Abstract
Immunity to mycobacterial infection is closely linked to the emergence of T cells that secrete cytokines, gamma interferon (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α), resulting in macrophage activation and recruitment of circulating monocytes to initiate chronic granuloma formation. The cytokine that mediates macrophage activation is IFN-γ, and, like IL-12, IL-18 was shown to activate Th1 cells and induce IFN-γ production by these cells. In order to investigate the role of IL-18 in mycobacterial infection, IL-18-deficient mice were infected with Mycobacterium tuberculosis and Mycobacterium bovis BCG Pasteur, and their capacities to control bacterial growth, granuloma formation, cytokine secretion, and NO production were examined. These mice developed marked granulomatous, but not necrotic, lesions in their lungs and spleens. Compared with the levels in wild-type mice, the splenic IFN-γ levels were low but the IL-12 levels were normal in IL-18-deficient mice. The reduced IFN-γ production was not secondary to reduced induction of IL-12 production. The levels of NO production by peritoneal macrophages of IL-18-deficient and wild-type mice did not differ significantly. Granulomatous lesion development by IL-18-deficient mice was inhibited significantly by treatment with exogenous recombinant IL-18. Therefore, IL-18 is important for the generation of protective immunity to mycobacteria, and its main function is the induction of IFN-γ expression.
Gamma interferon (IFN-γ) is a cytokine secreted by activated T cells and natural killer (NK) cells. It exerts various immunomodulatory effects on several types of cells (25) and is one of the major cytokines responsible for activating macrophages, which mediate nonspecific, cell-mediated, host defenses (12, 20). IFN-γ has been shown to be an important mediator of macrophage activation involved in controlling a number of intracellular pathogens, including Mycobacterium tuberculosis, Leishmania major, Leishmania donovani, and Listeria monocytogenes (2, 10, 14, 18). This protective immunity is closely associated with emergence kinetics and loss of CD4 T cells that secrete large amounts of IFN-γ (17). IFN-γ has been shown to play a critical role in protective immunity, as demonstrated by the severe disseminated form of tuberculosis observed in IFN-γ-gene-disrupted mice (4, 6). Recently, we also developed IFN-γ-gene-deficient mice (21, 22) and demonstrated that endogenous IFN-γ plays critical roles in macrophage fusion, cell recruitment, and granuloma assembly (21).
Interleukin-18 (IL-18) is a cytokine that has been identified in the livers of mice treated with Propionibacterium acnes and lipopolysaccharide (15). This cytokine was originally identified as an IFN-γ-inducing factor, which induces IFN-γ production by splenocytes, hepatic lymphocytes, and type 1 T helper (Th1) cell clones (11, 13, 16). IL-18 appears to have biological functions similar to those of IL-12, which is known to possess immunoregulatory activities. However, IL-18 was reported to be strikingly similar to the IL-1 family of cytokines (1) and showed no similarity to IL-12 (24).
In order to determine the in vivo functions of IL-18 in mycobacterial infection, we generated IL-18-deficient mice by gene targeting (23). We used these knockout (KO) mice as a model of systemic infection and showed that IL-18 is important for the development of protective immunity, although these mice infected with M. tuberculosis did not develop the acute disseminated form of infection.
MATERIALS AND METHODS
Mice.
We disrupted the IL-18 gene by homologous recombination in E14.1 embryonic stem cells, and a targeting vector was constructed to replace a 3.0-kb fragment of genomic DNA containing exons 3, 4, and 5 of the IL-18 gene with the neomycin resistance gene (23). Heterozygous mice were crossed to produce mice homozygous for the IL-18 gene mutation. IL-18-deficient C57BL/6 mice were born at the expected Mendelian ratios and were phenotypically normal and fertile. Their serum IL-18 concentrations, assessed by enzyme-linked immunosorbent assay (ELISA), were below the detectable level (the serum IL-18 concentration in normal C57BL/6 mice was 1,000 ± 20 pg/ml), indicating that the IL-18 gene mutation led to a lack of IL-18 production.
Experimental infections.
The virulent Kurono strain (ATCC 358121) and the avirulent strain Mycobacterium bovis BCG Pasteur (ATCC 27289) of M. tuberculosis were grown in Middlebrook 7H9 medium (Difco) to the mid-log phase (21). The cultured strains were filtered with a 4-μm-pore-size membrane filter (Millipore) before use so that they were dispersed evenly. IL-18-deficient and wild-type (WT) mice (10 mice/group) were infected intravenously (i.v.) via a lateral tail vein with an inoculum of 106 to 107 CFU of Kurono or BCG Pasteur strain suspended in 100 μl of phosphate-buffered saline (PBS). Mice were infected by an airborne route by placing them in the exposure chamber of an airborne infection apparatus (model 099CA4212; Glas-Col, Inc., Terre Haute, Ind.). The nebulizer compartment was filled with 5 ml of a suspension of 105 to 106 CFU of Kurono or BCG Pasteur strain at a concentration calculated to provide an uptake of ca. 200 to 500 viable bacilli by the lungs just after inhalation exposure during exposure for 60 min under the experimental conditions for this study. The survival of groups of mice for 100 days after infection with M. tuberculosis or M. bovis BCG Pasteur was recorded, and survival curves were plotted. The lungs from IL-18-KO, WT, and IFN-γ-KO mice (five mice each) (21) were retrieved from infected mice 10, 30, and 50 days after airborne infection, homogenized, diluted, plated on Ogawa slant medium, and incubated at 37°C for 21 days, and the colonies were counted.
Histology.
Some mice were sacrificed 50 days after infection, and some were monitored for up to 100 days postinfection. Tissue sections (5 μm thick) from paraffin blocks containing lung, liver, and spleen tissue were stained with hematoxylin and eosin or by the Ziehl-Neelsen method for acid-fast bacilli. We prepared every lung tissue with white nodular lesions. The sizes of the 10 granulomas were measured with a micrometer (Nikon Optical Co., Tokyo, Japan).
Cytokine assays.
In order to determine whether the splenic cells of IL-18-KO mice secrete IFN-γ, the spleens were harvested from the IL-18-deficient and WT mice and single cell suspensions were prepared. The cell suspensions were plated (5 × 105 cells/well) in 96-well culture plates and incubated for 3 days at 37°C in 5% CO2 in air, and the cells were stimulated with either medium alone or medium containing concanavalin A (ConA, 5 μg/ml), purified protein derivative (10 μg/ml), or live BCG organisms (103 CFU/well). The concentrations of IL-4, IL-10, IL-12, IL-1β, IFN-γ, and tumor necrosis factor alpha (TNF-α) in the culture supernatants of the cells incubated in the presence of the above reagents were measured by sandwich ELISA (Biosource International, Calif.).
RT-PCR.
Spleen tissue samples were taken from infected mice 14 or 50 days after infection, frozen in liquid nitrogen, and stored at −80°C until required for use, when RNA was extracted as described previously (3). Reverse transcriptase (RT)-PCR was carried out with gene-specific primer sets for inducible NO synthase (iNOS), TNF-α, IFN-γ, IL-12, IL-1β, and IL-18 (CLP Inc.) the respective sizes of which were 306, 276, 405, 850, and 447 bp. The size of β-actin used as a positive control was 514 bp. The same amounts of β-actin RNA from the spleen tissues used as an internal control were used in RT-PCR analysis.
Macrophage NO assay.
Peritoneal adherent macrophages (5 × 105/well) in RPMI 1640 supplemented with 10% (vol/vol) fetal calf serum were plated in 96-well culture plates, unstimulated or stimulated with recombinant IL-18 (100 ng/well; Pepro Tech) or IFN-γ (1,000 international units [IU]; Genzyme), and then cultured with the BCG or Kurono strain overnight. IFN-γ was added to determine whether it was able to activate macrophages from the KO mice. The supernatants were collected 36 h after the cultures were seeded and filtered, and the nitrite concentrations were determined by the Griess assay, as described previously (7).
Reconstitution of IL-18-deficient mice with exogenous IL-18.
Mice were injected subcutaneously with 10 μg of recombinant IL-18 (Pepro Tech) in PBS or PBS alone four times at weekly intervals. The biological activity of the recombinant IL-18 was evaluated by determining the 50% effective dose (12 ng/ml) by measuring the concentration-dependent stimulation of IFN-γ production by murine lymph node cells (4 × 105 cells/well) costimulated with ConA at a suboptimal concentration (750 ng/ml). The lungs from IL-18-KO mice treated subcutaneously with recombinant IL-18 were retrieved from the infected mice 7 weeks after airborne infection.
Statistical methods.
The values were compared by Student’s t test. For all statistical analyses, a P value of <0.01 was considered significant.
RESULTS
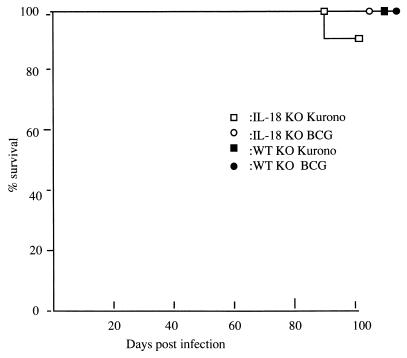
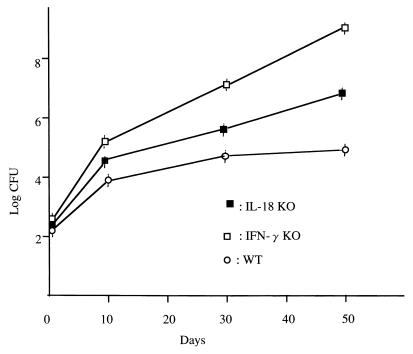
KO and WT littermates were infected i.v. (106 to 107 CFU) or by the airborne route (105 CFU) with virulent M. tuberculosis Kurono. All the KO and WT mice survived until the date of sacrifice (100 days after infection). When 107 CFU of the Kurono strain was given i.v. to five KO mice, one mouse died by 86 days postinfection (Fig. 1). Figure 2 shows the CFU in lung tissues of IL-18-KO, WT, and IFN-γ-KO mice inoculated with 106 CFU of the Kurono strain by the airborne route. The number of CFU in lung tissues from IL-18-KO mice was higher than that of WT mice but lower than that of IFN-γ-KO mice.
FIG. 1.
Survival curves of mice infected with M. tuberculosis or M. bovis BCG Pasteur. IL-18-KO and WT mice were infected i.v. with 106 CFU of the Kurono or BCG Pasteur strain. Data presented are from two separate experiments with 10 mice in each group.
FIG. 2.
CFU in lung tissues of IL-18-KO, WT, and IFN-γ-KO mice (12 mice each) exposed to 106 CFU of M. tuberculosis by the airborne route. At the indicated days after infection, four mice from each group were sacrificed and homogenates of lung tissues were plated. IFN-γ-KO mice were used as positive controls. Error bars indicate standard errors of the means.
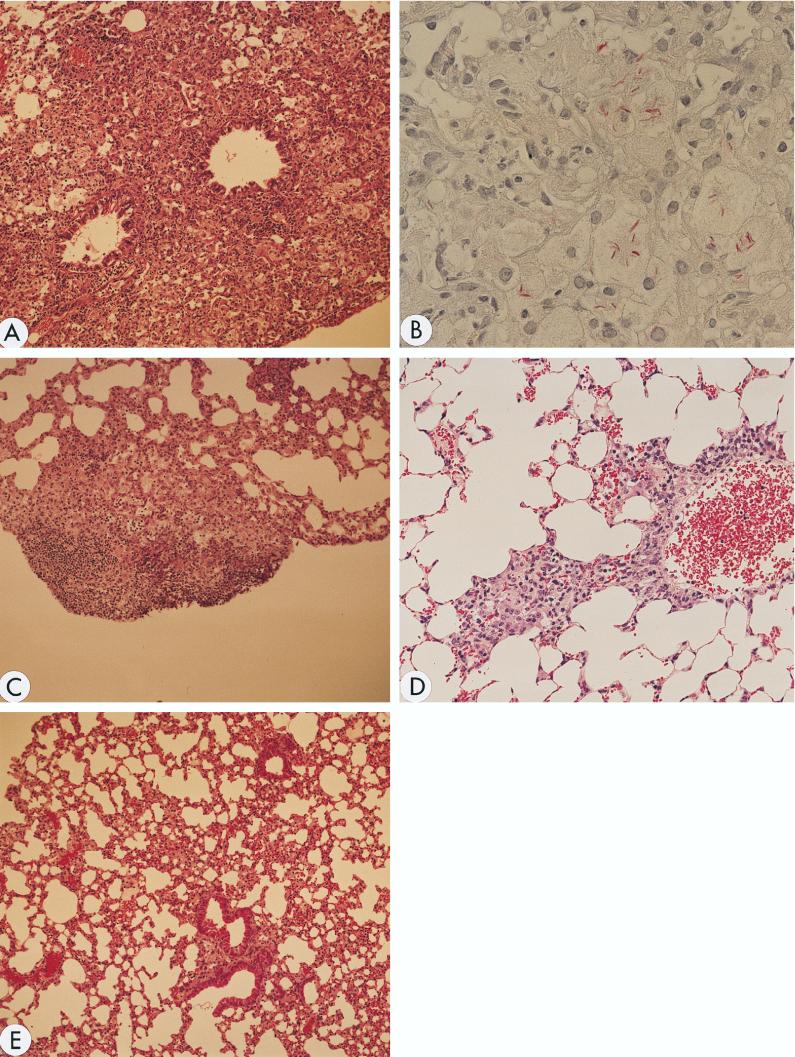
When 107 CFU of the Kurono strain was administered i.v. to KO mice, larger granulomas were observed in their lungs and spleens than in those of the WT controls (Fig. 3A). The average diameter of all granulomas in the lungs of IL-18-KO mice was 18,620 ± 430 μm, whereas that of WT mice was 5,880 ± 300 μm (P < 0.01). No necrotic lesions were present in the major organs. Hardly any granulomas were found in the organs of the WT mice. Similar pathological profiles were observed after infection by the airborne route, and numerous bacteria were present in the granulomatous lesions (Fig. 3B).
FIG. 3.
Histologic examination of lung tissues. Mice were killed 7 weeks after infection, and formalin-fixed sections were stained with hematoxylin and eosin (A, C, D, and E) and for acid-fast bacilli (B). (A) Tissue from IL-18-KO mice infected with the Kurono strain. A large, indiscrete granuloma with foamy macrophages is noted. Magnification, ×100. (B) Tissue from IL-18-KO mice infected with the Kurono strain. Mycobacteria stained red and are recognized in the granuloma by Ziehl-Neelsen staining. Magnification, ×600. (C) Tissue from WT mice infected with the Kurono strain. A small, discrete granuloma was formed. Magnification, ×100. (D) Tissue from IL-18-KO mice infected with the Kurono strain and treated four times subcutaneously with recombinant IL-18. The granuloma became smaller. Magnification, ×200. (E) Tissue from WT mice infected with BCG Pasteur. No granuloma was recognized. Alveolar septal thickening was noted. Magnification, ×100.
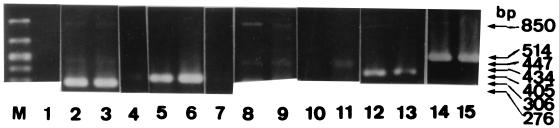
As the defect in the KO mice was genetically defined, the possibility of recovering immune response function by administering exogenous recombinant IL-18 was investigated. When IL-18 was given subcutaneously, the sizes of the granulomatous lesions were reduced significantly (mean diameter, 4,820 ± 153 μm), and there were virtually no bacteria in the lesions (Fig. 3C). Significantly less iNOS and TNF-α mRNA was produced by spleen cells of IL-18-KO mice than by those of untreated KO mice (Fig. 4). Thus, treatment with exogenous IL-18 reduced the bacterial load (104 versus 106 CFU), indicating that IL-18 plays an important role in the immune response to M. tuberculosis.
FIG. 4.
In vivo expression of various cytokines and iNOS mRNA in Kurono strain-infected mice by RT-PCR. The spleen tissues of IL-18-KO (lanes 1, 3, 4, 6, 7, 9, 11, 13, and 15) and WT (lanes 2, 5, 8, 10, 12, and 14) mice were removed 7 weeks after infection. M, size marker; lanes: 1, IL-18 from IL-18-KO mouse (no amplified band); 2 to 4, TNF-α (no TNF-α in IL-18-KO mice treated with recombinant IL-18 [lane 4]); 5 to 7, iNOS (almost no amplified band in IL-18-KO mice treated with recombinant IL-18 [lane 7]); 8 and 9, IL-12; 10 and 11, IL-1β; 12 and 13, IFN-γ; 14 and 15, β-actin.
In order to examine the major cytokine mRNA expression profiles of IL-18-KO mice, RT-PCR analysis of the infected spleens was carried out. No splenic IL-18 mRNA expression was detected in any IL-18-KO mouse (Fig. 4). IFN-γ, TNF-α, and iNOS mRNA was expressed to a moderate degree, but the IL-1β and IL-12 mRNA expression levels were somewhat lower. The IFN-γ, TNF-α, iNOS, IL-1β, and IL-12 mRNA expression levels in the infected mice were significantly lower than those in the WT mice.
In order to determine whether the absence of IL-18 influenced the induction and control of other cytokines, the levels of several cytokines in the culture supernatants of spleen cells were determined by sandwich ELISA. As shown in Table 1, spleen cells of KO mice produced significantly less IL-1β than those of WT mice.
TABLE 1.
Cytokine secretion by spleen cells of IL-18-KO mice
| Treatment | Amt (pg/ml) of cytokine secreted by IL-18-KO micea
|
|||||
|---|---|---|---|---|---|---|
| IL-1β | IL-4 | IL-10 | IL-12 | IFN-γ | TNF-α | |
| None | 0 (0) | 0 (0) | 0 (0) | 4 ± 0 (0) | 0 (0) | 50 ± 2 (40 ± 1) |
| BCG | 13 ± 1 (58 ± 1) | 0 (5 ± 0) | 156 ± 11 (120 ± 3) | 216 ± 11 (167 ± 5) | 61 ± 5 (490 ± 21) | 415 ± 15 (333 ± 12) |
| ConA | 0 (0) | 29 ± 3 (25 ± 1) | 52 ± 3 (60 ± 2) | 42 ± 5 (40 ± 1) | 87 ± 6 (420 ± 12) | 296 ± 3 (200 ± 9) |
| PPDb | 0 (12 ± 2) | 0 (16 ± 2) | 45 ± 4 (35 ± 2) | 111 ± 9 (142 ± 2) | 4 ± 0 (10 ± 0) | 423 ± 23 (370 ± 11) |
Cytokine values for WT mice are given in parentheses. Values are means ± standard errors of the means.
PPD, purified protein derivative.
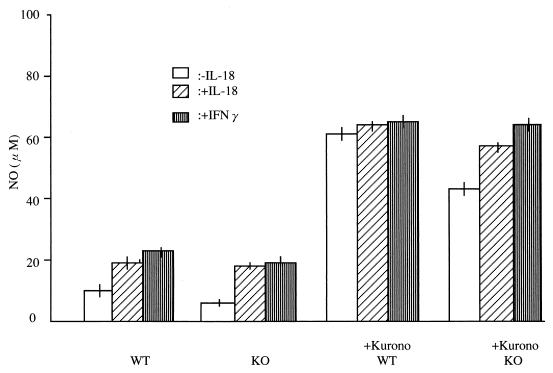
The NO levels in peritoneal macrophage supernatants were determined by the Griess assay and referred to a standard NaNO2 curve. The levels of NO production by unstimulated peritoneal macrophages from WT and KO mice were low (Fig. 5). When peritoneal macrophages from KO mice were stimulated overnight with either the BCG Pasteur or Kurono strain, the NO levels increased to 42 μM (KO mice) and 61 μM (WT control). When 100 ng of recombinant IL-18 was added to cultures of macrophages from KO mice, NO production increased to a moderate degree (57 μM). When 1,000 IU of IFN-γ was added to macrophages of KO and WT mice, NO production increased to 64 and 65 μM, respectively.
FIG. 5.
Nitric oxide production by the peritoneal macrophages from IL-18-KO and WT mice stimulated with the Kurono strain overnight in the presence or absence of recombinant IL-18 or with IFN-γ. Thereafter, the NO-producing ability of the macrophages was determined by the Griess reagent.
DISCUSSION
We have found that large granulomas were induced by inoculation of an M. tuberculosis strain but that necrotic lesions were not recognized in major organs. IFN-γ production by spleen cells from KO mice was reduced moderately, but neither IL-12, TNF-α, IL-4, nor IL-10 secretion by cells of KO and WT mice differed significantly. Therefore, the expression of Th2 cytokine mRNA, including IL-4 and IL-10 mRNA, does not contribute to the progress of mycobacterial infection in this experimental system. The splenic IFN-γ level in IL-18-KO mice was significantly lower than that in WT mice, demonstrating that IL-18 plays an important role in IFN-γ production. However, exogenous IFN-γ and IL-18 were still able to activate the peritoneal macrophages of IL-18-KO mice to various degrees. The IL-12 levels after mitogenic in vitro challenge of spleen cells from IL-18 KO and WT mice were almost the same, suggesting that the reduced IFN-γ production was not secondary to a low level of induction of IL-12 secretion. Together with the RT-PCR data, these data suggest that Th1 and NK cells of IL-18-KO mice are still able to produce IFN-γ, although the NK cell activity and Th1-mediated responses of these IL-18-deficient mice are compromised (23). It is interesting that mice devoid of IL-18 production can secrete IFN-γ to a moderate degree and exogenous IFN-γ still can activate macrophages from these mice. NK cells and NK T cells may secrete IFN-γ as a compensatory function.
IL-18 has been reported to protect mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production (9). When recombinant IL-18 was administered subcutaneously, the sizes of the granulomas were reduced significantly (P < 0.01). Therefore, changing the therapeutic regimens administered to the experimental model mice in our study may enable mycobacterial infection to be prevented completely. iNOS and TNF-α mRNA produced by spleen cells of IL-18-KO mice was significantly less than by untreated KO mice. Thus, treatment with exogenous IL-18 reduced the bacterial load, indicating that IL-18 plays an important role in the immune response to M. tuberculosis. This finding contrasts with the findings that the sizes of the granulomatous lesions in IFN-γ-KO and TNF-α-KO mice infected with M. tuberculosis were not reduced significantly by recombinant IFN-γ or TNF-α (8, 21). IL-18 may play a pivotal role in the immunotherapy of tuberculosis.
It has been reported that IL-18 induces IL-8 and IL-1β via TNF-α production from non-CD14-positive human blood mononuclear cells (19). In our experiments, IL-18-KO mice expressed far less IL-1β mRNA than did the WT counterpart. This finding was also confirmed by ELISA in that the level of IL-1β secretion was lower than that in WT mice. Further study will be required to explain this interesting observation.
In summary, we demonstrated that IL-18 influenced the course of mycobacterial infection in mice. The inflammatory lesions in IL-18-KO mice were no more severe than those observed in IFN-γ-KO (4, 6), IL-12-KO (5), and TNF-α-KO mice (8). Therefore, IL-18 does not seem to play a role in Mycobacterium-induced granuloma formation. Like IL-12-KO mice, IL-18-KO mice displayed reduced IFN-γ production in vivo relative to that in WT mice, although secretion of IL-12 by antigen-challenged spleen cells in vitro was almost normal. These findings suggest that IL-18 is an important factor involved in IFN-γ production in vivo and that IL-18 deficiency cannot be compensated for by IL-12 and other cytokines.
REFERENCES
- 1.Bazan J F, Timans J C, Kastelein R A. A newly defined interleukin-1? Nature. 1996;79:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 2.Belosevic M, Davis C E, Meltzer M S, Nacy C A. Regulation of activated macrophage antimicrobial activities: identification of lymphokines that cooperate with interferon gamma for induction of macrophage resistance to infection. J Immunol. 1988;141:890–896. [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the 1-arginine-dependent killing mechanism in IFN-γ stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 8.Kaneko, H., H. Yamada, Y. Kazumi, S. Mizuno, K. Sekikawa, and I. Sugawara. The role of tumor necrosis factor (TNF)-alpha in Mycobacterium-induced granuloma formation in TNF-alpha deficient mice. Lab. Invest., in press. [PubMed]
- 9.Kawakami K, Mahboob H Q, Zhang T, Okumura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 10.Kinderlen A F, Kaufmann S H, Lohmann-Matthes M L. Protection of mice against the intracellular bacteria Listeria monocytogenes by recombinant interferon. Eur J Immunol. 1984;14:964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- 11.Kohno K, Kataoka J, Otsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 12.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;11:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashimoto K. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type 1 T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 14.Murray H W, Rubin B Y, Rothermel C D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes: evidence that interferon gamma is the activating lymphokine. J Clin Invest. 1983;72:1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata T, Tanabe Y, Akita K, Torigoe K, Okura T, Fukuda S. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxin shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe T, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 17.Orme I. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 18.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 19.Puren A J, Fantuzzi G, Gu Y, Su M S, Dinarello C A. Interleukin-18 (IFN-γ-inducing factor) induces IL-8 and IL-1 β via TNF-α production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber R D, Celada A. A molecular characterization of interferon γ as a macrophage activating factor. Lymphokines. 1985;11:87–118. [Google Scholar]
- 21.Sugawara I, Yamada H, Kazumi Y, Doi N, Otomo K, Aoki T, Mizuno S, Udagawa T, Tagawa Y, Iwakura Y. Granulomas in interferon γ gene-disrupted mice are inducible by avirulent Mycobacterium, but not by virulent Mycobacterium. J Med Microbiol. 1998;47:871–877. doi: 10.1099/00222615-47-10-871. [DOI] [PubMed] [Google Scholar]
- 22.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/−, but not in TNF-α−/− mice: role for IFN-γ in activating apoptosis in hepatocytes. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 23.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 24.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 25.Vilcek J, Rinderknecht E, Sevastopoulos C G. Interferon γ: a lymphokine for all seasons. Lymphokines. 1985;11:1–32. [Google Scholar]