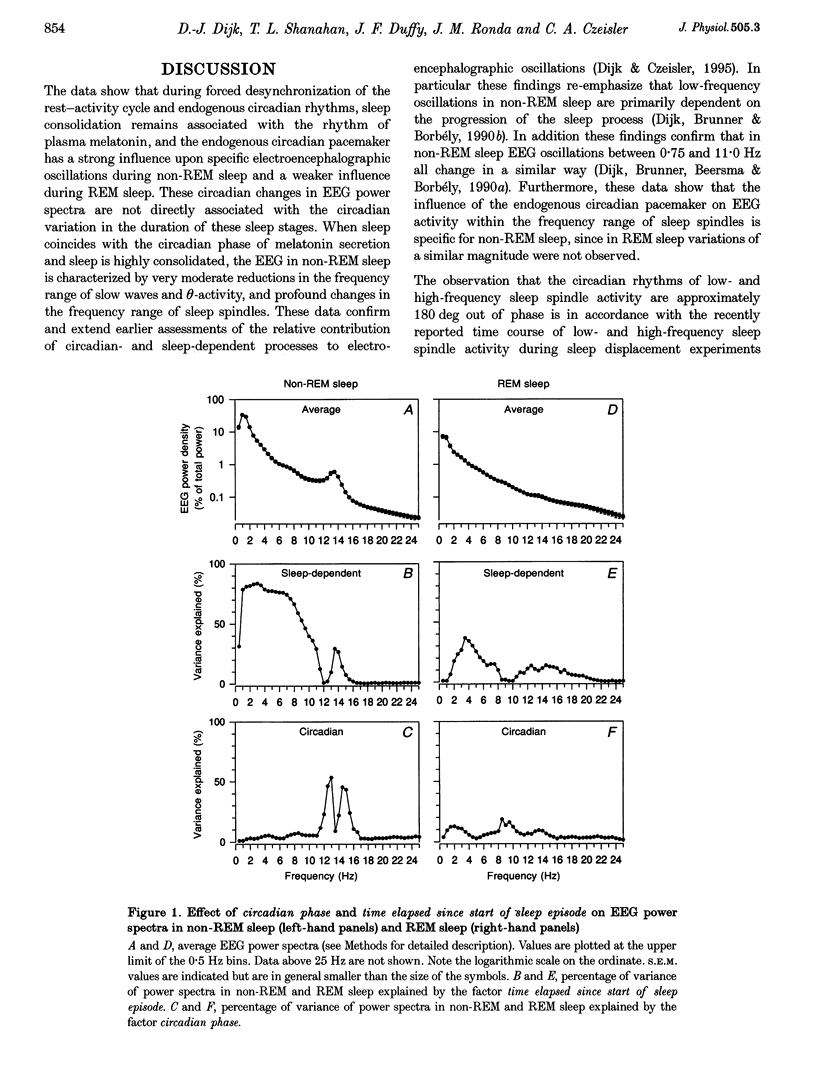
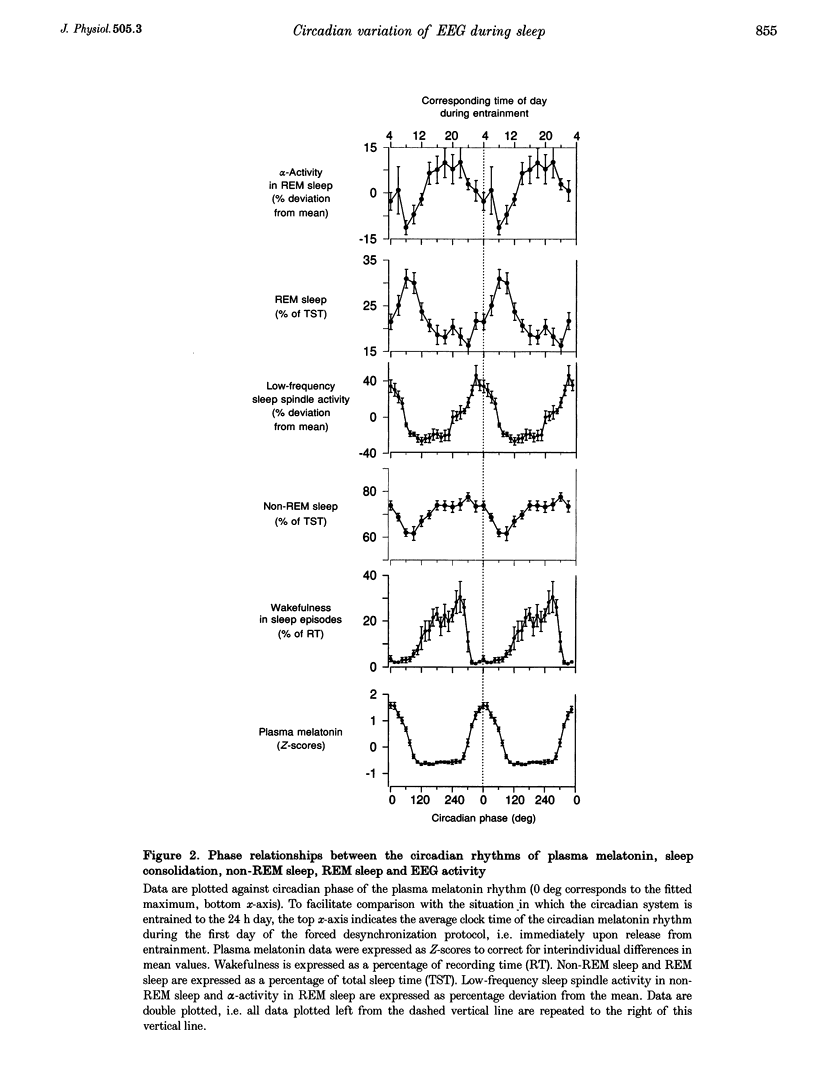
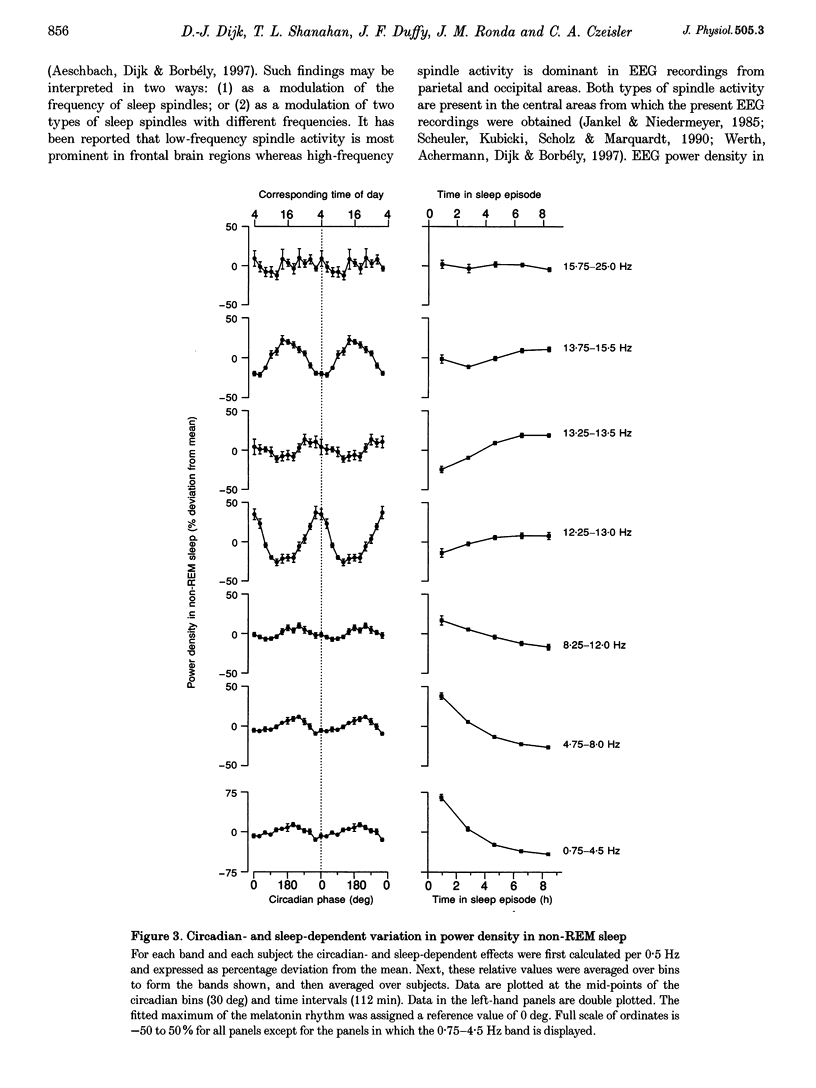

Selected References
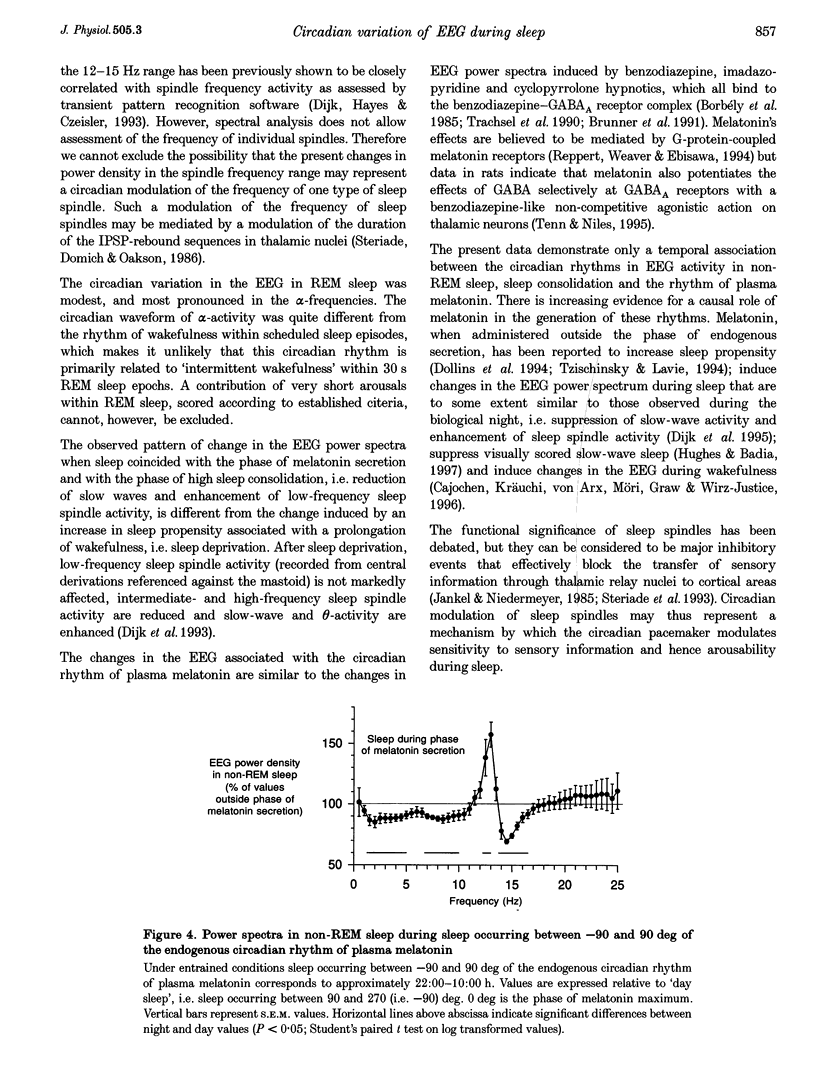
These references are in PubMed. This may not be the complete list of references from this article.
- Aeschbach D., Dijk D. J., Borbély A. A. Dynamics of EEG spindle frequency activity during extended sleep in humans: relationship to slow-wave activity and time of day. Brain Res. 1997 Feb 14;748(1-2):131–136. doi: 10.1016/s0006-8993(96)01275-9. [DOI] [PubMed] [Google Scholar]
- Borbély A. A., Mattmann P., Loepfe M., Strauch I., Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4(3):189–194. [PubMed] [Google Scholar]
- Brown E. N., Czeisler C. A. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992 Fall;7(3):177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Brunner D. P., Dijk D. J., Münch M., Borbély A. A. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104(1):1–5. doi: 10.1007/BF02244546. [DOI] [PubMed] [Google Scholar]
- Cajochen C., Kräuchi K., von Arx M. A., Möri D., Graw P., Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996 Apr 5;207(3):209–213. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- Dijk D. J., Brunner D. P., Beersma D. G., Borbély A. A. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990 Oct;13(5):430–440. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- Dijk D. J., Brunner D. P., Borbély A. A. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990 Mar;258(3 Pt 2):R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- Dijk D. J., Czeisler C. A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995 May;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D. J., Czeisler C. A. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994 Jan 17;166(1):63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk D. J., Hayes B., Czeisler C. A. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993 Oct 29;626(1-2):190–199. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- Hughes R. J., Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997 Feb;20(2):124–131. [PubMed] [Google Scholar]
- Jankel W. R., Niedermeyer E. Sleep spindles. J Clin Neurophysiol. 1985 Jan;2(1):1–35. doi: 10.1097/00004691-198501000-00001. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994 Nov;13(5):1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Shanahan T. L., Czeisler C. A. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab. 1991 Aug;73(2):227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986 Jan;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Tenn C. C., Niles L. P. Central-type benzodiazepine receptors mediate the antidopaminergic effect of clonazepam and melatonin in 6-hydroxydopamine lesioned rats: involvement of a GABAergic mechanism. J Pharmacol Exp Ther. 1995 Jul;274(1):84–89. [PubMed] [Google Scholar]
- Trachsel L., Dijk D. J., Brunner D. P., Klene C., Borbély A. A. Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology. 1990 Feb;3(1):11–18. [PubMed] [Google Scholar]
- Tzischinsky O., Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994 Oct;17(7):638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]


