Abstract
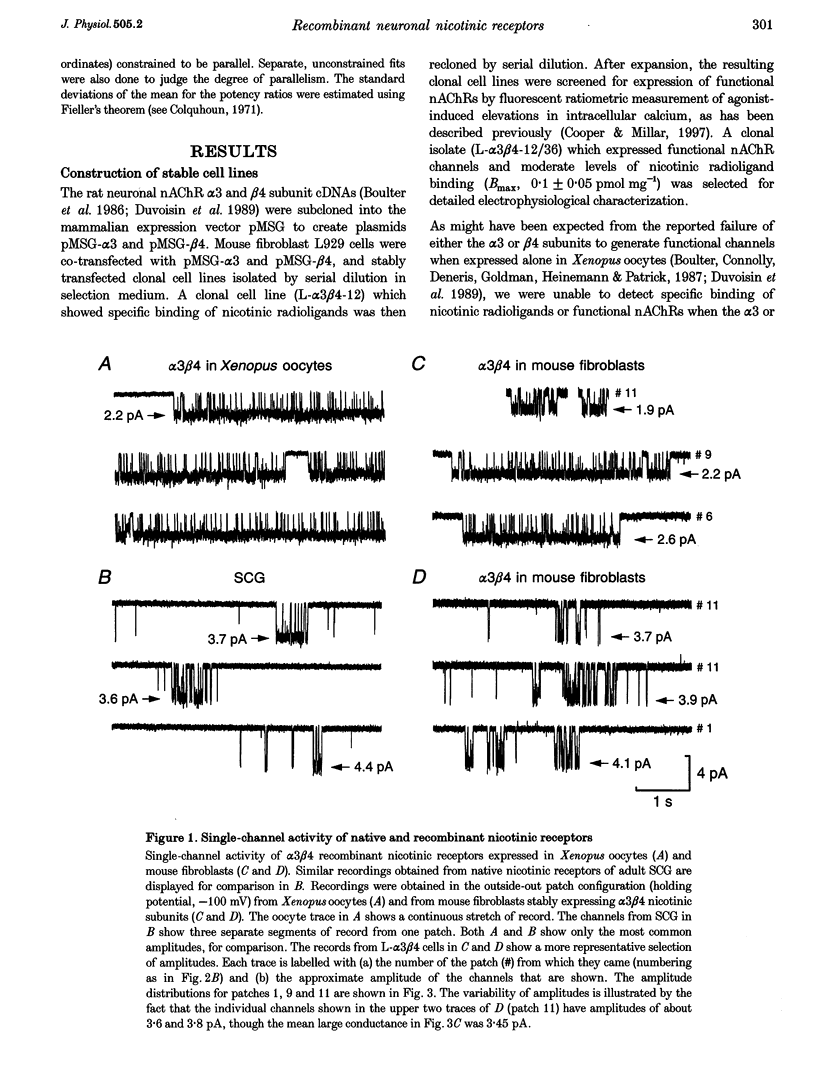
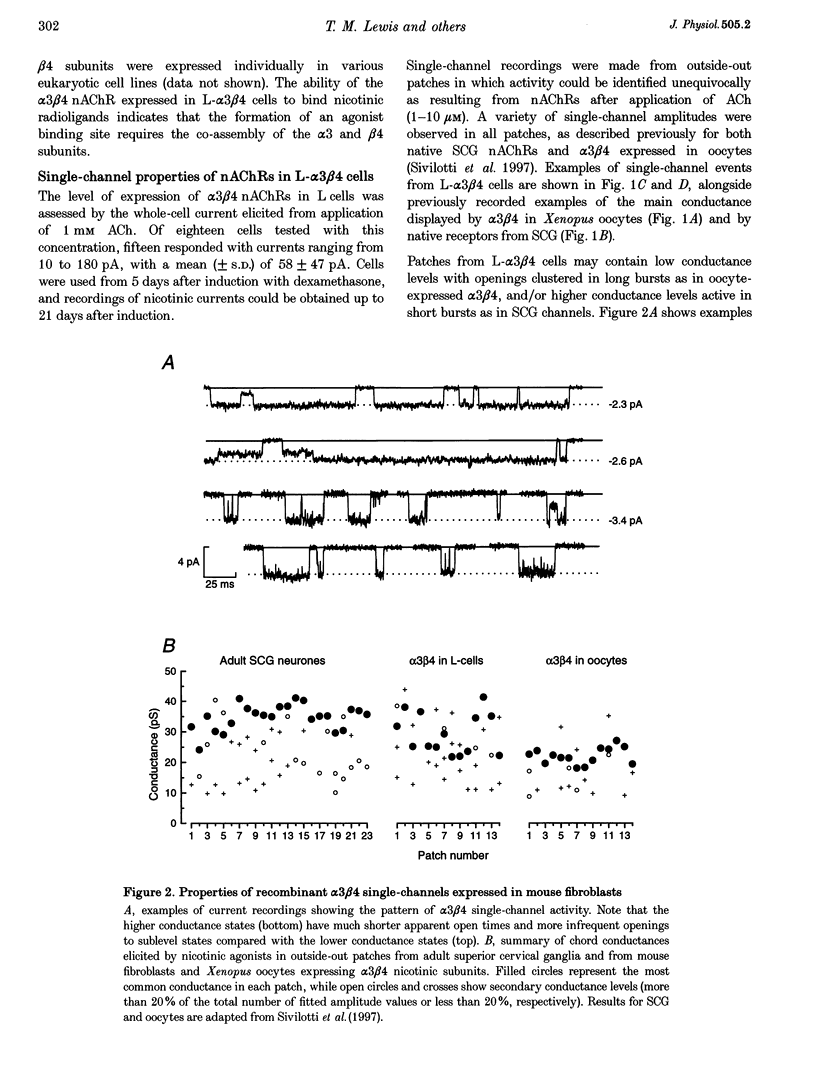
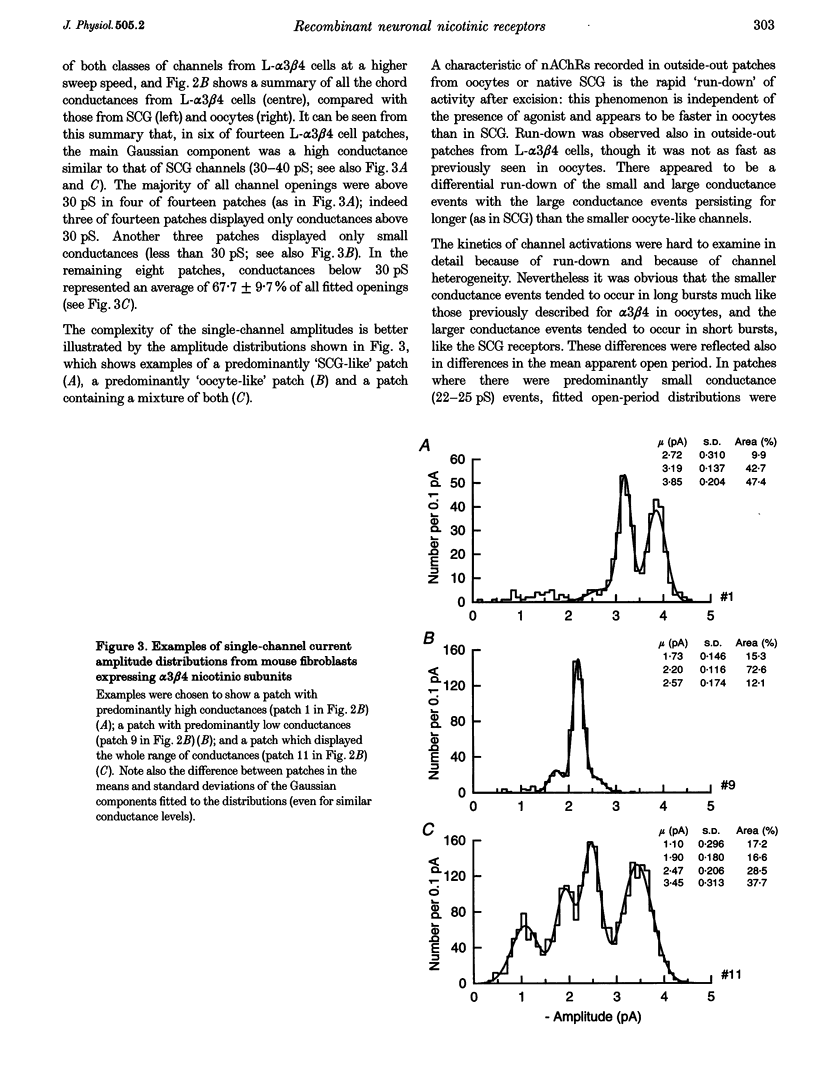
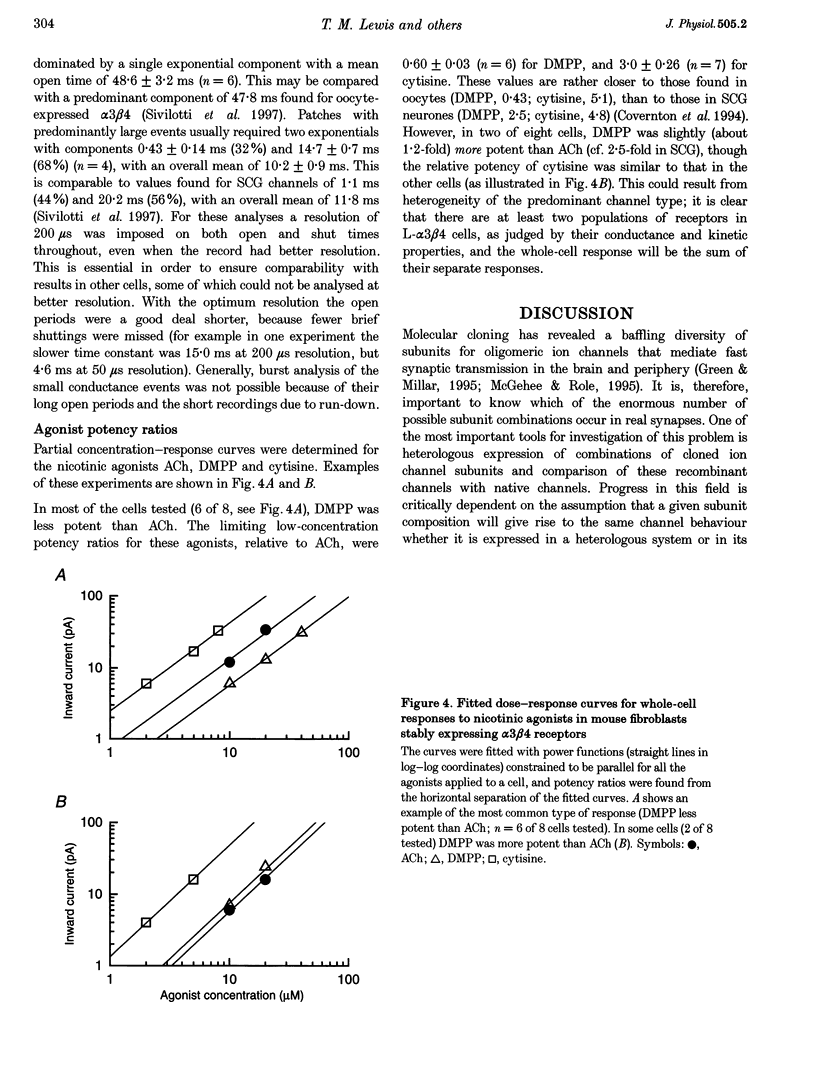
1. A stable mammalian cell line (L-alpha 3 beta 4) has been established which expresses the cloned rat neuronal nicotinic acetylcholine receptor (nAChR) subunits alpha 3 and beta 4, which are the most abundant in autonomic ganglia. Ion channel properties of nAChRs expressed in L-alpha 3 beta 4 cells were investigated by single-channel and whole-cell recording techniques, and compared with both rat alpha 3 beta 4 nAChRs expressed in Xenopus oocytes, and endogenous nicotinic receptors in rat superior cervical ganglion (SCG) neurones, using identical solutions for all cell types. 2. Acetylcholine (ACh) caused activation of single ion channel currents with a range of amplitudes. Some channels had high conductances (30-40 pS), and relatively brief lifetimes; these resembled the predominant native channel from SCG. Other channels had low conductances (20-26 pS) and long bursts of openings which were quite unlike native channels, but which were similar to channels formed by alpha 3 beta 4 in oocytes. Both types often occurred in the same patch. 3. Cytisine was about 3 times more potent than ACh (low-concentration potency ratio) in L-alpha 3 beta 4 cells, which is not dissimilar to the 5-fold potency ratio found in both SCG and oocytes, whereas 1,1-dimethyl-4-phenylpiperazinium (DMPP) was less potent than ACh in some cells (as in the oocyte), but more potent in others (as in SCG). 4. While the channels expressed in L-alpha 3 beta 4 cells do not mimic exactly those expressed in rat SCG, they differ considerably from the same subunit combination expressed in oocytes. Larger conductance, SCG-like channels were detected frequently in L-alpha 3 beta 4, but were rarely, if ever, seen in oocytes injected with alpha 3 and beta 4 mRNA. Our results indicate that ion channel properties such as single-channel conductance can be influenced by the choice of heterologous expression system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. T., Millar N. S. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor alpha7 subunit. J Neurochem. 1997 May;68(5):2140–2151. doi: 10.1046/j.1471-4159.1997.68052140.x. [DOI] [PubMed] [Google Scholar]
- Covernton P. J., Kojima H., Sivilotti L. G., Gibb A. J., Colquhoun D. Comparison of neuronal nicotinic receptors in rat sympathetic neurones with subunit pairs expressed in Xenopus oocytes. J Physiol. 1994 Nov 15;481(Pt 1):27–34. doi: 10.1113/jphysiol.1994.sp020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin R. M., Deneris E. S., Patrick J., Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989 Oct;3(4):487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Green W. N., Millar N. S. Ion-channel assembly. Trends Neurosci. 1995 Jun;18(6):280–287. [PubMed] [Google Scholar]
- Lansdell S. J., Schmitt B., Betz H., Sattelle D. B., Millar N. S. Temperature-sensitive expression of Drosophila neuronal nicotinic acetylcholine receptors. J Neurochem. 1997 May;68(5):1812–1819. doi: 10.1046/j.1471-4159.1997.68051812.x. [DOI] [PubMed] [Google Scholar]
- Mandelzys A., De Koninck P., Cooper E. Agonist and toxin sensitivities of ACh-evoked currents on neurons expressing multiple nicotinic ACh receptor subunits. J Neurophysiol. 1995 Sep;74(3):1212–1221. doi: 10.1152/jn.1995.74.3.1212. [DOI] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. J Physiol. 1991 Aug;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D. S., Role L. W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog Neurobiol. 1993 Oct;41(4):509–531. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Ragozzino D., Fucile S., Giovannelli A., Grassi F., Mileo A. M., Ballivet M., Alemà S., Eusebi F. Functional properties of neuronal nicotinic acetylcholine receptor channels expressed in transfected human cells. Eur J Neurosci. 1997 Mar;9(3):480–488. doi: 10.1111/j.1460-9568.1997.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Rust G., Burgunder J. M., Lauterburg T. E., Cachelin A. B. Expression of neuronal nicotinic acetylcholine receptor subunit genes in the rat autonomic nervous system. Eur J Neurosci. 1994 Mar 1;6(3):478–485. doi: 10.1111/j.1460-9568.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Sivilotti L. G., McNeil D. K., Lewis T. M., Nassar M. A., Schoepfer R., Colquhoun D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J Physiol. 1997 Apr 1;500(Pt 1):123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzer E., Ebbinghaus U., Storch A., Poteur L., Schrattenholz A., Kramer G., Methfessel C., Maelicke A. Stable expression in HEK-293 cells of the rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor. FEBS Lett. 1996 Nov 11;397(1):39–44. doi: 10.1016/s0014-5793(96)01115-5. [DOI] [PubMed] [Google Scholar]
- Wong E. T., Holstad S. G., Mennerick S. J., Hong S. E., Zorumski C. F., Isenberg K. E. Pharmacological and physiological properties of a putative ganglionic nicotinic receptor, alpha 3 beta 4, expressed in transfected eucaryotic cells. Brain Res Mol Brain Res. 1995 Jan;28(1):101–109. doi: 10.1016/0169-328x(94)00189-l. [DOI] [PubMed] [Google Scholar]


