Abstract
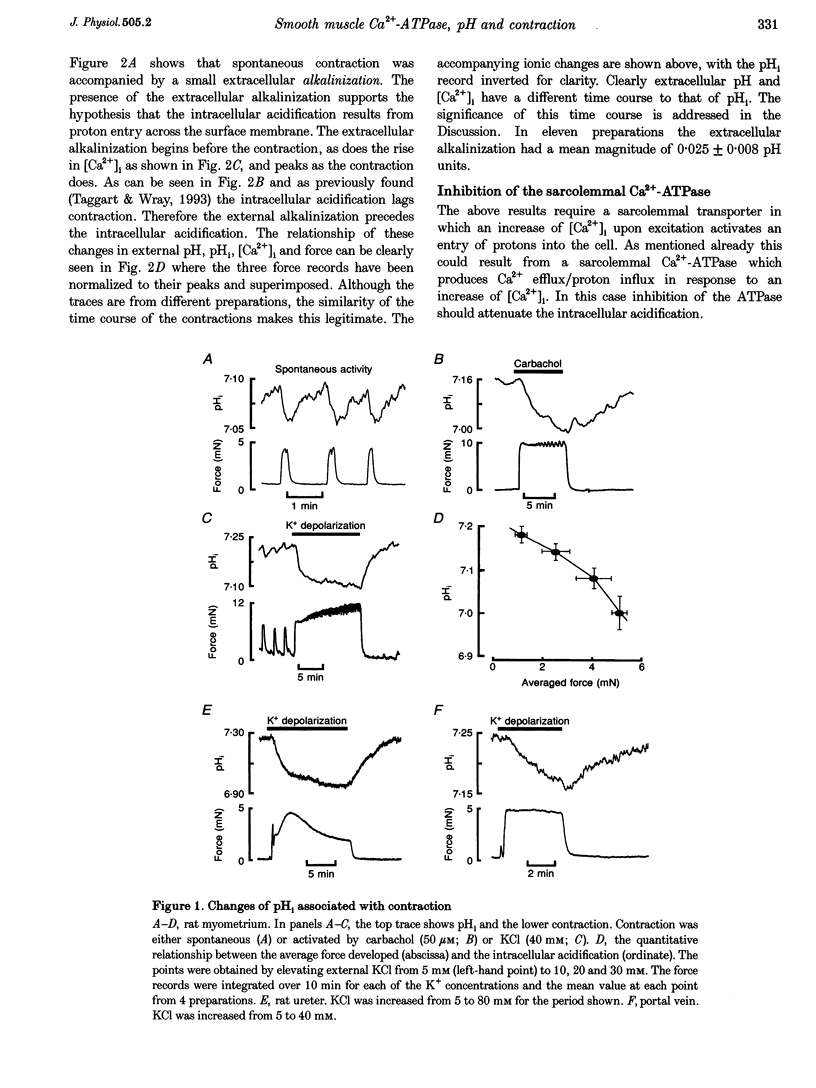
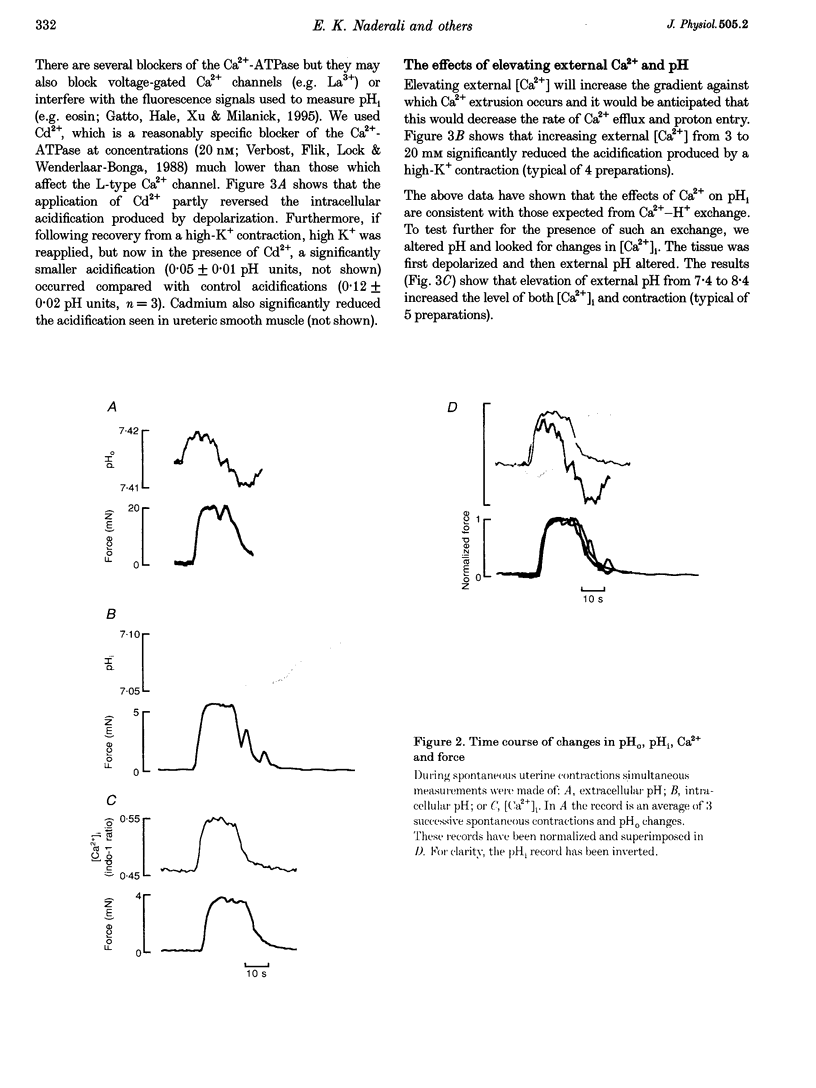
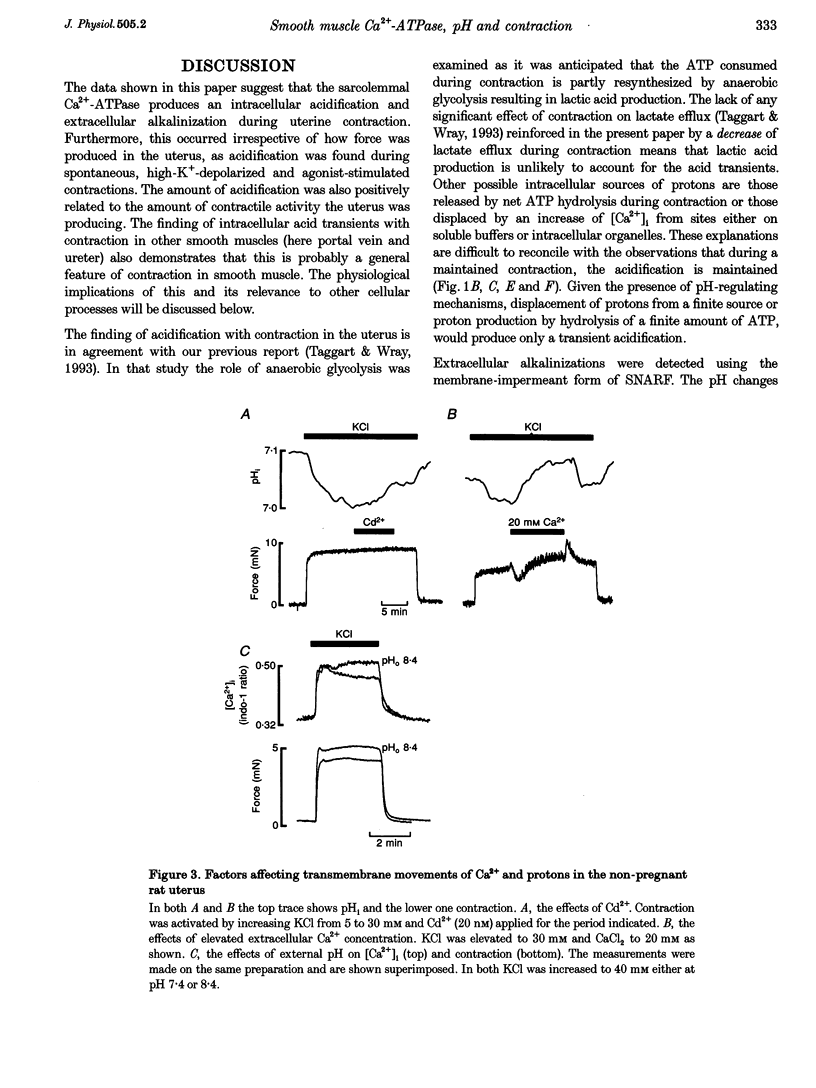
1. We have investigated the origin of the intracellular acid pH transients that accompany myometrial contraction. Intra- and extracellular pH were measured with SNARF and intracellular Ca2+ concentration ([Ca2+]i) with indo-1. 2. An intracellular acidification accompanied spontaneous contractions and those elicited by KCl depolarization or the addition of the agonists carbachol or prostaglandin F2 alpha. The size of the acidification increased with the magnitude of the contraction. 3. The intracellular acidification was accompanied by an extracellular alkalinization, showing that it results from proton movement across the surface membrane. Furthermore, it was decreased either by addition of Cd2+ (20 nM, an inhibitor of the sarcolemmal Ca(2+)-ATPase) or by elevating [Ca2+]o. 4. Extracellular alkalinization increased the magnitude of the rise of [Ca2+]i and force produced by KCl. 5. An intracellular acidification was also associated with contraction in the portal vein and ureter. 6. We conclude that the sarcolemmal Ca(2+)-ATPase produces a significant intracellular acidification while removing Ca2+. Both the acidification and decrease of [Ca2+]i will promote relaxation. Since Ca2+ and protons have opposite effects on many cellular processes, this dual regulation by these two ions may be of general importance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Mulvany M. J. Steady-state effects of arginine vasopressin on force and pHi of isolated mesenteric resistance arteries from rats. Am J Physiol. 1991 Dec;261(6 Pt 1):C1010–C1017. doi: 10.1152/ajpcell.1991.261.6.C1010. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Morris P. G., Pirolo J. S., Smith G. L. Metabolic consequences of increasing intracellular calcium and force production in perfused ferret hearts. J Physiol. 1986 Jul;376:121–141. doi: 10.1113/jphysiol.1986.sp016145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C., Wray S. The effects of extracellular pH and calcium change on force and intracellular calcium in rat vascular smooth muscle. J Physiol. 1995 Oct 15;488(Pt 2):281–291. doi: 10.1113/jphysiol.1995.sp020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga T. V., Taggart M. J., Wray S. An investigation into the mechanism whereby pH affects tension in guinea-pig ureteric smooth muscle. J Physiol. 1996 Jun 15;493(Pt 3):865–876. doi: 10.1113/jphysiol.1996.sp021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton C. A., Taggart M. J., Wray S., Smith G. L. Effects of pH and inorganic phosphate on force production in alpha-toxin-permeabilized isolated rat uterine smooth muscle. J Physiol. 1993 Jun;465:629–645. doi: 10.1113/jphysiol.1993.sp019697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas J. T., Arrieta J., Ye M., Flores G., Battle D. C. Intracellular acidification associated with changes in free cytosolic calcium. Evidence for Ca2+/H+ exchange via a plasma membrane Ca(2+)-ATPase in vascular smooth muscle cells. J Clin Invest. 1995 Apr;95(4):1480–1489. doi: 10.1172/JCI117819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiesland J., Baro I., Raimbach S., Eisner D. A., Wray S. Intracellular pH and buffering power measured in isolated single cells from pregnant rat uterus. Exp Physiol. 1991 Sep;76(5):815–818. doi: 10.1113/expphysiol.1991.sp003548. [DOI] [PubMed] [Google Scholar]
- Enyedi A., Minami J., Caride A. J., Penniston J. T. Characteristics of the Ca2+ pump and Ca2+-ATPase in the plasma membrane of rat myometrium. Biochem J. 1988 May 15;252(1):215–220. doi: 10.1042/bj2520215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagher U., Laudanski T., Schütz A., Sipowicz M., Akerlund M. The relationship between cadmium and lead burdens and preterm labor. Int J Gynaecol Obstet. 1993 Feb;40(2):109–114. doi: 10.1016/0020-7292(93)90368-7. [DOI] [PubMed] [Google Scholar]
- Gatto C., Hale C. C., Xu W., Milanick M. A. Eosin, a potent inhibitor of the plasma membrane Ca pump, does not inhibit the cardiac Na-Ca exchanger. Biochemistry. 1995 Jan 24;34(3):965–972. doi: 10.1021/bi00003a031. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T., Kishikawa T., Sugimori H. Effects of external calcium, magnesium, and temperature on spontaneous contractions of pregnant human myometrium. Biol Reprod. 1989 May;40(5):942–948. doi: 10.1095/biolreprod40.5.942. [DOI] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Niggli V., Sigel E., Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem. 1982 Mar 10;257(5):2350–2356. [PubMed] [Google Scholar]
- Taggart M. J., Burdyga T., Heaton R., Wray S. Stimulus-dependent modulation of smooth muscle intracellular calcium and force by altered intracellular pH. Pflugers Arch. 1996 Sep;432(5):803–811. doi: 10.1007/s004240050202. [DOI] [PubMed] [Google Scholar]
- Taggart M. J., Wray S. Occurrence of intracellular pH transients during spontaneous contractions in rat uterine smooth muscle. J Physiol. 1993 Dec;472:23–31. doi: 10.1113/jphysiol.1993.sp019933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart M., Austin C., Wray S. A comparison of the effects of intracellular and extracellular pH on contraction in isolated rat portal vein. J Physiol. 1994 Mar 1;475(2):285–292. doi: 10.1113/jphysiol.1994.sp020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Changes in the surface pH of voltage-clamped snail neurones apparently caused by H+ fluxes through a channel. J Physiol. 1988 Apr;398:313–327. doi: 10.1113/jphysiol.1988.sp017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Verbost P. M., Flik G., Lock R. A., Wendelaar Bonga S. E. Cadmium inhibits plasma membrane calcium transport. J Membr Biol. 1988 May;102(2):97–104. doi: 10.1007/BF01870448. [DOI] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]