Abstract
Background
Biosimilar etanercept presents itself as an innovative therapeutic opportunity for inflammatory and autoimmune diseases, however, its efficacy, safety, and immunogenicity in relation to the reference biological agent for the treatment of rheumatoid arthritis is still questioned. With this in mind, this study aimed to verify the efficacy, safety, and immunogenicity of the use of the biosimilar etanercept in relation to the reference biologic in patients over 18 years of age with rheumatoid arthritis.
Methods
A systematic review with meta-analysis was performed in accordance with the parameters of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) selecting only Phase III randomized clinical trials. The search strategy was constructed with the MeSH terms “Etanercept”, “Biological Products”, “Arthritis, Rheumatoid”, “Biosimilar Pharmaceuticals” and was performed in Medline via PubMed, Embase, the Cochrane Library, Web of Science, EBSCO and Lilacs in January 2023. The analysis measures were relative risk (RR) for dichotomous data and mean difference (MD) for continuous data. The statistical analysis for preparing meta-analyses was developed by the Review Manager 5.1.4 software.
Results
This systematic review selected 6 eligible studies with a sample population of n = 2355. The main efficacy outcomes showed that both drugs did not present statistically significant differences in ACR20, ACR50, and ACR70 responses within 6 months (RR 1.00; 95% CI = 0.94 to 1.07; RR 1.09; 95% CI = 0.94 to 1.26; RR 1.04; 95% CI = 0.82 to 1.31, respectively), with I2 ranging from 55 to 63% and 0.04 ≤ P ≥ 0.08. Adverse events were mostly mild or moderate, and serious adverse events were not statistically significant. Regarding immunogenicity, only 5.4% of the ADA-positive biosimilar group had positive neutralizing antibodies.
Conclusions
Thus, this review found that biosimilar etanercept had efficacy, safety, and immunogenicity similar to those for the biological reference.
Systematic review registration
This systematic review was registered on the PROSPERO platform under number CRD42020166610.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02715-w.
Keywords: Etanercept, Efficacy, Arthritis Rheumatoid, Systematic Reviews, Biosimilar pharmaceuticals
Introduction
Rheumatoid arthritis (RA) is a chronic and systemic inflammatory disease that mainly affects joints, such as the hands, wrists, elbows, knees, ankles, feet, shoulders, and cervical spine. With disease progression, patients develop an inability to perform daily activities [1]. Its cause is still unknown, but it is known that it is an autoimmune disease that can present in a mild, moderate, or severe form [2]. The incidence of RA is higher among people aged 50 years or older, and it affects twice as many women as men [3, 4].
The treatment of patients with RA involves a combination of educational, preventive, and nonpharmacological interventions, pharmacological treatment, and surgical procedures [1, 5]. Among the five classes of drugs available to treat RA (analgesics, nonsteroidal anti-inflammatory drugs, corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and targeted therapy with biological agents), biological agents represent notable advances in RA treatment, improving the quality of life of patients. These drugs act by blocking tumor necrosis factor (TNF) to inhibit the immune system and, consequently, reduce joint inflammation [3, 5].
Biological drugs are defined by the World Health Organization (WHO) as drugs extracted from biological fluids or tissues of animal origin or drugs obtained by biotechnological procedures [1, 6]. They are produced by biosynthesis in living cells by means of a microorganism, plant cell, or animal cell. However, biological, physical, and chemical tests are necessary to ensure quality in manufacturing processes [7].
Both biologics and biosimilars to etanercept are options when the response to one or more DMARDs is unsatisfactory for the treatment of RA [3]. Biosimilar medicines are biological products that are highly similar to reference products but are not identical [6]. Biosimilarity cannot be interpreted as evidence for interchangeability, as with generic drugs. This similarity is confirmed by randomized clinical trials in the pharmacokinetic, pharmacodynamic, safety, immunogenicity, and efficacy stages [8–10].
Because of the high costs associated with the use of biologics for the treatment of RA, the development and use of biosimilars have been proposed as a promising alternative to reduce the economic impact [9, 11]. In this context, the incorporation of biosimilars for RA is still a major challenge in health systems, and studies on the economic aspects as well as efficacy, safety, and immunogenicity are needed to guide the decision-making process [12].
Therefore, the aim of the present study was to compare the evidence on the efficacy, safety, and immunogenicity of biosimilar etanercept with those for the biologic etanercept (ETN) for patients over 18 years of age with RA.
Method
Study identification
This was a systematic review structured in accordance with the parameters of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [13] protocol and registered on the PROSPERO platform (CRD42020166610). The research question was developed using the PICOS framework: “What is the safety, efficacy, and immunogenicity of biosimilars compared with that for biologic etanercept for patients 18 years of age (adults and elderly) with rheumatoid arthritis?” (Supplementary material 1). The literature search strategy was constructed using the following keywords: “Etanercept”, “Biological Products”, “Arthritis, Rheumatoid”, “Biosimilar Pharmaceuticals” applied in a structured manner and with the necessary specificities for each electronic database: MEDLINE via PubMed, EMBASE, The Cochrane Library, Web of Science, EBSCO, and Lilacs. The search was performed in January 2023.
Eligibility criteria
The inclusion criteria were phase III randomized controlled clinical trials (phase III RCTs) that compared the biologic drug etanercept with biosimilars in patients aged 18 years or older with mild, moderate, or severe rheumatoid arthritis, without restrictions for time or language.
Phase I, II, and III RCTs associated with observational studies, case series, overviews, letters to the editor, qualitative studies, and articles with incomplete texts; studies that did not address the safety, efficacy, effectiveness, and immunogenicity of biologic/biosimilar etanercept; and studies that investigated only other drugs for the treatment of RA without evidence of the use of biologic/biosimilar etanercept were excluded. A search in gray literature was not relevant because only phase III RCTs were included in the analyses.
Selection of studies and data collection
Mendeley reference manager version 1.18 was used to organize the articles and remove duplicates, and the Rayyan QCRI platform was used to select the articles (https://rayyan.qcri.org) for which the title and abstract were read by two independent researchers (AMA and JMG). Disagreements were resolved independently by a third researcher (DCRP).
Data extraction was performed independently by two reviewers (AMA and JMG) using an extraction form in Microsoft Excel 2016, and discrepancies were resolved by consensus with a third researcher (JRB). Study registration was verified on the clinicaltrials.gov platform. When necessary, the authors of the selected articles were contacted to provide additional information, as were experts in the field of rheumatology. Manual searches were also carried out in the references of selected studies. The data extraction form included the following variables: authors, year of publication, country, study design, objective, population, number of participants, control group, exposed group, dosage, treatment follow-up time, efficacy, adverse events (AEs), immunogenicity, limitations, and main findings.
Among the outcomes analyzed, ACR20, ACR50, ACR70 (i.e., 20%, 50%, and 70% reduction in the number of swollen and painful joints, respectively) and improvement in 3 of the following 5 variables stipulated by the American College of Rheumatology (ACR) were considered for efficacy: acute phase inflammatory tests (C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), physician’s global assessment, patient’s global assessment, Health Assessment Questionnaire (HAQ) and visual analog pain scale [14]. For safety, serious and nonserious AEs were considered, differentiated by the classification used in the primary studies. For immunogenicity, the presence or absence of anti-drug antibodies (ADAs) was considered.
Risk of bias and quality of evidence
The methodological quality of the included studies was assessed using the Cochrane Collaboration risk of bias (ROB) tool (version 2.0), and the Robvis tool (risk of bias visualization) was used to generate signal plots and the summary plot [15, 16].
Risks of bias were independently assessed by two reviewers (AMA and JMG), and discrepancies were resolved by consensus with a third reviewer (ETS). ROB 2.0 consists of five risk of bias domains: randomization process; deviations from the intended interventions; lack of outcome data; result measurement; and selection of the reported outcome. The risk of bias for each domain was assessed as low, some concern, or high [15, 16].
GRADEpro online software was used to assess the quality of the evidence (https://gradepro.org/). Grading of Recommendations Assessment, Development, and Evaluation (GRADE) allows a critical evaluation of the quality of evidence for each outcome, considering four levels that represent the confidence of the estimate of the effects presented as very low, low, moderate, or high [17].
Statistical analysis
Review Manager® software, version 5.1.4, was used to prepare the meta-analyses for the outcomes evaluated, considering a follow-up period of up to 6 months and above. The disease activity score (DAS) results are presented descriptively, considering that it was not possible to summarize them through a meta-analysis. Sensitivity analysis was carried out in studies by a subgroup of countries from the same continent.
Relative risk was calculated for the dichotomous outcomes, and the differences in the standard deviations and the means were calculated for the continuous outcomes, with a 95% confidence interval (CI) provided for both. A random effects model was used due to the heterogeneity of the included studies. Heterogeneity was assessed using the Cochrane criteria, being statistically significant if P < 0.05 for the chi-square test, and I2 < 25% indicating low heterogeneity, 25 < I2 > 50% indicating moderate heterogeneity, and I2 > 50% indicating high heterogeneity [18].
Results
Characterization of the included studies
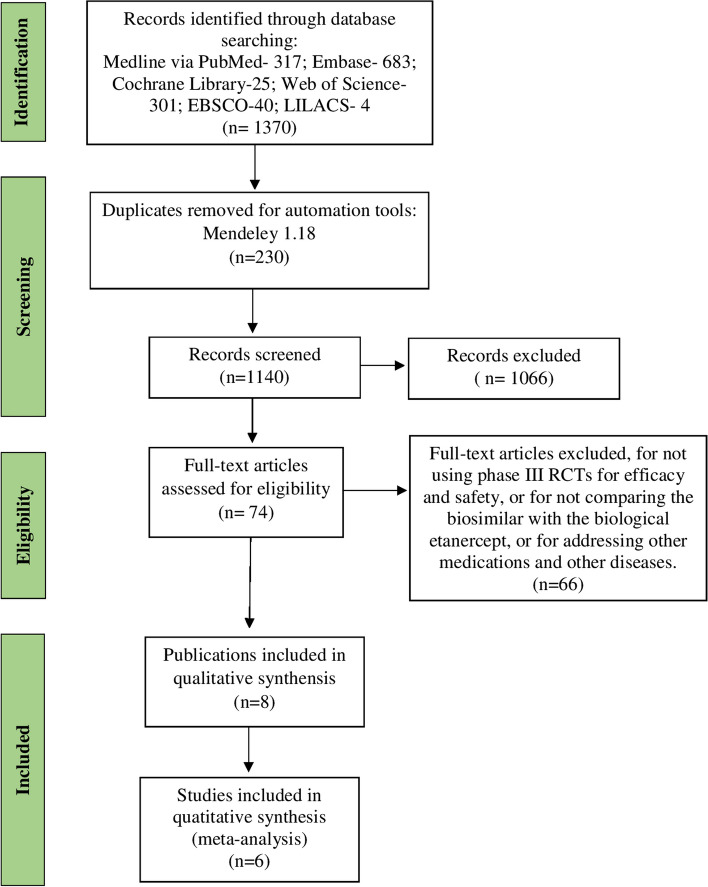
In the literature search, 1370 publications were retrieved, from which 8 publications were selected, 6 of which were multicenter studies, with follow-up times of 1 to 2 years (Fig. 1).
Fig. 1.
Flowchart for the inclusion of studies in the systematic review. Source: Adaptation of the PRISMA flow diagram
Biologic ETN was compared with the following biosimilars: HD203 (25 mg), GP2015 (50 mg), LBEC (50 mg) and SB4 (50 mg). Of the six studies, two used GP2015, two used LBEC, one used SB4 and one used HD203. All studies had as a prerequisite that the patients were already taking a stable dose of methotrexate (MTX) ranging from 7.5 to 25 mg/week, and three studies required folic acid (5 mg/week) together with MTX. Five studies used 50 mg of biosimilar/biologic etanercept once a week. The details of the studies are presented in Table 1.
Table 1.
Characterization of the studies included in the systematic review (n = 6)
| Author/year | Country | Study design | Follow-up time | Population | Outcomes | Dosage | Control group (biological)1 | Exposed group (biosimilar) 1 | Total participants |
|---|---|---|---|---|---|---|---|---|---|
| Bae et al. 2017 [19] | South Korea | Phase III RCT | 48 weeks | Patients ≥ 20 years with RA3 |
Primary: ACR20 at week 24 Secondary: ACR20 at weeks 12 and 48; ACR50 and 70 at weeks 12, 24, and 48; DAS286; EULAR4 response. AEs7. ADAs8 at weeks 24 and 48 |
25 mg of HD203 and ETN administered subcutaneously twice a week with MTX once a week (7.5–25 mg/week) | ETN (n = 147) |
HD203 (n = 147) |
n = 294 |
| Emery et al. 2015–2017 [20–22] | Ukraine, United Kingdom, Bulgaria, Colombia, Czech Republic, Hungary, South Korea, Lithuania, Mexico, and Poland | Phase III RCT with open-label period | 100 weeks | Patients aged 18 to 75 years with RA3 |
Primary: ACR20 at week 24 Secondary: ACR20, 50, and 70 up to week 52; DAS286-ESR; EULAR4 response. SDAI9 and CDAI10 scores. HAQ-DI12. AEs7. ADAs8 and neutralizing antibodies at weeks 0, 2, 4, 8, 12, 16, 24, and 52 |
50 mg of SB4 and ETN self-administered subcutaneously once a week with MTX (10–25 mg/week) and folic acid (5–10 mg/week) |
ETN (n = 297, open label n = 119) |
SB4 (n = 299, open label n = 126) |
n = 596 open label n = 245 |
| Jaworski et al. 2019 [23] | Mexico, Russian Federation, the United States of America, and 13 European countries 2 | Phase III RCT | 48 weeks | Patients ≥ 18 years of age with RA34 |
Primary: DAS286-CRP up to week 24 Secondary: DAS286, EULAR4 response, ACR20/50/70 response, HAQ-DI12 in week 24 and week 48; FACIT11; AEs7. ADAs8 and neutralizing antibodies up to 48 weeks |
GP2015 50 mg and ETN self-administered subcutaneously once a week with MTX (10–25 mg/week) and folic acid (≥ 5 mg/week) | ETN (n = 166) | GP2015 (n = 175) | n = 376 |
| Matsuno et al. 2018 [24] | South Korea and Japan | Phase III RCT | 52 weeks | Patients aged between 20 and 75 years with RA3 |
Primary: DAS286-ESR at week 24 Secondary: DAS286 at weeks 12 and 52, ACR20 at weeks 12, 24, and 52, ACR50, ACR70 and EULAR4 response at weeks 12, 24, and 52. AEs up to week 54. ADAs8 and neutralizing antibodies at weeks 0, 12, 24, and 52 |
50 mg of LBEC0101 and ETN-RP5 subcutaneously once a week with MTX (7.5 to 15 mg/week in Korea and 6 to 16 mg/week in Japan, with dose approval in the country) | ETN (n = 187) | LBEC0101 (n = 187) | n = 374 |
| Matucci-Cerinic et al. 2018 [25] | Mexico, the Russian Federation, the United States of America, and 13 European countries 2 | Phase III RCT | 24 weeks | Patients ≥ 18 years with RA34 |
Primary: DAS286-CRP week 24 Secondary: EULAR4, DAS286 ACR20/50/70, HAQ-DI12, FACIT11; AEs7; ADAs8 at weeks 2, 4, 12, and 24 |
50 mg of GP2015 and ETN self-administered subcutaneously once a week with MTX (10–25 mg/week) and folic acid (≥ 5 mg/week) | ETN (n = 190) | GP 2015 (n = 186) | n = 376 |
| Park et al. 2019 [26] | South Korea and Japan | Phase III RCT, with open-label period | 100 weeks | Patients aged 41 to 65 years with RA4 |
Primary: DAS286-ESR/CRP at weeks 76 and 100 Secondary: ACR 20, 50, 70, EULAR4 response at weeks 52, 76, and 100 |
50 mg LBEC0101 and ETN self-administered subcutaneously once a week with MTX (7.5–15 mg/week) | ETN (n = 187, open label 78) | LBEC0101 (n = 187, open label 70) |
n = 374 n = 148 no open label |
1Number of participants at baseline
2Bulgaria, Czech Republic, Estonia, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Serbia, Slovakia, Spain, United Kingdom
3American College of Rheumatology Criteria, 1987
4Criteria of the European American College of Rheumatology (EULAR) 2010
5Etanercept Reference Product–ETN-RP
6Disease Activity Score 28–DAS28
7Adverse Events–AEs
8Antidrug Antibodies–ADAs
9Simplified Disease Activity Index–SDAI
10Clinical Disease Activity Index–CDAI
11Functional Assessment of Chronic Illness Therapy–FACIT
12Health Assessment Questionnaire Disability Index–HAQ-DI
Source: Prepared by the authors
Risk of bias and quality of evidence
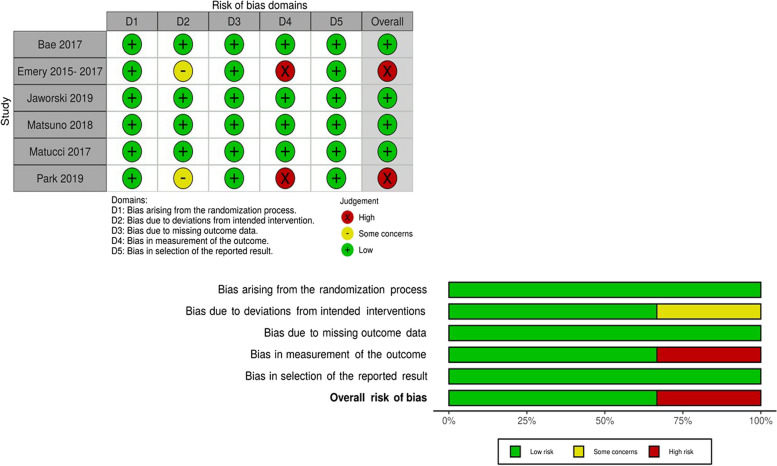
All studies were assessed as having a low risk of bias (Fig. 2). Regarding the critical aspects, in the allocation confidentiality domain, only Emery et al. [20–22] did not clarify the allocation confidentiality procedure. Regarding the blinding of participants and staff and the blinding of outcome assessors, Emery et al. [20–22] and Park et al. [26] presented a high risk of bias due to the open-label extension without blinding the study investigators. In the incomplete outcomes and selective reporting of outcomes domains, Park et al. [26] did not report the loss of a patient, and Matucci-Cerinic et al. [25] did not mention the secondary outcomes listed in the registered protocol.
Fig. 2.
Risk of bias of the studies included in the systematic review. Source: Prepared by the authors
The quality of the evidence, as determined using GRADE, was considered high for almost all outcomes. There was only a one-point reduction in inconsistency due to the high heterogeneity in the ACR20, ACR50, and ACR70 outcomes (Supplementary material 1).
Efficacy
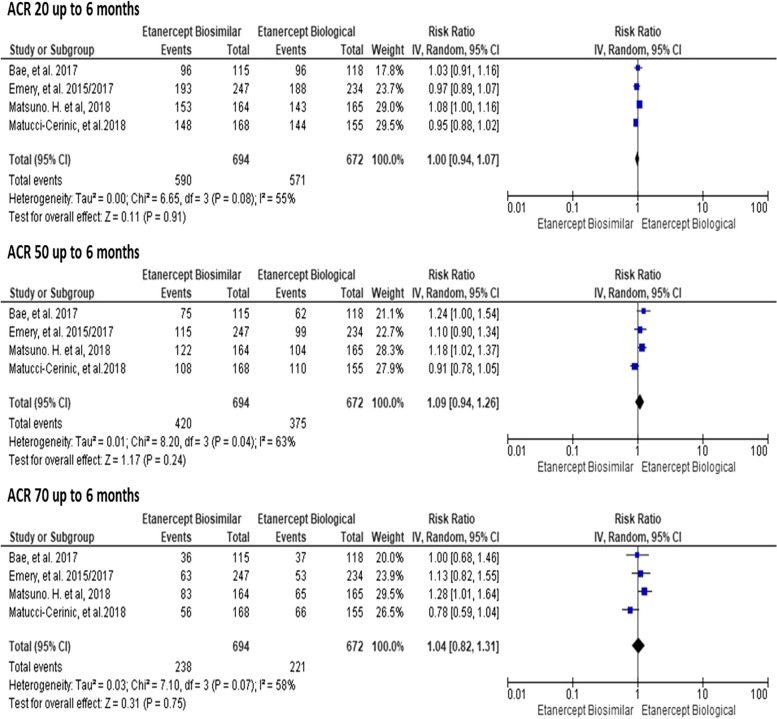
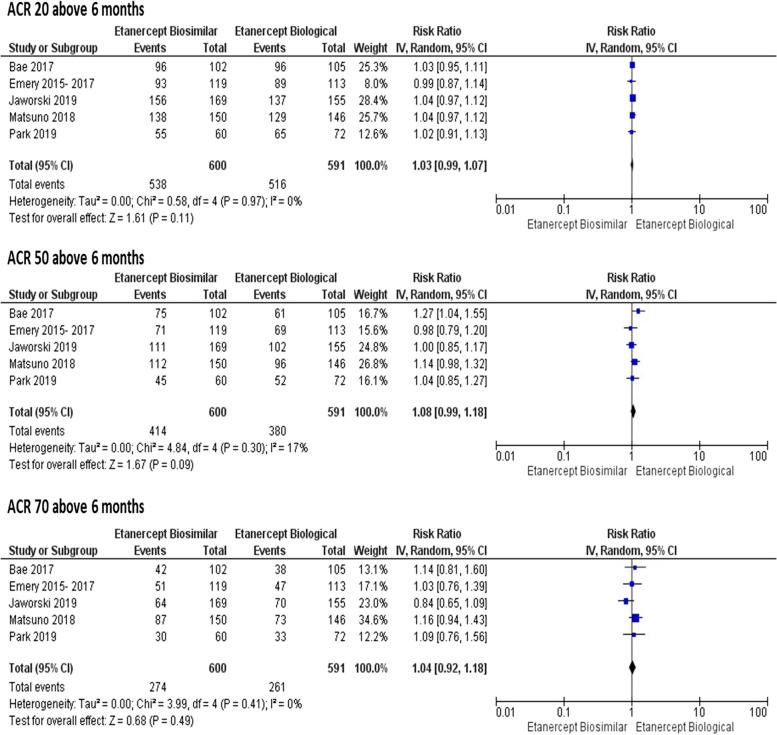
There were no statistically significant differences between the drugs in the ACR20, ACR50, and ACR70 responses up to 6 months (RR 1.00; 95% CI = 0.94 to 1.07; RR 1.09; 95% CI = 0.94 to 1.26; RR 1.04; 95% CI = 0.82 to 1.31, respectively), with I2 ranging from 55 to 63% and 0.04 ≤ p ≥ 0.08 (Fig. 3); there were also no differences for these outcomes (ACR20, ACR50, ACR70) after 6 months (RR 1.03; 95% CI = 0.99 to 1.07; RR: 1.08; 95% CI = 0.99 to 1.18; and RR 1.04; 95% CI = 0.92 to 1.18, respectively), with I2 ranging from 0 to 17% and 0.30 ≤ P ≥ 0.97 (Fig. 4).
Fig. 3.
Meta-analyses of ACR20, ACR50, and ACR70 up to 6 months. Source: Prepared by the author
Fig. 4.
Meta-analyses of ACR20, ACR50, and ACR70 above 6 months. Source: Prepared by the authors
For the evaluation of efficacy based on the DAS28 score (disease activity score in 28 joints), a decrease in the number of painful and overly inflamed joints was observed at baseline and after the intervention, but without statistically significant differences between the groups. In addition, all studies showed clinical disease remission, with predominantly good or moderate EULAR (European League Against Rheumatism) response in the biosimilar group (ranging from 85.5% to 97.6%) and in the biologic etanercept group (ranging from 89.7 and 97.4%).
HAQ scores were not statistically significant in either group. The mean difference (MD) between the groups up to 6 months was (MD 0.01; 95% CI = − 0.05 to 0.07; I2 = 0%; P = 0.89) and above 6 months was (MD 0.03; 95% CI = − 0.05 to 0.11; I2 = 0%; p = 0.42), without significant heterogeneity. Only the study by Bae et al. [19] reported HAQ scores at 48 weeks were not with a significant difference in means (RR 0.04; 95% CI = − 0.08 to 0.22), compared with those in other studies (Supplementary material 1).
Safety
The following severe AEs cited in primary studies [19–26] were considered in the analysis: acute pyelonephritis, arthralgia, acute cholecystitis and osteomyelitis, pneumonia, vertebral compression fracture, and severe infection (such as sepsis, abscess, opportunistic infections or invasive fungal infection, including histoplasmosis). For this review, nonsevere events were those stipulated as moderate and mild in the primary studies.
The results of the meta-analyses of nonsevere AEs evaluated up to 6 months were as follows: increase in alanine aminotransferase (RR 1.20; 95% CI = 0.65 to 2.20; P = 0.37), urinary tract infection (RR 0.77; 95% CI = 0. 36 to 1.63; P = 0.48), injection site reaction (RR 0.34; 95% CI = 0.20 to 0.57; P = 0.67) and nasopharyngitis (RR 0.89; 95% CI = 0.52 to 1.53; P = 0.18); only one study showed moderate heterogeneity (I2 = 42%). The nonserious AEs evaluated above 6 months were cough (RR 1.20; 95% CI = 0.65 to 2.20; P = 0.37), upper respiratory tract infection (RR 1.00; 95% CI = 0.70 to 1.44; P = 0.44), injection site reaction (RR: 0.31; 95% CI = 0.15 to 0.63; P = 0.48), erythema at the injection site (RR 0.20; 95% CI = 0.12 to 0.34; P = 0.77), rash at the injection site (RR 0.21; 95% CI = 0.10 to 0.42; P = 0.52), and nasopharyngitis (RR 1.10; 95% CI = 0.79 to 1.53; P = 0.72), with low heterogeneity (I2 = 0% and P > 0.05) among all events analyzed in this meta-analysis (Supplementary material 1).
The severe AEs analyzed were not statistically significant and had low heterogeneity up to 6 months (RR 0.47; 95% CI = 0.10 to 2.10; I2 = 8%; P = 0.30) and above 6 months (RR: 1.23; 95% CI = 0.85 to 1.78; I2 = 0%; P = 0.45) (Supplementary material 1). Studies [19–22, 24, 25] also reported eight deaths: four related to the biosimilar and four related to biologic ETN. However, one death was not associated with the use of ETN (family history and habits) [25].
Immunogenicity
The presence of positive anti-drug antibodies (ADAs) was evidenced in all studies, totaling 110 patients in the ETN group and 74 patients in the biosimilar group. Five studies [19–22, 25, 26] reported 11 patients (10%) in the biologic ETN group and two studies [19–22] reported 4 patients (5.4%) in the biosimilar group positive for the presence of neutralizing antibodies (Supplementary material 1). However, two studies [23, 24] did not detect positive neutralizing antibodies in any of the patients.
Sensitivity analysis
Sensitivity analyses were conducted to explore potential outcomes in the subgroup that analyzed only Asian countries (South Korea and Japan) [19, 24, 26]. Study exclusion by country did not produce significantly different relative risk (RR) in most cases (ACR. 20, 50, 70 up to 6 months, ACR 20, 70 above 6 months, HAQ above 6 months, Nasopharyngitis above 6 months, Upper Tract Respiratory infection above 6 months, Injection site reaction above 6 months, and serious adverse events above 6 months (Supplementary material 1), however, significant statistical differences were evidenced for rheumatoid arthritis in the grouped RR results for ACR 50 above 6 months. Three studies [19, 24, 26] (RR 1.14; 95% CI = 1.03 to 1.27; I2 = 0%; P = 0.01) (Supplementary material 1).
Discussion
This systematic review with meta-analysis revealed similar statistical analyses results for efficacy, safety, and immunogenicity. The evaluation of efficacy using the ACR, DAS28, and HAQ showed similar changes between the two groups. Nonserious AEs occurred mostly after 6 months; however, serious AEs were mostly identified within 6 months. ADAs were reported in a small proportion of patients.
Regarding efficacy, there was no statistically significant difference in the DAS28, ACR, and HAQ outcomes between biosimilar and biological treatments. The scientific literature comparing biosimilars with biological etanercept in phase III RCTs is still sparse. Nevertheless, some evidence with synthetic anti-TNF drugs corroborated the findings herein, for example, Costa et al. [27], who evaluated infliximab and MTX, and Machado et al. [28], who evaluated adalimumab, both for the treatment of RA.
Regarding efficacy, there was no statistically significant difference in the DAS28, ACR, and HAQ outcomes between biosimilar and biological treatments. Clinical trials demonstrated that the response to treatment with the biosimilar, measured by the ACR20/50/70 criteria, is not inferior to that observed with the ETN biologic. Furthermore, the reduction in DAS28 was similar between the two groups, corroborating the efficacy of the biosimilar in reducing disease activity. The scientific literature comparing biosimilars with biological etanercept in phase III RCTs is still sparse, especially with larger sample sizes and studies monitoring long-term post-marketing efficacy. Nevertheless, some evidence with synthetic anti-TNF drugs corroborated the findings herein, for example, Costa et al. [27], who evaluated infliximab and MTX, and Machado et al. [28], who evaluated adalimumab, both for the treatment of RA.
The heterogeneity was high for ACR20, ACR50, and ACR70 outcomes within 6 months but was low above 6 months. This can be explained by the greater loss to follow-up with the prolongation of phase III RCTs. However, caution is required in the interpretation of long-term outcomes because studies with a follow-up above 6 months are scarcer. Previous evidence has suggested high heterogeneity with synthetic anti-TNF above 6 months [29–32]. These divergences may be associated with the relative statistical variability and methodological differences between studies and their outcomes, which may occur due to intrastudy or interstudy variance [33].
In the sensitivity analysis, ACR 50 over 6 months showed a 14% increase in risk for biosimilar etanercept in relation to biological etanercept in Asian countries, despite being a small percentage in the study by Bae et al. [19] This direction was ratified, as it was found that there was a greater chance of type I error when analyzing the ACR50 response rates, thus reaching a result that could actually have happened by chance.
Regarding safety, Tweehuysen et al. [34] and Glintborg et al. [35] reported that the use of the biosimilar did not have a negative impact on RA disease activity, a finding that is similar to the results of this study, in which most AEs were mild or moderate in severity [36]. However, in this review, urinary tract infection (UTI) and an increase in alanine aminotransferase (ALT) are notable AEs because they were not present in analyses above 6 months. These differences were also identified for another anti-TNF biological drug certolizumab; however, more studies are needed to evaluate other clinical comorbidities not associated with the drug [37].
Some AEs were only present in the analyses above 6 months, for example, cough, upper respiratory tract infection (URTI), erythema at the injection site, and rash at the injection site. In this regard, Hans-Peter et al. [38] reported that infections that cause AEs are very common after the use of biologic ETN. I Greenblatt et al. [39] reported that AEs associated with intramuscular injections affect blood vessels, nerves, and muscles. However, these AEs can be minimized through the knowledge and skills of patients; thus, it is necessary for guidelines and training to be provided to patients who self-administer etanercept injections.
The low incidences of ADAs and positive neutralizing antibody reactions after the administration of biosimilars are consistent with immunogenicity results for patients with RA in other studies [34, 36, 40–43]. In fact, there are product-specific factors that affect immunogenicity, such as the original nature of the product (synthetic or human), impurities, product aggregates, formulation, glycosylation, and container closure system [44–50].
The study by Tweehuysen et al. [34] found that the biosimilar etanercept SB4 was less immunogenic than the reference biologic. However, it is necessary to investigate in future studies the factors that contribute to the lower immunogenicity profile of SB4.
MTX was an important synthetic DMARD used in select clinical studies because when associated with biosimilar or biologic drugs, etanercept in first-line therapy achieved satisfactory clinical responses for safety and efficacy. Vollenhoven et al. [51] reported that MTX monotherapy generated only 20 to 40% clinical improvement in patients with moderate to severe RA. In this sense, combinations of biosimilar drugs and DMARDs may be valid strategies in patients with RA who show an unsatisfactory response to the strict use of synthetic or biological DMARDs [7].
The growing evidence of the lack of statistical significance for many of the efficacy and safety outcomes of etanercept biosimilars compared to the biologic ETN raises an alert regarding statistical analysis. In half of the individual studies, sample size calculation methods were not reported [19, 25, 26]; among those that did report, two were based on predefined criteria for power and effect size [23, 25], and one study determined sample size using historical data for equivalence testing [20]. Conversely, more than half (83.33%) of the studies described statistical power (2 studies at 80% [19, 20] and 3 studies at 90% [23–25]) as well as the adequacy of this power to detect the expected effect sizes. It is essential to note that when studies have low statistical power, they are more likely to produce imprecise estimates or false-negative results, failing to detect an effect when it truly exists. This occurs because statistical power is influenced by several factors, including sample size, event rate in dichotomous outcomes, and data variability in continuous outcomes.
It is noteworthy that, in this study, the introduction of biosimilars can contribute to a substantial reduction in the costs of biological treatments, without compromising the quality of care offered to patients with RA [52]. This also contributes to the continued monitoring of the long-term efficacy and safety of biosimilars through post-marketing studies and patient registries, as well as to the acceptance and confidence of health professionals and patients in biosimilars in their successful integration into therapeutic regimens, having significant implications for clinical practice and the incorporation of health policy [7].
Limitations
Some limitations were identified in this systematic review with meta-analysis: (1) the low number of primary multicenter phase III RCTs comparing biosimilars with biologic etanercept; (2) inconsistency of evidence due to high heterogeneity in some outcomes; (3) variability in the follow-up time of outcomes above 6 months; (4) absence of blinding in the switching studies; and (5) the lack of subgroup analyses in different ethnic populations worldwide.
Implications for research and clinical practice
The introduction of biosimilars may allow the pharmaceutical market to further reduce healthcare costs due to greater discounts [52]. Furthermore, health education and communication strategies positively influence patients’ expectations about the transition to a biosimilar, resulting in better acceptance rates due to the attribution of drug effects [34].
In light of the uncertainty regarding the real-world similarity of biosimilars, the results of this review are consistent with previous findings: the use of biosimilars or biologics does not have a significant impact on efficacy, safety, or immunogenicity [40]. More RCTs should be performed to identify different clinical indications for the treatment of RA, with a larger sample to confirm the real long-term efficacy, safety, and immunogenicity.
Conclusions
This is the first systematic review with meta-analysis that evaluated the efficacy, safety, and immunogenicity of biosimilars with those for biologic etanercept; it is an innovative study because it measures associations in phase III RCTs that have high scientific evidence.
No unexpected or new events were observed during the study. The long-term administration of biosimilars was associated with continued efficacy and was well tolerated in patients with RA. Furthermore, treatment with a biosimilar not only improves the clinical outcomes but also the functional outcomes of patients with RA. This study found evidence that biosimilars have safety, efficacy, and immunogenicity similar to those for the reference biologic. Surveillance and post-sale registration studies are necessary to monitor the efficacy and safety of biosimilars consumed in the long term and in different regions and ethnic populations under treatment.
Supplementary Information
Supplementary Material 1: Table S1. PRISMA 2020 checklist statement: an updated guideline for reporting systematic reviews. Chart 1. PICOS framework for the systematic review. Chart 2. Search strategy for the systematic review. Figure S1. Meta-analyses of HAQ up to and above 6 months. Figure S2. Meta-analyses of non-serious adverse events up to 6 months. Figure S3. Meta-analyses of non-serious adverse events above 6 months. Figure S4. Meta-analyses of serious adverse events up to/over 6 months. Figure S5. Subgroup meta-analyses for Asian countries of ACR20, ACR50 and ACR70 up to 6 months. Figure S6. Subgroup meta-analyses for Asian countries of ACR20, ACR50 and ACR70 above 6 months. Figure S7. Subgroup meta-analyses for Asian countries of non-serious adverse events and serious adverse above 6 months. Figure S8. Subgroup meta-analyses for Asian countries of HAQ above 6 months. Table S2. Grade analyses for the outcomes comparing biosimilar etanercept with biologic etanercept in patients with rheumatoid arthritis. Table S3. Characterization of positive ADA immunogenicity among patients with rheumatoid arthritis in the ETN (biologic) and biosimilar groups. Table S4. Characterization of positive neutralizing antibodies in patients with rheumatoid arthritis in the ETN (biologic) and biosimilar groups.
Acknowledgements
Evidence Program for Health Policies and Technologies (PEPTS) Oswaldo Cruz Foundation (Fiocruz) Brasília and the Department of Pharmaceutical Assistance of the Ministry of Health of Brazil.
Authors’ contributions
AMA and JMG performed the search, removed duplicate articles, read the titles and abstracts of the articles, extracted data, and independently assessed the risks of bias. DCRP acted as a third researcher to resolve discrepancies in the selection of studies and guided the study process. JRB participated in the extraction of data from the selected studies and resolved discrepancies in the data extraction performed by the other two researchers (AMA and JMG). ETS resolved divergences in the assessment of the risks of bias and provided guidance on the use of the necessary research tools. All authors read and approved the final version of the manuscript.
Funding
AMA, JMG, and JRB were funded by the project GEREB 003 FIO 18-Subproject 1 (Qualification of the Capacity for Governance and Management of Pharmaceutical Assistance within the SUS) through the Department of Pharmaceutical Assistance of the Ministry of Health and Fiocruz Brasília.
Data availability
All data generated or analyzed during this study are included in this article, together with its supplementary information file (Supplementary material 1).
Declarations
Ethics approval and consent to participate
This study is a systematic review and was registered on the PROSPERO platform under the number CRD42020166610.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–96. 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–8. 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, Davis JM 3rd, Hunder GG, Therneau TM, Gabriel SE. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–9. 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Protocolo Clínico e Diretrizes Terapêuticas da Artrite Reumatóide . Brasília: Ministério da Saúde; 2020. Available from: http://conitec.gov.br/images/Consultas/Relatorios/2020/Relatrio_Artrite_Reumatoide_CP_21_2020.pdf. 2020.
- 6.World Organization Health (WHO). Glossário com definições da Organização Mundial de Saúde. International Alliance of Patients Organizations. Biored Brasil. 2016. Available from: https://www.bioredbrasil.com.br/glossario-com-definicoes-da-organizacao-mundial-da-saude-oms/#:~:text=Medicamentos.
- 7.Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–75. 10.1136/ard.2009.126532. Epub 2010 May 5. Erratum in: Ann Rheum Dis. 2011 Aug;70(8):1519 [DOI] [PMC free article] [PubMed]
- 8.Sigurdardottir V, Svärd A. Repeated switches between reference product etanercept and biosimilar do not affect disease activity or retention rate of etanercept over 24 months - a cohort study with historical controls. Joint Bone Spine. 2019;86(4):529–30. 10.1016/j.jbspin.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Gomes E, Rosseto R, Pinheiro L, Hasenclever L, Paranhos J. Desenvolvimento de Biossimilares no Brasil. Front J Soc Technol Environ Sci. 2016;5(1):31–42. Available from: http://periodicos.unievangelica.edu.br/index.php/fronteiras/.
- 10.Weise M, Bielsky MC, De Smet K, Ehmann F, Ekman N, Giezen TJ, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–7. 10.1182/blood-2012-04-425744. [DOI] [PubMed] [Google Scholar]
- 11.Niazi SK. Biosimilars and Interchangeable Biologics: Tactical Elements. Boca Raton: CRC Press, Taylor & Francis Group: CRC Press, Taylor & Francis Group; 2016. 575 p.
- 12.Mota, L.M.H.d., Kakehasi, A.M., Gomides, A.P.M. et al. 2017 recommendations of the Brazilian Society of Rheumatology for the pharmacological treatment of rheumatoid arthritis. Adv Rheumatol (London, England). 2018;58(1):2. 10.1186/s42358-018-0005-0. [DOI] [PubMed]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus H, Strand V, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–35. 10.1002/art.1780380602. [DOI] [PubMed]
- 15.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. Cochrane Database of Systematic Reviews. 2016;Issue 10(Suppl 1). 10.1002/14651858.CD201601.
- 17.Brasil. Diretrizes metodológicas: Sistema GRADE – Manual de graduação da qualidade da evidência e força de recomendação para tomada de decisão em saúde. Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Ciência e Tecnologia. – Brasília: Ministério da Saúde, 2014. 72f. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_metodologicas_sistema_grade.pdf.
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Bae SC, Kim J, Choe JY, Park W, Lee SH, Park YB, et al. A phase III, multicentre, randomised, double-blind, active-controlled, parallel-group trial comparing safety and efficacy of HD203, with innovator etanercept, in combination with methotrexate, in patients with rheumatoid arthritis: the HERA study. Ann Rheum Dis. 2017;76(1):65–71. 10.1136/annrheumdis-2015-207613. [DOI] [PubMed] [Google Scholar]
- 20.Emery P, Vencovský J, Sylwestrzak A, Leszczyński P, Porawska W, Baranauskaite A, Tseluyko V, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2015;76(1):51–7. 10.1136/annrheumdis-2015-207588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emery P, Vencovský J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, Tseluyko V, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2017;56(12):2093–101. 10.1093/rheumatology/kex269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery P, Vencovský J, Sylwestrzak A, Leszczyński P, Porawska W, Stasiuk B, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017;76(12):1986–91. 10.1136/annrheumdis-2017-211591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworski J, Matucci-Cerinic M, Schulze-Koops H, Buch MH, Kucharz EJ, Allanore Y, et al. Switch from reference etanercept to SDZ ETN, an etanercept biosimilar, does not impact efficacy, safety, and immunogenicity of etanercept in patients with moderate-to-severe rheumatoid arthritis: 48-week results from the phase III, randomized, double-blind EQUIRA study. Arthritis Res Ther. 2019;21(1):130. 10.1186/s13075-019-1907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuno H, Tomomitsu M, Hagino A, Shin S, Lee J, Song YW. Phase III, multicentre, double-blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann Rheum Dis. 2018;77(4):488–94. 10.1136/annrheumdis-2017-212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matucci-Cerinic M, Allanore Y, Kavanaugh A, Buch MH, Schulze-Koops H, Kucharz EJ, et al. Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study. RMD Open. 2018;4(2):e000757. 10.1136/rmdopen-2018-000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MC, Matsuno H, Kim J, Park SH, Lee SH, Park YB. Long-term efficacy, safety and immunogenicity in patients with rheumatoid arthritis continuing on an etanercept biosimilar (LBEC0101) or switching from reference etanercept to LBEC0101: an open-label extension of a phase III multicentre, randomised, double-blind, parallel-group study. Arthritis Res Ther. 2019;21(1):122. 10.1186/s13075-019-1910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa JO, Lemos LLP, Machado MAA, Almeida AM, Kakehasi AM, Araújo VE et al . Infliximabe, metotrexato e sua combinação no tratamento da artrite reumatoide: revisão sistemática e metanálise. Rev Bras Reumatol. 2015;55(2):146–158. 10.1016/j.rbr.2014.10.009.
- 28.Machado, Marina Amaral de Ávila et al. Adalimumabe no tratamento da artrite reumatoide: uma revisão sistemática e metanálise de ensaios clínicos randomizados. Revista Brasileira de Reumatologia. 2013, v. 53, n. 5, pp. 419–430. Available from: <>. Epub 23 May 2014. ISSN 1809–4570.
- 29.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10(42):iii-iv, xi-xiii, 1–229. 10.3310/hta10420. [DOI] [PubMed]
- 30.Navarro-Sarabia F, Ariza-Ariza R, Hernández-Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. J Rheumatol. 2006;33(6):1075–81 (Epub 2006 May 1). [PubMed] [Google Scholar]
- 31.Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordström DC, Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS ONE. 2012;7(1):e30275. 10.1371/journal.pone.0030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;17(9):52. 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos EJF, Cunha M. Interpretação Crítica dos Resultados Estatísticos de uma Meta‐Análise: Estratégias Metodológicas. Millenium, 2013;0(44):85‐98.
- 34.Tweehuysen L, Huiskes VJB, van den Bemt BJF, Vriezekolk JE, Teerenstra S, van den Hoogen FHJ, et al. Open-label, non-mandatory transitioning from originator etanercept to biosimilar SB4: six-month results from a controlled cohort study. Arthritis Rheumatol. 2018Sep;70(9):1408–18. 10.1002/art.40516. [DOI] [PubMed] [Google Scholar]
- 35.Glintborg B, Sørensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–31. 10.1136/annrheumdis-2016-210742. [DOI] [PubMed] [Google Scholar]
- 36.Lee YJ, Shin D, Kim Y, Kang J, Gauliard A, Fuhr R. A randomized phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel®) in healthy subjects. Br J Clin Pharmacol. 2016;82(1):64–73. 10.1111/bcp.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brasil. Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas. Artrite reumatoide: Portaria SAS/MS nº 710, de 27 de junho de 2013, retificada em 06 de março de 2014 e 10 de junho de 2014. Brasília; 2014. Available from: http://conitec.gov.br/images/Protocolos/Artrite-Reumatoide.pdf.
- 38.Hofmann HP, Kronthaler U, Fritsch C, Grau R, Müller SO, Mayer R, et al. Characterization and non-clinical assessment of the proposed etanercept biosimilar GP2015 with originator etanercept (Enbrel(®)). Expert Opin Biol Ther. 2016;16(10):1185–95. 10.1080/14712598.2016.1217329. [DOI] [PubMed] [Google Scholar]
- 39.Greenblatt DJ, Allen MD. Intramuscular injection-site complications. JAMA. 1978;240(6):542–4. [PubMed] [Google Scholar]
- 40.Amgen. Enbrel US Prescribing Information. Vol. 50, Interactions. 1998. Available from: http://pi.lilly.com/us/zyprexa-pi.pdf.
- 41.Dore RK, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(1):40–6. [PubMed]
- 42.Hoshino M, Yoshio T, Onishi S, Minota S. Influence of antibodies against infliximab and etanercept on the treatment effectiveness of these agents in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2012;22(4):532–40. 10.1007/s10165-011-0567-8. [DOI] [PubMed] [Google Scholar]
- 43.Mazilu D, Opriş D, Gainaru C, et al. Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment. Biomed Res Int. 2014;2014:702701. 10.1155/2014/702701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100(2):354–87. 10.1002/jps.22276. (Epub 2010 Aug 25). [DOI] [PubMed] [Google Scholar]
- 45.Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21(6):897–903. 10.1023/b:pham.0000029275.41323.a6. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–7. 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noguchi A, Mukuria CJ, Suzuki E, Naiki M. Immunogenicity of N-glycolylneuraminic acid-containing carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. J Biochem. 1995;117(1):59–62. 10.1093/oxfordjournals.jbchem.a124721. [DOI] [PubMed] [Google Scholar]
- 48.Sheeley DM, Merrill BM, Taylor LC. Characterization of monoclonal antibody glycosylation: comparison of expression systems and identification of terminal alpha-linked galactose. Anal Biochem. 1997;247(1):102–10. 10.1006/abio.1997.2036. [DOI] [PubMed] [Google Scholar]
- 49.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364(1):8–18. 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Flynn GC, Chen X, Liu YD, Shah B, Zhang Z. Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol Immunol. 2010;47(11–12):2074–82. 10.1016/j.molimm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, Chatzidionysiou K, Bratt J; Swefot study group. Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet. 2012;379(9827):1712–20. 10.1016/S0140-6736(12)60027-0. [DOI] [PubMed]
- 52.Dörner T, Strand V, Cornes P, Gonçalves J, Gulácsi L, Kay J, Kvien TK, Smolen J, Tanaka Y, Burmester GR. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75(6):974–82. 10.1136/annrheumdis-2016-209166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. PRISMA 2020 checklist statement: an updated guideline for reporting systematic reviews. Chart 1. PICOS framework for the systematic review. Chart 2. Search strategy for the systematic review. Figure S1. Meta-analyses of HAQ up to and above 6 months. Figure S2. Meta-analyses of non-serious adverse events up to 6 months. Figure S3. Meta-analyses of non-serious adverse events above 6 months. Figure S4. Meta-analyses of serious adverse events up to/over 6 months. Figure S5. Subgroup meta-analyses for Asian countries of ACR20, ACR50 and ACR70 up to 6 months. Figure S6. Subgroup meta-analyses for Asian countries of ACR20, ACR50 and ACR70 above 6 months. Figure S7. Subgroup meta-analyses for Asian countries of non-serious adverse events and serious adverse above 6 months. Figure S8. Subgroup meta-analyses for Asian countries of HAQ above 6 months. Table S2. Grade analyses for the outcomes comparing biosimilar etanercept with biologic etanercept in patients with rheumatoid arthritis. Table S3. Characterization of positive ADA immunogenicity among patients with rheumatoid arthritis in the ETN (biologic) and biosimilar groups. Table S4. Characterization of positive neutralizing antibodies in patients with rheumatoid arthritis in the ETN (biologic) and biosimilar groups.
Data Availability Statement
All data generated or analyzed during this study are included in this article, together with its supplementary information file (Supplementary material 1).