Abstract
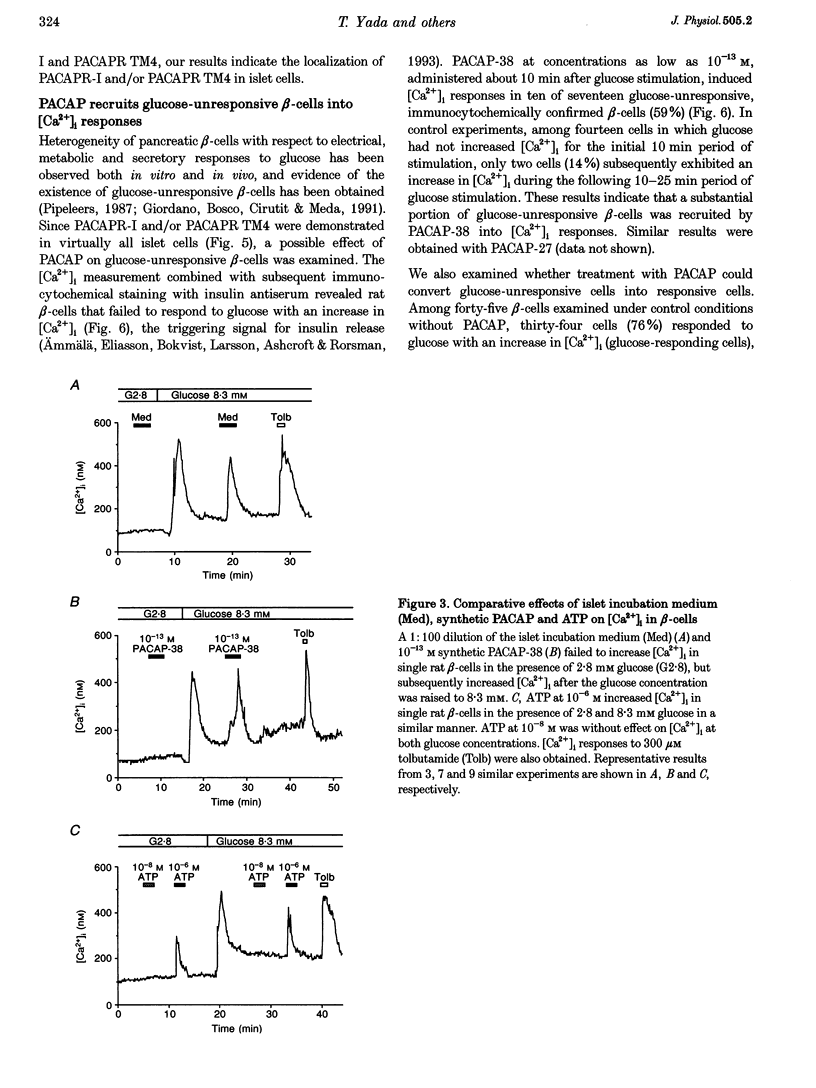
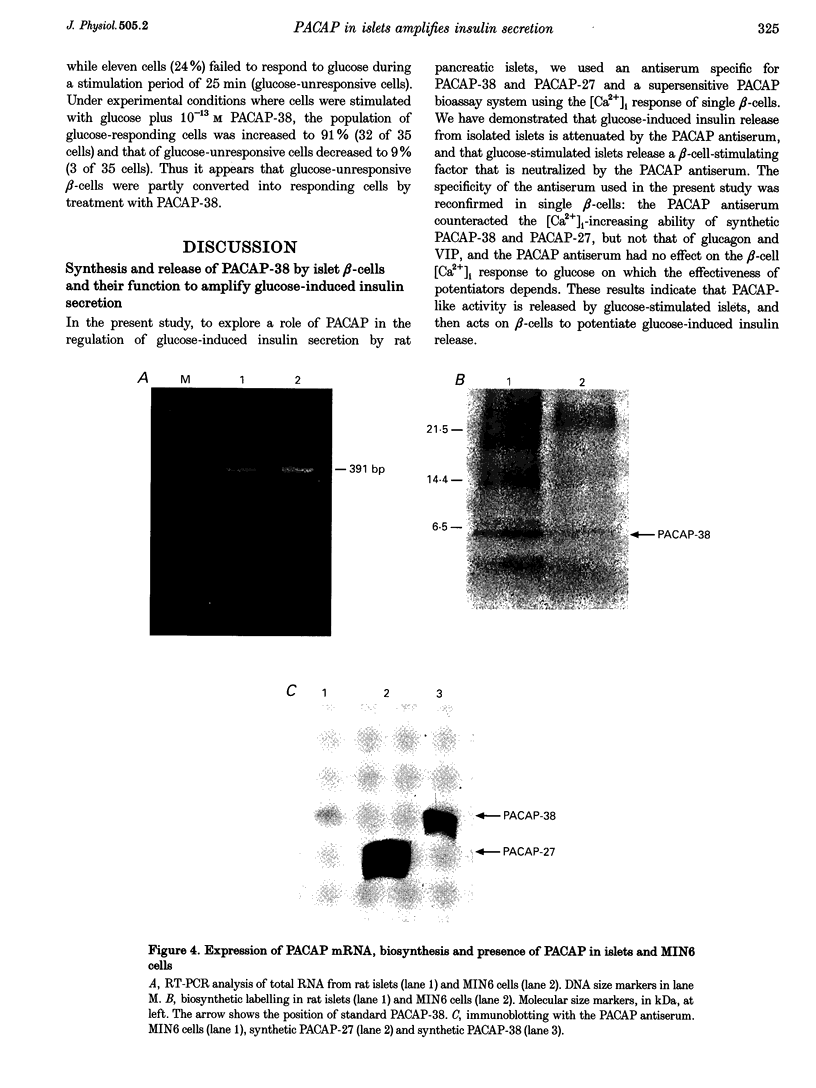
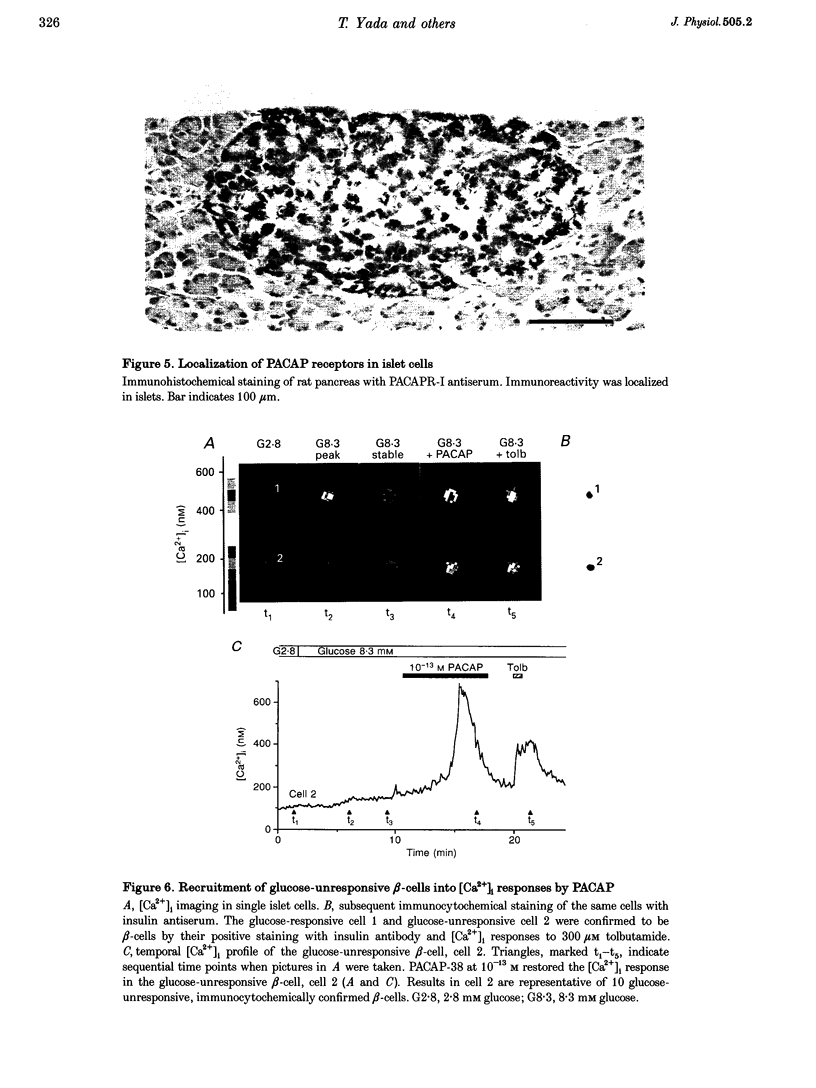
1. We examined whether pituitary adenylate cyclase-activating polypeptide with 38 or 27 residues (PACAP-38 or PACAP-27) serves as an intra-islet regulator of glucose-induced insulin secretion in rats. PACAP antiserum specific for PACAP-38 and PACAP-27 was used to neutralize the effect of endogenous PACAP in islets. PACAP release from islets was bioassayed using the response of cytosolic Ca2+ concentration ([Ca2+]i) in single beta-cells, monitored by dual-wavelength fura-2 microfluorometry. Expression of PACAP mRNA was studied by reverse transcription-polymerase chain reaction (RT-PCR), while expression of PACAP was studied by metabolic labelling and immunoblotting. Localization of PACAP receptors was studied immunohistochemically. 2. High glucose-stimulated insulin release from isolated islets was attenuated by PACAP antiserum but not by non-immune sera. 3. The islet incubation medium with high glucose (Med) possessed a capacity, which was neutralized by PACAP antiserum, to increase [Ca2+]i in beta-cells. PACAP antiserum also neutralized the [Ca2+]i-increasing action of synthetic PACAP-38 and PACAP-27, but not that of vasoactive intestinal polypeptide (VIP) and glucagon. 4. Both Med and synthetic PACAP increased [Ca2+]i in beta-cells only in the presence of stimulatory, but not basal, glucose concentrations. In contrast, ATP, a substance that is known to be released from beta-cells, increased [Ca2+]i in beta-cells at both and stimulatory glucose concentrations. 5. Expression of PACAP mRNA and biosynthesis of PACAP-38 were detected in islets and a beta-cell line, MIN6. 6. Immunoreactivity for PACAP-selective type-I receptor was observed in islets. 7. [Ca2+]i measurements combined with immunocytochemistry with insulin antiserum revealed a substantial population of glucose-unresponsive beta-cells, many of which were recruited by PACAP-38 into [Ca2+]i responses. 8. These results indicate that PACAP-38 is a novel islet substance that is synthesized and released by islet cells and then, in an autocrine and/or paracrine manner, potentiates and arouses beta-cell responses to glucose, thereby amplifying glucose-induced insulin secretion in islets.
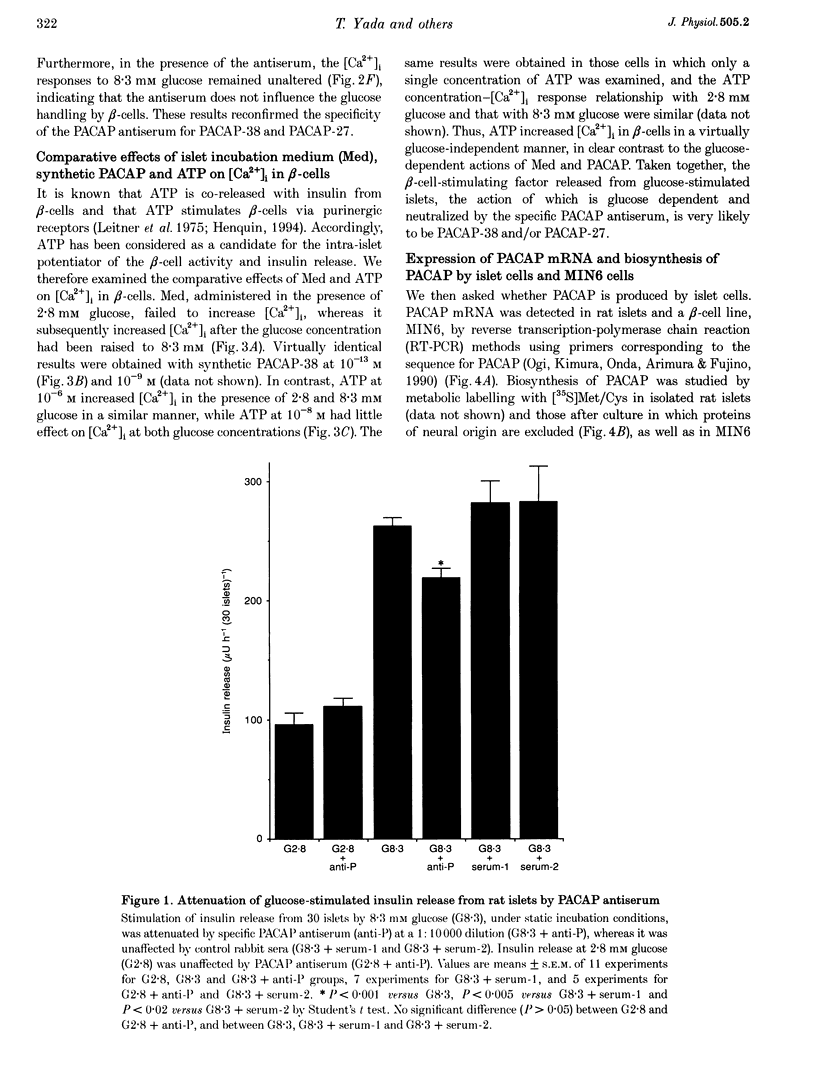
Full text
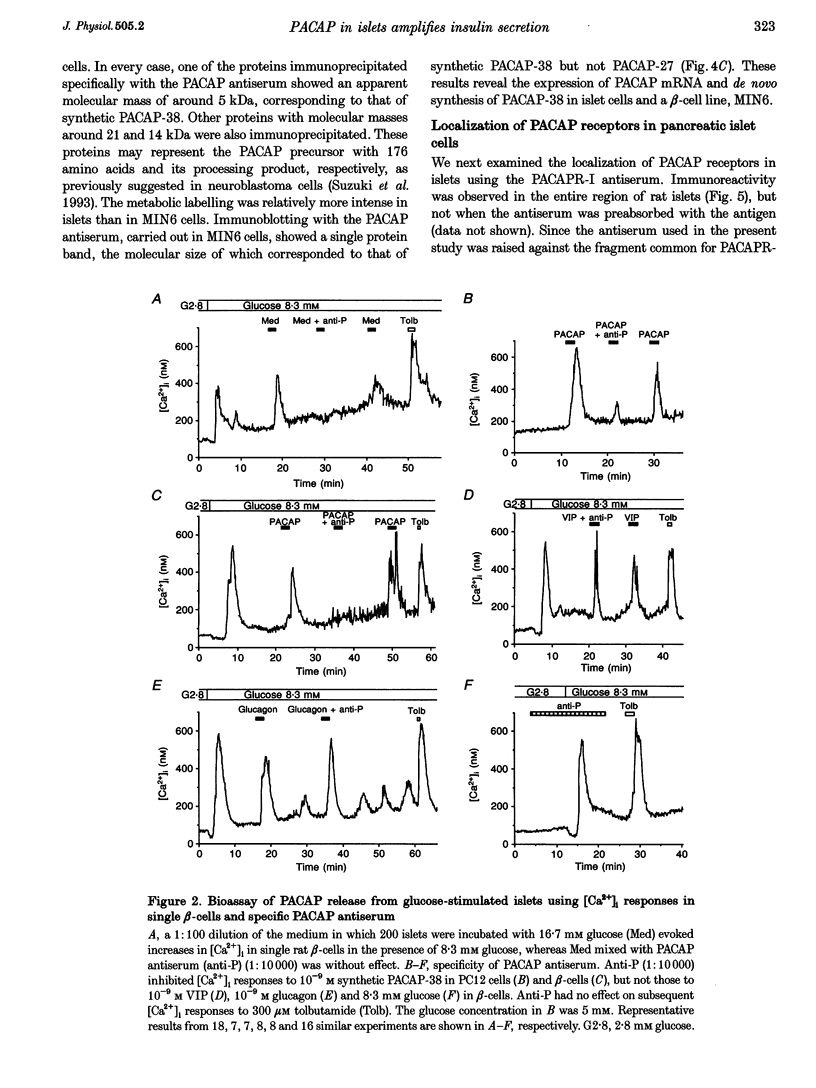
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A., Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995 Jan;16(1):53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Chatterjee T. K., Sharma R. V., Fisher R. A. Molecular cloning of a novel variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor that stimulates calcium influx by activation of L-type calcium channels. J Biol Chem. 1996 Dec 13;271(50):32226–32232. doi: 10.1074/jbc.271.50.32226. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992 Mar 15;267(8):5108–5113. [PubMed] [Google Scholar]
- Fridolf T., Sundler F., Ahrén B. Pituitary adenylate cyclase-activating polypeptide (PACAP): occurrence in rodent pancreas and effects on insulin and glucagon secretion in the mouse. Cell Tissue Res. 1992 Aug;269(2):275–279. doi: 10.1007/BF00319618. [DOI] [PubMed] [Google Scholar]
- Giordano E., Bosco D., Cirulli V., Meda P. Repeated glucose stimulation reveals distinct and lasting secretion patterns of individual rat pancreatic B cells. J Clin Invest. 1991 Jun;87(6):2178–2185. doi: 10.1172/JCI115251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz G. G., 4th, Kühtreiber W. M., Habener J. F. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature. 1993 Jan 28;361(6410):362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Yoshida H., Mizuta M., Mizuno N., Fujii Y., Gonoi T., Miyazaki J., Seino S. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2679–2683. doi: 10.1073/pnas.91.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Kawai K., Ohse C., Watanabe Y., Suzuki S., Yamashita K., Ohashi S. Pituitary adenylate cyclase activating polypeptide stimulates insulin release from the isolated perfused rat pancreas. Life Sci. 1992;50(4):257–261. doi: 10.1016/0024-3205(92)90332-j. [DOI] [PubMed] [Google Scholar]
- Klinteberg K. A., Karlsson S., Ahrén B. Signaling mechanisms underlying the insulinotropic effect of pituitary adenylate cyclase-activating polypeptide in HIT-T15 cells. Endocrinology. 1996 Jul;137(7):2791–2798. doi: 10.1210/endo.137.7.8770899. [DOI] [PubMed] [Google Scholar]
- Köves K., Arimura A., Görcs T. G., Somogyvári-Vigh A. Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology. 1991 Aug;54(2):159–169. doi: 10.1159/000125864. [DOI] [PubMed] [Google Scholar]
- Köves K., Arimura A., Somogyvári-Vigh A., Vigh S., Miller J. Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology. 1990 Jul;127(1):264–271. doi: 10.1210/endo-127-1-264. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Holz G. G., Habener J. F. Pituitary adenylate cyclase-activating polypeptide induces the voltage-independent activation of inward membrane currents and elevation of intracellular calcium in HIT-T15 insulinoma cells. Endocrinology. 1995 Apr;136(4):1530–1536. doi: 10.1210/endo.136.4.7895663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J. W., Sussman K. E., Vatter A. E., Schneider F. H. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975 Mar;96(3):662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- Lutz E. M., Sheward W. J., West K. M., Morrow J. A., Fink G., Harmar A. J. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993 Nov 8;334(1):3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., Coy D. H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989 Oct 16;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Ogi K., Kimura C., Onda H., Arimura A., Fujino M. Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1990 Dec 31;173(3):1271–1279. doi: 10.1016/s0006-291x(05)80924-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Halter J. B., Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981 Mar;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. The biosociology of pancreatic B cells. Diabetologia. 1987 May;30(5):277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- Straub S. G., Sharp G. W. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J Biol Chem. 1996 Jan 19;271(3):1660–1668. doi: 10.1074/jbc.271.3.1660. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Harada M., Kitada C., Ohkubo S., Matsumoto H., Watanabe T., Coy D. H., Tsuda M., Arimura A., Fujino M. Production of immunoreactive pituitary adenylate cyclase activating polypeptide (PACAP) by human neuroblastoma cells, IMR-32: detection and characterization with monoclonal and polyclonal antibodies against different epitopes of PACAP. J Biochem. 1993 May;113(5):549–556. doi: 10.1093/oxfordjournals.jbchem.a124081. [DOI] [PubMed] [Google Scholar]
- Usdin T. B., Bonner T. I., Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994 Dec;135(6):2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Yada T., Hamakawa N., Yaekura K. Two distinct modes of Ca2+ signalling by ACh in rat pancreatic beta-cells: concentration, glucose dependence and Ca2+ origin. J Physiol. 1995 Oct 1;488(Pt 1):13–24. doi: 10.1113/jphysiol.1995.sp020942. [DOI] [PMC free article] [PubMed] [Google Scholar]