Abstract
Osteoporosis, a metabolic disorder, remains challenging to treat due to limited understanding of its underlying mechanism. The annual cycle of “cyclic physiological osteoporosis (CPO)” and its full reversal in male deer represents a unique natural model for studying this condition. Deer antlers, weighing up to 25 kg/pair, derive over 60% of their mineral contents from deer skeleton during mineralization. Based on the literature, we propose to divide CPO and its reversal into two phases: Phase I (approximately 115 days): from hard antler casting to the end of antler linear growth, marked by simultaneous robust antler ossification and CPO development; and Phase II (up to 165 days): from end of Phase I to the onset of antler skin shedding, characterized by complete antler mineralization and CPO reversal. This review analyzes the paradoxical occurrence of robust antler ossification and skeleton CPO within the same endocrine microenvironment during phase I; total antler mineralization and full reversal of deer skeleton CPO in phase II. Furthermore, we will discuss potential insights for osteoporosis treatment using deer materials from the period of Phase II. Our goal is to identify novel substances and therapies that could be applied in clinical setting to effectively treat osteoporosis.
Keywords: Osteoporosis reversal, Deer skeleton, Deer antler, Bone formation, Bone resorption
Introduction
Osteoporosis is a common skeletal metabolism disorder characterized by reduced bone mass and microarchitectural deterioration in bone tissues, leading to increased risks of fracture [1–4]. It is predicted that osteoporosis symptom is becoming more serious problem worldwide along with demographic change and lifestyle transformation [5–7]. Currently, there are limited options to effectively treat osteoporosis [8, 9], as the mechanism underlying the condition is far from fully understood.
Animal models have played and continue to play an indispensable role in revealing the mechanisms underlying different clinical symptoms. Thus far, the best-established animal model for osteoporosis research is in mice, though there are occasional reports in other animals such as sheep, cats, dogs, pigs, hens, and non-human primates [10–13]. Although these artificially created models are of great value for understanding the etiology of osteoporosis, each can mimic only certain aspects of human osteoporosis. Gratefully, there exists a natural case, namely the male deer whose skeleton suffers severe osteoporosis annually and then fully recovers, a phenomenon causally related with the antler growth cycle. Banks et al. [14] termed this phenomenon “cyclic physiological osteoporosis” (CPO). We believe that the male deer might offer a unique natural model for the mechanistic study of osteoporosis and its reversal. Thus far, any in depth study into the molecular mechanism underlying this unique CPO is still lacking. Especially in recent years, deer CPO seems to have become a relatively neglected area, evidenced by the scarcity of relevant publications. A comprehensive review related to this topic would call attention to revisit this field, which, we believe, holds the potential for the effective treatment, and even reversal, of human osteoporosis.
Deer antlers are large bony organs, and as such, their mineralization requires huge amounts of calcium (Ca) and phosphorus (P) in a short period (around 3 to 4 months) [15]. According to Chapman [16], if a pair of red deer hard antlers weighs 13 kg and fully formed in 130 days, it corresponds to an average daily increase of 100 g of bone. The skeleton of an adult male red deer weighs about 26 kg and 80% of it is produced in the first 18 months of life. This corresponds to an average daily increase of bone of about 34 g which is far less than that required for antler growth [16]. As more than half of the minerals in the hard antler are supplied by the deer skeleton during the antler mineralization period [17], one can imagine the magnitude of the mineral loss in the male deer skeleton. To our surprise, however, these skeletons are able to fully recover from this severe osteoporosis within a month [18, 19]. This article reviews the literature related to deer CPO and provides insights into the potential use of this unique natural model for research and treatment of osteoporosis in clinics.
Deer bone CPO and associated biological changes
Minerals of deer skeleton turnover are closely tied up to different antler growth phases. Muir et al. [20] recorded that loss of bone minerals in four-year-old red deer stags starts during the rapid antler growth phase between 28 and 112 days after hard antler casting; by the cessation of antler linear growth, osteoporosis starts to reverse, such that the deer bone soon returns to a normal hardness [20].
Based on the currently available literatures, antler mineralization, CPO and CPO recovery of deer skeleton can be divided into two phases: Phase I – from the day of hard antler casting to termination of antler linear growth (around 110 to 115 days), CPO occurs in this Phase; and Phase II – from termination of Phase I to the commencement of velvet skin shedding (around 110 to 165 days), reversal of CPO occurs in this phase. Phase I falls in the period of low concentrations of plasma testosterone (normally T < 1 ng/ml), whereas Phase II is related to sharply increased levels of androgen hormones (normally T > 10 ng/ml) [21–23]. Full mineralization of the hard antler in Phase II is caused by a high level of testosterone, as antlers of castrated stags can still grow but cannot mineralize [24, 25] and this abnormality can be rescued by injection of exogenous androgen [26, 27].
Phase I. Antler mineralization and CPO as two contrasting processes under the same endocrine milieu
The most rapid antler growth period each year takes place when deer circulating testosterone level is at its nadir, and this low androgen level readily explains why demineralization of deer skeleton takes place in this period. Unexpectedly, within the same period, a robust ossification process in the growing antler tissue occurs, which is contradictory with the currently held view that osteoporosis in humans coincides with low level of sex hormones [28–31].
Robust ossification in growing antlers
During the period between April and July each year, antlers grow extremely fast: elongation rate up to 2 cm/day [20, 32–34]. The skeletal mass of a stag is estimated to be about 15% of its liveweight, given a skeletal mass of around 45 kg in a large Cervid of 300 kg and an antler mass of 25 kg (formed in 110 days), the antler mass would be more than half that of the skeletal mass on a dry-weight basis [16]. Therefore, the rate of antler bone formation far exceeds that of the deer skeleton.
Krauss et al. [35] described the formation of bone structures in the growing antler in Phase I. In the beginning, a tubular framework initially composed of mineralized cartilage develops; this is then converted into a tubular framework composed of micro-lamellar bone with a low degree of collagen fibril. This framework has a honeycomb-like appearance with the cylindrical pores oriented along the main antler axis. Later, the tube lumina are filled with rod-shaped primary osteons, whose collagen fibrils are mainly oriented along the pores, thus improving the antler’s mechanical properties. This peculiar structural framework may enable the antler to achieve both rapid growth and intensive ossification.
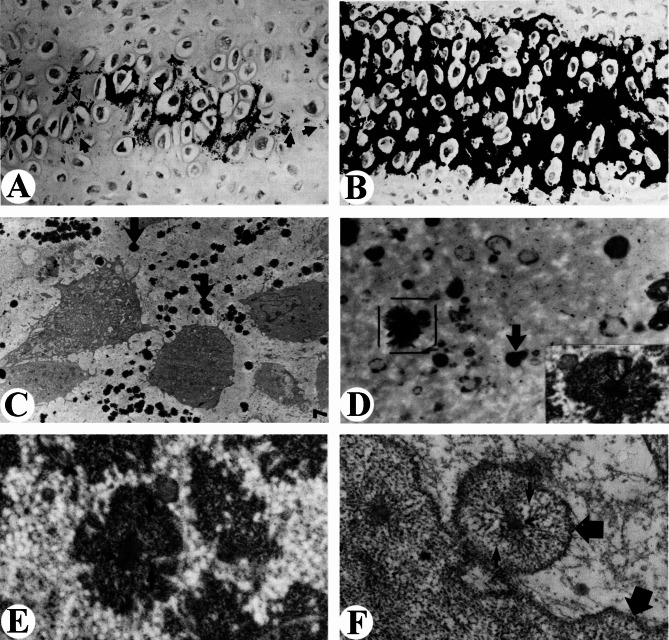
Banks and Newbrey [36, 37] compared antler cartilage with cartilage of growth plate. Calcification of antler cartilage occurs in the central region of cartilaginous trabeculae that consist of cells at various stages of development and maturation. Cartilage calcification, noted initially in discrete foci of the perilacunar matrix, spreads 360° around mid-trabecular chondrocytes (Fig. 1A). Calcification spreads proximately as well as toward the trabecular periphery. The continued growth and fusion of individual calcification foci results in conversion of the cartilaginous trabeculae into solid masses of calcified cartilage (Fig. 1B). Calcified cartilage serves as a temporary scaffold upon which woven bone is deposited, both of which are removed gradually and continuously until total replacement by lamellar bone is completed.
Fig. 1.
Cartilage mineralization in the growing antler of white-tailed deer. (A) Initiation of mineralization. Individual foci of mineralization (solid arrow) appear in the perilacunar matrix. Mineralization foci grow and coalesce (open arrow). Continued growth (star) encircles the chondrocytes until encirclement with mineral is complete (double star). Mineralization begins in mid-trabecular and spreads toward trabecular margins. (B) Mineralization of cartilage trabeculae. Mineralization foci have grown until almost all of the cartilaginous spicule contains mineral (A and B, after Banks and Newbrey, 1982a; reproduced with the permission of the publisher). C and D. Ultrastructure of chondrocytes in the calcification zone. (C) The calcification appears as discrete radial foci (arrows) that surrounds the cells. Eventually, these foci will fuse to form a mineralized sphere completely surrounding the cells. (D) Higher magnification of matrix vesicles in the early calcification zone. The first hydroxyapatite crystals are seen on the surface of matrix vesicles (dark arrow). Inset: a calcified site, at the centre there is a matrix vesicle (large open arrow) and many condensed ruthenium red (RR) granules immediately surrounding the vesicle (C and D, after Newbrey and Banks, 1982b; reproduced with the permission of the publisher). E and F. Ultrastructure of matrix vesicle. (E) The matrix vesicle in the antler cartilage, residing in the centre of a condensed accumulation of RR-positive proteoglycan granules (arrows). (F) The matrix vesicle in the rat growth plate cartilage. A centralized matrix vesicle and surrounding proteoglycan granules (small arrows) are apparent. A lamina limitans (large arrows) surrounds the perimeter of the matrix vesicle. (E and F, after Banks and Newbrey, 1982b; reproduced with the permission of the publisher)
The matrix vesicles (MVs) are the focal initiators of both somatic and antler cartilage matrix calcification [36, 38, 39], and present in the cartilage region of the proliferative zone (Fig. 1C) in the antler. The distribution of MVs and the subsequent foci of calcification within antler cartilage differ from that within calcifying somatic cartilage. Whereas MVs and calcification foci are generally confined to the longitudinal septa in the somatic process, these entities occur as radially positioned structures around the chondrocytes in the antler cartilage (Fig. 1D). In the calcifying antler cartilage, MVs reside at the center of a condensed accumulation of proteoglycan granules (Fig. 1E), whereas in the growth plate cartilage, centralized MVs and surrounding proteoglycan granules are apparent, but a demineralization demarcation zone surrounds the perimeter of the matrix vesicles (Fig. 1F).
To identify the mineralization-related genes in the antler cartilaginous zones, Steger et al. [40] developed an “antler cDNA microarray” using fetal cartilage as a control. They found that matrix of these two types of cartilage is biochemically similar. Bone matrix genes are highly expressed in the growing antler: 2- to 10-fold higher in antler than in the fetal cartilage; and far higher (10- to 30-fold or more) than in the ribs and vertebrae of deer. Therefore, antler tissue has much greater mineralization potential than somatic tissues.
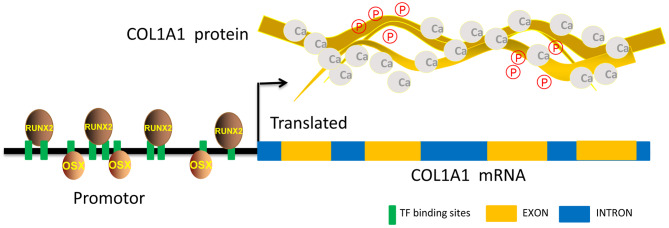
COL1A1 is known to be the strongest extracellular matrix for mineral deposition [41]. The promoter region of the deer COL1A1 gene contains a large number of binding sites for the transcription factors RUNX2 and OSX [42], the two most relevant transcription factors for bone development (Fig. 2) [40]. A 175 bp sequence spanning the OSX binding site sp1 in the first intron of the COL1A1 gene is totally conserved in ruminants and humans [43] and contains a SNP site that has been used as a diagnostic marker for the susceptibility to osteoporosis in humans [44].
Fig. 2.
Multiple binding sites for RUNX2 and OSX in the promoter region of COL1A1 gene. COL1A1 protein is the strongest extracellular matrix for mineral deposition, particularly Ca++ and P5+
Bone metabolism-related-genes, osteocalcin, RUNX2 and OSX, are highly expressed in the ossified part of growing antlers, unlike in the ribs and vertebrae where the levels of these genes remain low. In addition, the 6- to 7-fold lower levels of hydroxyproline and ethanolamine-phosphate and the 40% less free inorganic phosphate in the antler versus in the vertebral disc indicate the occurrence of intensive bone deposition in the antler at the expense of deer skeleton. The very high glucose level in the antler bone (5-fold higher than the vertebral body) is a consequence of the high anabolic activity along the RUNX2–osteocalcin–insulin pathway [40, 45].
Lopez-Pedrouso et al. [46] examined proteins expressed in the antler tip (high proliferation and differentiation region) and compared with those in the mid-part of the antler (robust bone calcification region). They found that the metabolic rate in the mineralizing part is more intensive than that in the growth center, as the antioxidant proteins in the mineralizing part are more than those in the growth center. The low hydroxyproline content in the antler bone indicates much lower collagen breakdown than in the vertebrae [40].
Ker et al. [47] examined gene expression in growing antler cells and compared them with that in human bone cells. They found that antler cells mineralize (17 times) much faster than human cells under identical culture conditions. Among the identified genes, S100A10 is the key factor responsible for rapid mineralization. Therefore, rapid mineralization in antlers is underpinned by specific genes.
Resorption of deer skeleton
One would think that osteoporosis of deer skeleton, if it were to occur, would take place during the second intensive antler mineralization phase (Phase II), as over half of the minerals are deposited in the antler tissue during such a short period (around 7 weeks). Surprisingly, demineralization of deer skeleton takes place only during the period of rapid antler growth (Phase I), whereas recovery from the deer skeleton’s porotic state starts at the transition from Phase I to Phase II [48–51].
The factors that cause the resorption of minerals in the deer skeleton remain largely unknown. Based on the current knowledge, we believe that deposition of Ca++ in the calcifying antlers at appreciable quantities would create the tendency to lower the plasma Ca++ level, which would trigger the secretion of parathyroid hormone (PTH) to restore this stable level. Therefore, mineral resorption in deer skeleton may be a passive process. As a consequence, not only would Ca++ absorption from the intestine increase, but Ca++ resorption from the skeleton would also occur in order to maintain this strict physiological Ca++ level [51], , as this Ca++ level is linked to the cardiovascular and nervous systems and slight deviation from the physiological level would be expected to have serious consequences [52, 53].
Resorption period and the extent in different deer bones.
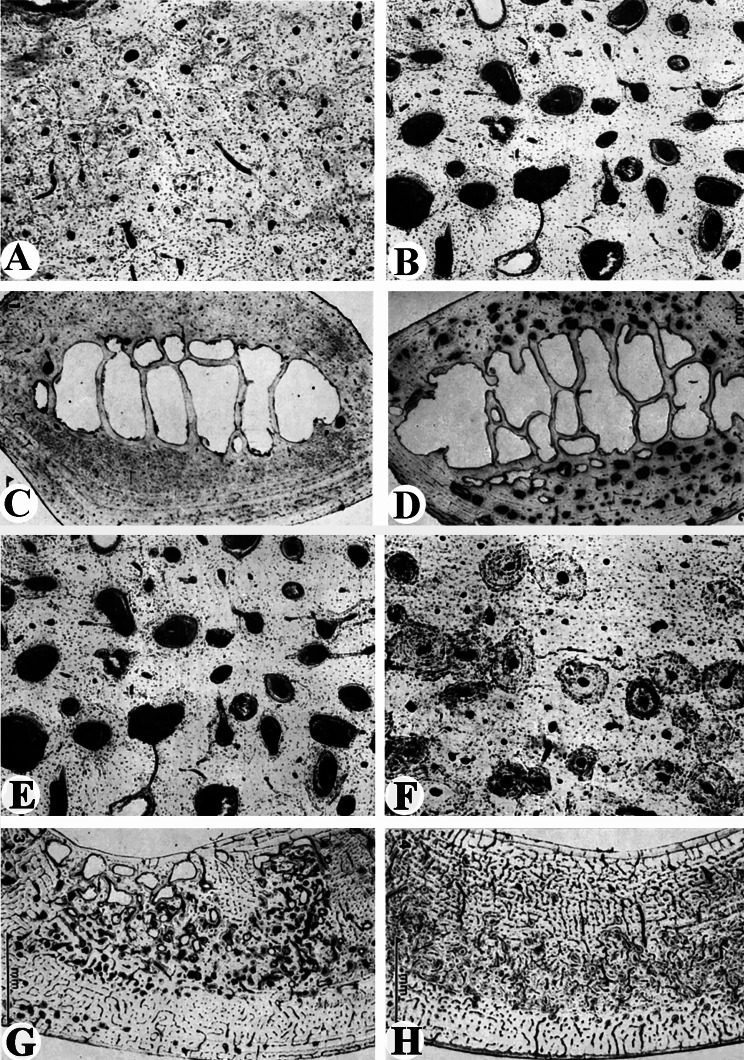
During hard antler phase, Banks et al. [49] found that the osteonal zone of the compacta has a stable configuration, a conspicuous scarcity of remodeling foci (Fig. 3A). The porosity of the compacta became more pronounced during the peak of antler growth, as marked by an increase in the number of remodeling foci (Fig. 3B). Hillman et al. [50] obtained the similar results: dormant period of remodeling in the skeleton occurs in the hard antler phase, and the highest bone remodeling takes place in the peak of antler growth. The highest resorption rate occurred in the ribs at 23% with lower levels of 13% in the metacarpus and 10% in the metatarsus. Resorption in ribs ranges from 0.1 to 3% during the hard antler period. These authors confirmed that the sternebrae underwent an even more marked CPO than the ribs.
Fig. 3.
Osteoporosis in ribs in mule deer in different periods. (A) Costal compacta prior to antler growth. The osteons of the compacta possess a mature configuration. Note that there is a conspicuous lack of resorption spaces indicating the stable nature of the bone during this period of the antler cycle. (B) Remodeling foci of osteonal bone during the antler growth period. The rapid turnover of mineral represented by this section demonstrates the intense osteoclastic and osteoblastic activity. Refilling osteons outnumbered the resorption spaces. (A and B. after Banks et al., 1968; reproduced with the permission of the publisher). (C) Cross section of rib during period of hard antler phase, indicating lack of the internal bone remodeling and the lamellar structure of the rib. (D) Cross section of rib taken during peak of antler growth. Resorption spaces are present throughout the cortex. (C and D. after Hillman et al., 1973; reproduced with the permission from the publisher). Reversal of osteoporosis in mule deer skeleton following cessation of antler growth (lower). E and F: Compacta of ribs. (E) In the antler growth period; refer to Fig. 3B. (F) In the hard antler phase. Note that the absence of resorption spaces is a conspicuous feature of the compacta; many osteons are not fully calcified and appear much darker than the surrounding bone due to a greater uptake of dye. (E and F, after Banks et al., 1968a; reproduced with the permission of the publisher). G and H: Cross section through the lateral cortex of the metatarsus. (G) Taken during the peak of antler growth. Note that conspicuous resorption spaces are the dominant feature. (H) Taken after the cessation of antler growth. Note that newly formed osteons are present in the middle portion of the cortex. (G and H, after Hillman et al., 1973; reproduced with the permission from the publisher)
After cessation of antler growth, ribs are characterized by a decrease in resorption foci and a small number of filling osteons (Fig. 3C). During the period of rapid antler growth, there are many resorption spaces within the ribs. The porosity, however, is more evident in the lateral cortex of the rib (Fig. 3D). Remodeling in the metacarpus, metatarsus and tibia do not occur to the extent observed in the rib and sternebrae. The amount and pattern of resorption are indicative of an adaptive mechanism since there is greater stress on the weight-bearing long bones than the ribs [54].
Meister [55] described the pronounced osteoporosis in different bones during antler growth period. In the metacarpus, large cavities develop from enlarged Haversian canals. In the femur, periosteal cellular connective tissue deeply penetrates bone shaft, and adjacent Haversian systems are destroyed from the outside by dense rows of osteoclasts. In the metatarsus, pronounced osteoporosis with large cavities develops from enlarged Haversian canals, within which syncytial formation of osteoclastic elements are detected. In the radio-ulna, large cavities are developed from enlarged Haversian canals, Volkmann’s canals are slightly enlarged, and both osteoblasts and osteoclasts are encountered.
-
2)
Degree of resorption in different types of bone.
Banks et al. [49] considered that trabecular bone was the principal location of the labile minerals of the skeleton. Chapman [16] reported that the trabecular bone of deer skeleton has a stable configuration in the hard antler stage. However, during antler growth period, trabecular remodeling conforms to the various stages of antler growth. The percentage of trabecular bone and the mean width of the trabeculae were lowest (15% and 1.9 mm respectively) in Phase I and highest (21% and 2.9 mm respectively) at the end of Phase II [56]. Trabecular bone is more metabolically active than cortical bone in terms of the rate of mineral turnover. This explains why a greater CPO always firstly occurs in trabecular bone compared with cortical bone. Besides trabecular bones, cortical lamellar bone was also subject to a varying degree of osteoporosis, although to a lesser extent [18, 57].
-
3)
Seasonal changes in density and mineral composition of deer skeleton.
Through gravimetric measurements, Banks et al. [49] found that fully hydrated densities of deer bone ranged from 1.71 to 2.04 g per ml and the ash values ranged from 0.82 to 1.25 g per ml. Chemical analyses showed that the percentage of Ca++ averaged 37.7%; P5+ averaged 15.7%; and Mg++ averaged 0.56%. The lowest values occurred during the peak of antler growth and the highest values in the hard antler period.
Hillman et al. [54] reported that remodeling in deer skeleton at the cessation of antler growth decreases to a low level comparable to the antler growth. In ribs, the lowest values of ash weight occur during the mid-period of antler growth. The ratios of Ca++, Mg++ and P5+ in bone ash do not vary during the antler growth cycle, indicating no change in bone composition during periods of high remodeling.
Unidirectional flow of minerals from deer skeleton to growing antlers
Minerals of the deer skeleton contribute to antler ossification was confirmed using radio-labeled minerals [58]. Radioactive Ca++ (45Ca) and strontium (89Sr) were injected prior to the commencement of antler growth to allow their deposition in the deer skeleton. Eight weeks after the injections, the antlers had grown 10–13 cm in length and contained appreciable amounts of 45Ca++and 89Sr++, indicating that these deposited elements were mobilized from the skeleton to the growing antlers. Muir et al. [14] used 45CaCl2 to study the kinetics of Ca++ metabolism and found that Ca++, released from deer skeleton, appeared to be irreversibly lost from circulation into the antlers and thus can be treated in the same way as loss of Ca++ from the body in milk.
Some seemingly unexplainable phenomena related to bone metabolism.
Phase I antler ossification occurs despite in an environment with basal testosterone level.
Rapid bone ossification in most mammalian species takes place during adolescence when the circulating level of sex hormones is sharply elevated [59]. Indeed, osteoporosis as a characteristic bone metabolism in the post-menopausal women or in aged men [28, 60]. Likewise, intensive mineral deposition in Phase II antlers is also driven by high levels of circulating testosterone, as evidenced by the intensive mineralization of antlers associated with concurrent sharp increase in testosterone level and its abrogation by castration. Surprisingly, minerals are also robustly deposited in rapid-growing antlers when circulating testosterone is in its nadir (barely detectable) and when deer skeleton is intensively demineralizing. The mechanism underlying this phenomenon is still a mystery.
Growing antlers are enveloped with velvet skin. Velvet skin and periosteum/perichondrium are richly innervated with sensory nerves [61–63]. Steger et al. [40] inferred that calcitonin gene-related peptide (CGRP), a diffusible neuropeptide, may play a central role in initiating the process of antler ossification in Phase I. CGRP is abundant in the sensory nerve terminals and leaves the sensory nerves and infiltrates cartilage through vascular channels (antler cartilage is a richly vascularized tissue [64]) to enter the circulation. The infiltrated CGRP stimulates high expression of both RUNX2 and OSX in the antler tissue [65, 66]. The regulatory role of CGRP in RUNX2 expression has also been reported in osteoblasts [67–69]. The upregulated RUNX2 and OSX activate downstream target genes, particularly COL1A1 and osteocalcin, for mineralization and matrix synthesis. The expected result of the above events is the trapping of minerals from the bloodstream by COL1A1 and other mineral-binding matrix proteins in the growing antlers. Lowering the circulating Ca++ level would trigger the secretion of PTH to maintain the circulating Ca++ level via increasing absorption of Ca++and other minerals from feed and the skeleton system. Through this putative way, robust antler ossification is achieved in an environment of low levels of sex hormones.
In addition to the CGRP theory, autophagy may also play a role in Phase I of the antler ossification process. The effect of autophagy in bone homeostasis is known to be exerted through osteoblasts, osteocytes, bone marrow mesenchymal stem cells and osteoclasts [70–72]. Impaired autophagy in osteoblasts leads to decreased bone mass [73]. Inhibiting autophagy in osteocytes results in bone tissue senescence [74]. Autophagy also contributes to pre-osteoblast differentiation, osteoblast-osteocyte transition, and formation and function of osteoclasts [75]. In one of our previous studies [65], we identified numerous autophagy-related genes (ATGs) expressed in regenerating antlers from reserve mesenchyme (RM) to mineralized cartilage (MC). Among these genes, Gamma-Aminobutyric Acid Receptor-Associated Proteins (GABARAPs) and UKL1 are highly expressed. GABARAPs are ATG8 family proteins, along with LC3 and UKL1, that are highly relevant to bone metabolism via autophagy. Administration of GABARAP to a rat osteoarthritis model significantly increased the expression levels of Col II and SOX9; upregulated the mediators of the autophagy pathway, and suppressed the PI3K/AKT/mTOR pathway in the chondrocytes [76]. We believe that uniquely devised autophagy regulation in bone-related cells (osteoblasts, osteocytes and osteoclasts etc.) solely based on the findings of full osteoporosis reversal in the deer model would revolutionize the treatment of osteoporosis in the clinic setting.
-
2)
Demineralization of deer skeleton occurs despite adequate provisions of mineral reserves in the diet.
Despite high Ca++ intake, extensive demineralization of deer skeleton still happens during Phase I [20]. Parfitt [77] noticed that the requirement for bone-derived minerals during antler growth seems to be complicated by the need to mobilize such stores from the skeleton, even though high quantities of the minerals are available from the diet. Based on the Ca++ kinetic studies with stags consuming forages, Muir et al. [78] found that the skeleton may supply 30–60% of the required Ca++, even when Ca++ intake is considerably greater than the requirement. The skeletal contribution may be more important where the diet is inadequate. Collectively, these phenomena might suggest that resorption of minerals from the skeleton is not directly driven by the requirement of antler ossification, rather by the low androgen environment that is permissive for bone-loss.
Phase II. Total mineralization in antler bone and full reversal of CPO in deer skeleton as two contrasting processes under the same endocrine environment
The increase in deer skeletal bone density around the transition from Phase I to Phase II suggests that bone accretion in the skeleton must have commenced around this point. This implies that during this period, deer have to cope with demands for minerals at two sites: the antler bone and the deer skeleton, although antler bone would have priority, particularly when mineral availability is limited. To fully mineralize a pair of 25 kg antlers, and if over 50% of minerals are deposited in the antlers during Phase II, over 10 kg of minerals must be gained from the diet to satisfy the antler requirement only, not to mention for CPO reversal of the deer skeleton. Thus, the ability to gather so large quantity of minerals in such a short period (within a month) is definitely extraordinary.
Total mineralization of the antler tissue
López-Pedrouso et al. [46] made a comparison between the mid-part of the antler (mineralizing part) and the Phase II ribs and identified 30 proteins that were overabundant in the antler and 58 proteins overabundant in the ribs (in CPO reversal). Among the overexpressed proteins in the rib, the highest was COL1A2; and the most abundant in the mineralizing portion of the antler was COL1A1. Remarkably, both COL1A1 and COL1A2 genes are 10 and 20 times overexpressed in the antler bone than in the rib of the same animal, respectively [40, 79].
In deer, when the seasonal rise in testosterone is blocked by an anti-androgen drug, antler growth continues but mineralization is impaired significantly [80]. Therefore, antler growth and mineralization are two relatively separate events. Uniquely, after the Phase II the antler becomes so heavily mineralized that it completely occludes its blood supply, which in turn “kills” the antler tissue. To date, the process of full antler mineralization has not been described at the histological level, nor has the underlying molecular mechanism been explored.
Full reversal of deer CPO
Full reversal of deer CPO during Phase II is a unique feature, and this phenomenon does not occur in human osteoporotic bone. A complete recovery and refilling of remodeling foci suggests that the stag possesses unique control mechanisms for mineral metabolism [40].
Banks et al. [49] provided convincing evidence that the absorbed foci in the skeleton are completely refilled after the cessation of antler growth in mule deer. At the peak of antler growth, the costal cortical bone of ribs is severely absorbed and osteoporotic (Fig. 3E). During hard antler period, there is a reduction or a complete absence of resorption space (Fig. 3F). Many osteons are recognized as newly deposited but have progressed to a more mature state. Hillman et al. [54] reported that, at the peak of antler growth in mule deer, the metacarpus and metatarsus contained many areas of resorption being arranged in an irregular pattern. Resorption is more prominent in the metatarsus and extends toward the endosteal surface (Fig. 3G). In contrast, at the late stage or completion of Phase II, the absorbed holes are greatly reduced, and many new, incompletely ossifying osteons are present (Fig. 3H).
Parfitt [57] reported that during the period of most rapid antler growth, there is a sixfold increase in cortical porosity, which is completely refilled after the antlers are fully grown; this cycle is repeated year after year with seemingly no cumulative adverse effect [77]. The author hypothesized that the cyclical cortical porosity of the deer represents a mechanism for spreading a sharply increase in need for Ca++ over a longer period. Antlers grow in the spring, when limited Ca++ is available from feed [81]. Providing some Ca++ from the skeleton means that less is needed from the diet during that period [82]. In order to “repay the debt” incurred by bone absorption, increased intestinal Ca++ absorption continues throughout summer and autumn. This strategy enables a sharply increased demand for Ca++ to be repaid to the skeleton over a longer period.
Borsy et al. [83] examined gene expression status in deer ribs in the periods of CPO, CPO reversal, and bone homeostasis (hard antler phase). They identified a number of DEGs, which are also expressed in human pathological osteoporosis. Furthermore, the authors identified 4 DEGs that were markedly different between osteoporotic and non-osteoporotic bone in the same (trib2, fabp3 and fabp4) or opposite ways (igsf4) in deer and human. Among them, trib2 is highly expressed in growing antlers; fabp4 highly expressed in osteoporotic bone in both deer and human, whereas fabp3 moderately expressed in bone tissues of deer and human.
In the Phase II period, both full antler mineralization and recovery of deer bone CPO occur simultaneously. Thus, deer must find feed rich in minerals, effectively absorb and conserve them. Besides from the diets, deer may also absorb minerals from their calcified hard antlers. Although hard antlers are apparently dead tissue, Rolf and Enderle [84] found that a polished hard antler of fallow deer contains a well intact system of capillaries that are connected to the vascular system of the deer body via pedicle tissue. Deer may retrieve some calcium from it through internal channels, particularly in the osteoporosis reversal period.
What would happen if deer fail to obtain a Ca++-rich diet? Firstly, we believe that antler would likely have priority to utilize the limited mineral sources, and this would cause partial reversal of the CPO that would render deer skeleton prone to fracture. Secondly, an inadequate supply of minerals may even not meet the needs for antler mineralization, and thus result in the formation of sub-mineralized antlers. Deer carrying such antlers would likely be disadvantaged when combating during the rutting season. The phenomenon that high incidence of osteoporotic lesions in male moose (having largest antlers in the extant deer species) may in part be explained by a seasonal osteoporotic condition induced by antler growth [85]. If Ca++and P5+ lost during antler growth are not fully replenished from the summer, autumn and winter diet before the next round of antler growth starts, the resorption could lead to permanent osteoporosis or reduced antler growth, either way would likely reduce the fitness of the affected individuals [86]. Seasonal CPO would gradually be supplanted by permanent osteoporosis, which has been postulated to have finally caused the demise of Irish elk, the deer that carried the largest antlers ever (over 40 kg) in deer species [86].
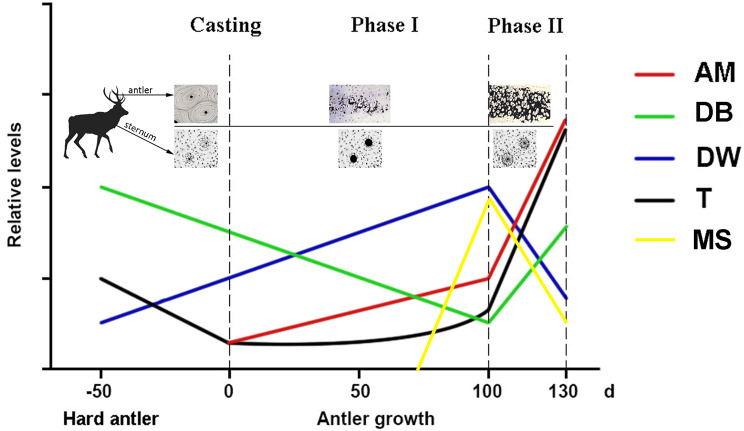
Based on the currently available data, we summarized the changes in relevant parameters over the period of Phase I and Phase II in a chart. These include degree of antler mineralization, density of deer skeleton, testosterone levels, antler cortical mineralization surface, and deer bodyweight (Fig. 4). The chart clearly shows that the inflection points of these parameters all happen to be at the transition point between Phase I and Phase II. Nonetheless, the transition date between two phases must be considered as approximate and would vary according to deer species, age and even geographical location.
Fig. 4.
Inflection points for differential antler mineralization and deer bone cyclic osteoporosis during the periods of Phase I and Phase II. AM, antler mineralization; DB, deer bone; DW, deer bodyweight; T, testosterone; MS, cortical mineralizing surface
In the chat, demineralization of deer skeleton starts at the time of the casting of hard antlers, gone through the whole Phase I period before reaching the phase transition point; remineralization was then switched on and lasted the entire Phase II. Antler mineralization starts as soon as initiation of antler growth and gradually increases in a slow-slope-manner until reaching this transition point; then the process is sharply increased and lasted the entire Phase II. Cortical mineralizing surface starts to increase around day 74 after the hard antler casting when primary osteon formation is initiated. It reaches its peak around day 100 and then slowly decreased when osteonal growth was completed by 130 days [87]. Circulating testosterone level at the initial antler growth is at its nadir in the whole year and remains low during Phase I although in the later stage slowly elevated to certain level when reaching this point; then the level is sharply increased and the trend lasted the entire Phase II. Change in deer bodyweight happens to be also at this point: before it, deer bodyweight increases continuously and after it the bodyweight starts to fall [88], possibly due to the decreased food intake by stags due to sharply increased circulating testosterone level. Zhang et al. [89] conducted a metabolomic analysis on sika deer antler at different growth stages, ranging from 25 to 130 days after hard antler casting. They found that the entire antler growth period could be divided into two distinct stages: the first stage from beginning of antler growth to 100 days, and the second stage from 100 days to 130 days. This division based on metabolic changes aligns with our classification well in this review, providing molecular-level support to our classification.
Facts and a perspective for use of phase II deer products to treat osteoporosis
Currently drugs used to treat osteoporosis in clinic are targeted at stimulation of bone formation using PTH, or inhibition of bone resorption using calcitonin, bisphosphonates, estrogens, and selective estrogen receptor modulators [90–93]. Due to the severity of side effects of these drugs, many patients start to choose an alternative or complementary medicine (such as TCM).
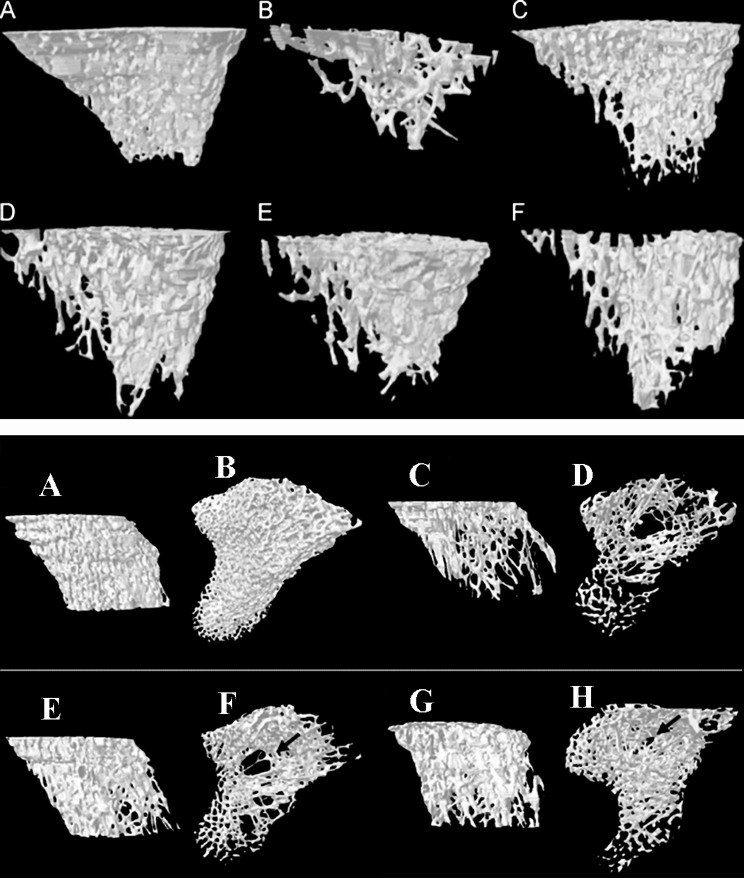
In recent years, research has been carried out intensively to verify the claimed antler efficacies and reveal the underlying mechanisms, particularly on osteoporosis. These studies have demonstrated that antler extracts can substantially alleviate ovariectomy (OVX)-induced osteoporosis in model animals [94–96]. Tseng et al. [28, 97] found that only upper and mid portions of antlers, but not the base portion, can increase the microarchitecture of the trabecular bone in OVX-rats (Fig. 5 upper panel), indicating antler effects on osteoporosis vary in different portions. Overall, antler products have dual roles in anti-osteoporosis: stimulating bone formation and inhibiting bone resorption. However, to date the phenomenon of deer CPO has not been taken into consideration for treating osteoporosis.
Fig. 5.
Effects of velvet antler (VA) extracts on tibial bone density of ovariectomized rats. Upper panel (drawn after Fig. 6 in Tseng et al. [28]): effects of different antler portions (upper, mid and base) on bone microarchitecture. Note that only upper and mid portions had significant and dose-dependent effects. (A) sham-operated; (B) control (ovariectomized, OVX); (C) estradiol (OVX+ES); (D)-(F) VA groups: (D) upper portion (OVX+VAU), (E) middle portion (OVX+VAM) and (F) basal VA portion (OVX+VAB). Lower panel (drawn after Fig. 4 in Tseng et al. [97]): effects of mid-portion of VA on bone microarchitecture. Note that mid-portion of the VA-B showed significant effects on bone density. (A) sham-operated; (B) control (ovariectomized, OVX); (C) middle portion of VA and blood (OVX+VA-B); (D) estradiol (OVX+ES); the arrow indicates loss of the trabecular bone structure
We believe that full incorporation of the phenomenon of deer CPO for the treatment of osteoporosis would greatly benefit human health. Phase II deer products include antlers, male deer bone and blood; and have not been used for the treatment of osteoporosis. It is known that Phase II antlers exhibit unprecedented rates of calcification (up to 250 g/day) far exceeding that of Phase I antlers, thus should contain more anabolic substances for bone formation. Interestingly, Phase II antlers are mainly the ones re-grown from the left-over remnants of Phase I antlers after harvesting [98]. These re-grown antlers are cheap but must be removed before they become hard for safety reasons.
Phase II deer bone experiences rapid reversal of CPO, and should contain more potent anabolic substances for bone formation than any other stage bones. Phase II deer bones are available more than in any other seasons in venison-producing countries, thus are readily availability. Phase II blood should contain the most effective anabolic substances for treating osteoporosis, as both intensive antler calcification and reversal of CPO happen to be in this period. Indeed, Tseng et al. [97] found that the mid portion of antler extracts ware more effective at treating osteoporosis (Fig. 5 lower panel) as they contain more blood (antler: blood = 1:0.2). As more male deer are slaughtered in this period, thus source of Phase II blood is not an issue.
Very recently, we conducted a comparative study between Phase I and Phase II blood on effects of osteoporosis using OVX-rats. The preliminary results demonstrate that the Phase II blood exhibit significantly greater efficacy on treating osteoporosis than their Phase I counterparts. The active compounds would likely be identified if the effects of Phase II deer products for alleviating osteoporosis symptoms are further confirmed as expected. This may pave the way for novel approaches in treating osteoporosis, a debilitating disease.
Acknowledgements
Not applicable.
Abbreviations
- ALP
Alkaline phosphatase
- APP
Antler polypeptides
- ATGs
Autophagy-related genes
- BMMs
Bone marrow macrophages
- BMP2
Bone morphogenetic protein 2
- CGRP
Calcitonin gene-related peptide
- COL-I
Collagen I
- CPO
Cyclic physiological osteoporosis
- DEGs
Differentially expressed genes
- FABP4
Fatty acid-binding protein
- MC
Mineralized cartilage
- MW
Molecular weight
- OCN
Osteocalcin
- OP
Osteoporosis
- PTH
Parathyroid hormone
- RM
Reserve mesenchyme
- Runx2
Runt-related transcription factor 2
- TCM
Traditional Chinese medicine
- VA
Velvet antler
Author contributions
Conceptualization: C.L. Writing—original draft: C.L. Fig. preparation: W.W., G.Z., and H.B. Writing—review and editing: Y.S., G.M., W.L., H.L., and J.W. Funding acquisition: C.L. and G.Z. All authors have read and approved the article.
Funding
This work was supported by the National Natural Science Foundation of China (No. U20A20403, 32300708) and Science and Technology Development Plan Project of Jilin Province (20240602094RC). We thank Dr. Peter Fennessy for critically reading through the paper.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Li, Email: w.li@jlau.edu.cn.
Gerry Melino, Email: melino@uniroma2.it.
Yufang Shi, Email: shiyufang@gmail.com.
References
- 1.Chen C, Wu B, Yu H, Dai Z, Yan L, Cai D, et al. Oral dehydroepiandrosterone supplementation enhances osteoporotic fracture healing in the OVX rats. Bone. 2024;187:117201. 10.1016/j.bone.2024.117201. [DOI] [PubMed] [Google Scholar]
- 2.Aspray TJ, Hill TR. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019;91:453–76. 10.1007/978-981-13-3681-2_16. [DOI] [PubMed] [Google Scholar]
- 3.Kamel HK. Postmenopausal osteoporosis: etiology, current diagnostic strategies, and nonprescription interventions. Journal of managed care pharmacy: JMCP. 2006;12(6 Suppl A):S4-9; quiz S26-8. 10.18553/jmcp.2006.12.S6-A.S4 [DOI] [PMC free article] [PubMed]
- 4.Oheim R, Schinke T, Amling M, Pogoda P. Can we induce osteoporosis in animals comparable to the human situation? Injury. 2016;47(Suppl 1):S3–9. 10.1016/s0020-1383(16)30002-x. [DOI] [PubMed] [Google Scholar]
- 5.Huber FA, Bunnell KM, Garrett JW, Flores EJ, Summers RM, Pickhardt PJ, et al. AI-based opportunistic quantitative image analysis of lung cancer screening CTs to reduce disparities in osteoporosis screening. Bone. 2024;186:117176. 10.1016/j.bone.2024.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melton LJ. 3rd. Epidemiology worldwide. Endocrinol Metab Clin North Am. 2003;32(1):1–13. 10.1016/s0889-8529(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Melton LJ. 3rd. Involutional osteoporosis. N Engl J Med. 1986;314(26):1676–86. 10.1056/nejm198606263142605. [DOI] [PubMed] [Google Scholar]
- 8.Brown JP, Seoul. Korea). 2021;36(3):544–52. 10.3803/EnM.2021.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529–55. 10.1016/s0749-0690(02)00022-8. [DOI] [PubMed] [Google Scholar]
- 10.Bilezikian JP. Gender specificity and osteoporosis. J Gend Specif Med. 2000;3(7):6–12. [PubMed] [Google Scholar]
- 11.Thorndike EA, Turner AS. In search of an animal model for postmenopausal diseases. Front Biosci. 1998;3:c17–26. 10.2741/a260. [DOI] [PubMed] [Google Scholar]
- 12.Turner RT. Skeletal adaptation to external loads optimizes mechanical properties: fact or fiction. Curr Opin Orthop. 2001;12(5):384–8. 10.1097/00001433-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Wu J, Hu Y, Zhou J, Li Q, Zhang Y, et al. Exogenous iron caused osteocyte apoptosis, increased RANKL production, and stimulated bone resorption through oxidative stress in a murine model. Chem Biol Interact. 2024;399:111135. 10.1016/j.cbi.2024.111135. [DOI] [PubMed] [Google Scholar]
- 14.Banks WJ Jr., Epling GP, Kainer RA, Davis RW. Antler growth and osteoporosis. II. Gravimetric and chemical changes in the costal compacta during the antler growth cycle. Anat Rec. 1968;162(4):399–406. 10.1002/ar.1091620402. [DOI] [PubMed] [Google Scholar]
- 15.Baxter BJ, Andrews RN, Barrell GK. Bone turnover Associated with Antler growth in red deer(Cervus elaphus). Anat Rec. 1999;256:14–9. [DOI] [PubMed] [Google Scholar]
- 16.Chapman DI. Antlers-bones of contention. Mammal Rev. 1975;5(4):121–72. [Google Scholar]
- 17.Muir PD, Sykes AR, Barrell GK. Calcium metabolism in red deer (Cervus elaphus) offered herbages during antlerogenesis: kinetic and stable balance studies. J Agric Sci. 1987;109:357–64. [Google Scholar]
- 18.Landete-Castillejos T, Kierdorf H, Gomez S, Luna S, García AJ, Cappelli J, et al. Antlers - evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone. 2019;128:115046. 10.1016/j.bone.2019.115046. [DOI] [PubMed] [Google Scholar]
- 19.Bubenik G, Bubenik A, editors. Horns, pronghorns, and Antlers. New York: Springer-; 1990. [Google Scholar]
- 20.Muir PD, Sykes AR, Barrell GK. Growth and mineralisation of antlers in red deer (Cervus elaphus). N Z J Agric Res. 1987;30:305–15. [Google Scholar]
- 21.Bubenik GA, editor. Endocrine regulation of the antler cycle. Antler Development in Cervidae.; 1982; Caesar Kleberg Wildl. Res. Inst., Kingsville,TX.
- 22.Suttie JM, Fennessy PF, Lapwood KR, Corson ID. Role of steroids in antler growth of red deer stags. J Exp Zool. 1995;271(2):120–30. 10.1002/jez.1402710207. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Liu Z, Zhao S. Variation of testosterone and estradiol levels in plasma during each developmental stage of sika deer antler. Acta Theriol Sin. 1988;8(3):224–31. [Google Scholar]
- 24.Goss RJ. Deer antlers. Regeneration, function and evolution. New York, NY: Academic; 1983. [Google Scholar]
- 25.Akhtar RW, Liu Z, Wang D, Ba H, Shah SAH, Li C. Identification of proteins that mediate the role of androgens in antler regeneration using label free proteomics in sika deer (Cervus nippon). Gen Comp Endocrinol. 2019;283:113235. 10.1016/j.ygcen.2019.113235. [DOI] [PubMed] [Google Scholar]
- 26.Morris J, Bubenik G, editors. The effects of androgens on the development of antler bone. Antler Development in Cervidae; 1982; Caesar Kleberg Wildlife Research Institute, Kingsville, Texas.
- 27.Goss RJ. Inhibition of growth and shedding of antlers by sex hormones. Nature. 1968;220:83–5. [DOI] [PubMed] [Google Scholar]
- 28.Tseng T, Mu C, Hsu C. The correlation between renal function and bone mineral density. Minerva Urol Nefrol. 2014;66(3):153–6. [PubMed] [Google Scholar]
- 29.Guo K, Wang T, Luo E, Leng X, Yao B. Use of Network Pharmacology and Molecular Docking Technology To Analyze the Mechanism of Action of Velvet Antler in the treatment of postmenopausal osteoporosis. Evid Based Complement Alternat Med. 2021;2021:7144529. 10.1155/2021/7144529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng R, Lin H, Li Z, Chen D, Lin X, Zhang Z, et al. Mechanisms of intervertebral disc degeneration treatment with deer antlers based on Network Pharmacology and Molecular Docking. Evid Based Complement Alternat Med. 2022;2022:8092848. 10.1155/2022/8092848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Wang A, Cao Z, Li J, Zheng M, Xu Y. Mechanism of Pilose Antler in treatment of osteoporosis based on Network Pharmacology. J Healthc Eng. 2022;2022:5298892. 10.1155/2022/5298892. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Li C, Clark DE, Lord EA, Stanton JA, Suttie JM. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat Rec. 2002;268(2):125–30. 10.1002/ar.10120. [DOI] [PubMed] [Google Scholar]
- 33.Fennessy P, Moore H. Red deer velvet antler growth and harvesting. Wellington, New Zealand: Aglink MAF; 1981. p. 261. [Google Scholar]
- 34.Goss RJ. Problems of antlerogenesis. Clin Orthop. 1970;69:227–38. [PubMed] [Google Scholar]
- 35.Krauss S, Wagermaier W, Estevez JA, Currey JD, Fratzl P. Tubular frameworks guiding orderly bone formation in the antler of the red deer (Cervus elaphus). J Struct Biol. 2011;175(3):457–64. 10.1016/j.jsb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Banks WJ, Newbrey JW, editors. Antler development as a unique modification of mammalian endochondral ossification. Antler Development in Cervidae; 1982; Caesar Kleberg Wildl. Res. Inst., Kingsville, Texas.
- 37.Banks WJ, Newbrey JW, editors. Light microscopic studies of the ossification process in developing antlers. Antler Development in Cervidae; 1982; Caesar Kleberg Wildl. Res. Inst., Kingsville, Texas.
- 38.Su G, Zhang D, Li T, Pei T, Yang J, Tu S, et al. Annexin A5 derived from matrix vesicles protects against osteoporotic bone loss via mineralization. Bone Res. 2023;11(1):60. 10.1038/s41413-023-00290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Zhao Q, Tang J, Lei X, Zhang J, Li Y, et al. Enzyme-activated biomimetic vesicles confining mineralization for bone maturation. ACS Appl Mater Interfaces. 2024;16(26):33005–20. 10.1021/acsami.4c03978. [DOI] [PubMed] [Google Scholar]
- 40.Stéger V, Molnár A, Borsy A, Gyurján I, Szabolcsi Z, Dancs G, et al. Antler development and coupled osteoporosis in the skeleton of red deer Cervus elaphus: expression dynamics for regulatory and effector genes. Mol Genet Genomics. 2010;284(4):273–87. 10.1007/s00438-010-0565-0. [DOI] [PubMed] [Google Scholar]
- 41.Guex AG, Puetzer JL, Armgarth A, Littmann E, Stavrinidou E, Giannelis EP, et al. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017;62:91–101. 10.1016/j.actbio.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uehara N, Shibusawa N, Mikami Y, Kyumoto-Nakamura Y, Sonoda S, Kato H, et al. Bone metastatic mammary tumor cell-derived extracellular vesicles inhibit osteoblast maturation via JNK signaling. Arch Biochem Biophys. 2023;750:109821. 10.1016/j.abb.2023.109821. [DOI] [PubMed] [Google Scholar]
- 43.Jin H, van’t Hof RJ, Albagha OM, Ralston SH. Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet. 2009;18(15):2729–38. 10.1093/hmg/ddp205. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez L, Oriola J, Jo J, Ferró T, Pons F, Peris P, et al. Collagen type I alpha1 gene Sp1 polymorphism in premenopausal women with primary osteoporosis: improved detection of Sp1 binding site polymorphism in the collagen type 1 gene. Clin Chem. 1999;45(6 Pt 1):904–6. [PubMed] [Google Scholar]
- 45.Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, et al. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem. 2011;286(21):19149–58. 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Pedrouso M, Lorenzo JM, Landete-Castillejos T, Chonco L, Pérez-Barbería FJ, García A, et al. SWATH-MS quantitative proteomic analysis of deer Antler from two regenerating and mineralizing sections. Biology. 2021;10(7). 10.3390/biology10070679. [DOI] [PMC free article] [PubMed]
- 47.Ker DFE, Wang D, Sharma R, Zhang B, Passarelli B, Neff N, et al. Identifying deer antler uhrf1 proliferation and s100a10 mineralization genes using comparative RNA-seq. Stem Cell Res Ther. 2018;9(1):292. 10.1186/s13287-018-1027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks W, Davis R. Observations on the relationship of antlerogenesis to bone morphology and composition in the Rocky Mountain mule Der (Odocoileus hemionus hemionus). Anat Rec. 1966;154:312. [Google Scholar]
- 49.Banks W, Epling G, Kainer R, Davis R. Morephological and morphometric changes in the costal Compacta during the Antler Growth cycle. Anat Rec. 1968;162:387–98. [DOI] [PubMed] [Google Scholar]
- 50.Hillman D. Some highlights from the 5th Annual Ruminant Health-Nutrition Conference. Feeding for maximum production (a nutritionist’s viewpoint). Vet Med Small Anim Clin. 1967;62(4):372. [PubMed]
- 51.Wasserman RH, Fullmer CS. On the molecular mechanism of intestinal calcium transport. Adv Exp Med Biol. 1989;249:45–65. 10.1007/978-1-4684-9111-1_5. [DOI] [PubMed] [Google Scholar]
- 52.Xu S, Zhang M, Cong J, He Y, Zhang L, Guo Y, et al. Reduced blood circulating calcium level is an outstanding biomarker for preeclampsia among 48 types of human diseases. QJM. 2022;115(7):455–62. 10.1093/qjmed/hcab222. [DOI] [PubMed] [Google Scholar]
- 53.Jayasena CN, Modi M, Palazzo F, De Silva A, Donaldson M, Meeran K, et al. Associations of serum 25-hydroxyvitamin D with circulating PTH, phosphate and calcium in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf). 2013;78(6):838–43. 10.1111/cen.12062. [DOI] [PubMed] [Google Scholar]
- 54.Hillman JR, Davis RW, Abdelbaki YZ. Cyclic bone remodelling in deer. Calcif Tissue Res. 1973;12:323–30. [DOI] [PubMed] [Google Scholar]
- 55.Meister WW. Changes in histological structure of the Long bones of White-tailed deer (Odocoileus Virginianus) during the growth of the antlers. Anat Rec. 1956;124:709–21. [DOI] [PubMed] [Google Scholar]
- 56.McIntosh. A quantitative microradiographic study of cyclic morphological changes in trabecular bone of mule deer (Odocoileus hemionus hemionus). Fort Collins, Colorado: Colorado State University; 1969. [Google Scholar]
- 57.Parfitt AM. Vitamin D receptor genotypes in osteoporosis. Lancet. 1994;344(8936):1580. [PubMed] [Google Scholar]
- 58.TAFT EB, HALL TC, AUB JC. The growth of the deer antler. New York State Conservationist. 1956;10:4–5. [Google Scholar]
- 59.Raisz LG, Bingham PJ. Effect of hormones on bone development. Annu Rev Pharmacol. 1972;12:337–52. 10.1146/annurev.pa.12.040172.002005. [DOI] [PubMed] [Google Scholar]
- 60.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40. 10.1016/s0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 61.Gray C, Hukkanen M, Konttinen YT, Terenghi G, Arnett TR, Jones SJ, et al. Rapid neural growth: calcitonin gene-related peptide and substance P-containing nerves attain exceptional growth rates in regenerating deer antler. Neuroscience. 1992;50(4):953–63. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Sheard PW, Corson ID, Suttie JM. Pedicle and antler development following sectioning of the sensory nerves to the antlerogenic region of red deer (Cervus elaphus). J Exp Zool. 1993;267(2):188–97. 10.1002/jez.1402670212. [DOI] [PubMed] [Google Scholar]
- 63.Wislocki GB, Singer M. The occurrence and function of nerves in the growing antlers of deer. J Comp Neurol. 1946;85:1–19. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Suttie JM. Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus). Anat Rec. 1994;239(2):198–215. 10.1002/ar.1092390211. [DOI] [PubMed] [Google Scholar]
- 65.Ba H, Wang D, Wu W, Sun H, Li C. Single-cell transcriptome provides novel insights into antler stem cells, a cell type capable of mammalian organ regeneration. Funct Integr Genomics. 2019;19(4):555–64. 10.1007/s10142-019-00659-2. [DOI] [PubMed] [Google Scholar]
- 66.Guo Q, Liu Z, Zheng J, Zhao H, Li C. Substances for regenerative wound healing during antler renewal stimulated scar-less restoration of rat cutaneous wounds. Cell Tissue Res. 2021;386(1):99–116. 10.1007/s00441-021-03505-9. [DOI] [PubMed] [Google Scholar]
- 67.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC Jr, et al. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138(4):1008–15. 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 68.Wu C, Zheng Z, Ren W, Deng T, Li Y, Yang L, et al. Mm9_circ_009056 enhances osteogenesis by targeting BMP7 via CGRP-mediated miR-22-3p. Biochem Biophys Res Commun. 2018;501(1):199–205. 10.1016/j.bbrc.2018.04.215. [DOI] [PubMed] [Google Scholar]
- 69.Han N, Jiang BG, Wang TB, Zhang PX, Kou YH, Zhang DY. [Effect of calcitonin gene-related peptide on RUNX2 expression in primary rat osteoblasts]. Beijing Da Xue Xue bao Yi xue ban = J Peking Univ Health Sci. 2011;43(5):652–6. [PubMed] [Google Scholar]
- 70.Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, et al. Autophagy in stem cells. Autophagy. 2013;9(6):830–49. 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082–92. 10.1080/15548627.2020.1822097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceccariglia S, Cargnoni A, Silini AR, Parolini O. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy. 2020;16(1):28–37. 10.1080/15548627.2019.1630223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Li D, Ma Z, Qian Z, Kang X, Jin X, et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy. 2018;14(10):1726–41. 10.1080/15548627.2018.1483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019;7:28. 10.1038/s41413-019-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy. 2014;10(1):7–19. 10.4161/auto.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu L, Wu W, Sun X, Zhang P. Glucocorticoids enhanced Osteoclast Autophagy through the PI3K/Akt/mTOR signaling pathway. Calcif Tissue Int. 2020;107(1):60–71. 10.1007/s00223-020-00687-2. [DOI] [PubMed] [Google Scholar]
- 77.Parfitt AM. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part III of IV parts; PTH and osteoblasts, the relationship between bone turnover and bone loss, and the state of the bones in primary hyperparathyroidism. Metabolism. 1976;25(9):1033–69. 10.1016/0026-0495(76)90133-5. [DOI] [PubMed] [Google Scholar]
- 78.Muir P, Sykes A. Effect of winter nutrition on antler development in red deer (Cervus elaphus): a field study. N Z J Agric Res. 1988;31:145–50. [Google Scholar]
- 79.Hu P, Wang T, Liu H, Xu J, Wang L, Zhao P, et al. Full-length transcriptome and microRNA sequencing reveal the specific gene-regulation network of velvet antler in sika deer with extremely different velvet antler weight. Mol Genet Genomics. 2019;294(2):431–43. 10.1007/s00438-018-1520-8. [DOI] [PubMed] [Google Scholar]
- 80.Muir PD, Barrell GK, Sykes AR. Modification of antler growth in red deer stag by the use of synthetic progestagen. Proc N Z Soc Anim Prod. 1982;42:145–7. [Google Scholar]
- 81.RP H. J B-L. Calcium and common sense. New York: Doubleday; 1988. [Google Scholar]
- 82.Cowan RL, Hartsook EW, Whelan JB. Calcium-strontium metabolism in white tailed deer as related to age and antler growth. Proc Soc Exp Biol Med. 1968;129(3):733–7. 10.3181/00379727-129-33412. [DOI] [PubMed] [Google Scholar]
- 83.Borsy A, Podani J, Stéger V, Balla B, Horváth A, Kósa JP, et al. Identifying novel genes involved in both deer physiological and human pathological osteoporosis. Mol Genet Genomics. 2009;281(3):301–13. 10.1007/s00438-008-0413-7. [DOI] [PubMed] [Google Scholar]
- 84.Rolf HJ, Enderle A. Hard fallow deer antler: a living bone till antler casting? Anat Rec. 1999;255(1):69–77. [DOI] [PubMed] [Google Scholar]
- 85.Hindelang M, Peterson RO. Osteoporotic skull lesions in moose at Isle Royale National Park. J Wildl Dis. 1996;32(1):105–8. 10.7589/0090-3558-32.1.105. [DOI] [PubMed] [Google Scholar]
- 86.Moen RA, Pastor J, Cohen Y. Antler growth and extinction of Irish elk. Evol Ecol Res. 1999;1:235–49. [Google Scholar]
- 87.Gomez S, Garcia AJ, Luna S, Kierdorf U, Kierdorf H, Gallego L, et al. Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone. 2013;52(1):506–15. 10.1016/j.bone.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Ward J, Bennett C, Stevens D. The 100 kg weaner: combining genetics and nutrition to transform our venison production system. 2021 Conference Proceedings of the Sheep and Beef Cattle Veterinarians and the Deer Veterinarians Branches of the NZVA. 2021.
- 89.Zhang R, Rong M, dong Y, Wang T, Xing X. Metabolomic analysis of sika deer antler in different growth stages. Acta Vet Et Zootechnica Sinica. 2022;53(12):4518–26. 10. 11843/j. issn. [Google Scholar]
- 90.Somjen D, Katzburg S, Sharon O, Grafi-Cohen M, Knoll E, Stern N. The effects of estrogen receptors α- and β-specific agonists and antagonists on cell proliferation and energy metabolism in human bone cell line. J Cell Biochem. 2011;112(2):625–32. 10.1002/jcb.22959. [DOI] [PubMed] [Google Scholar]
- 91.Moshi MR, Nicolopoulos K, Stringer D, Ma N, Jenal M, Vreugdenburg T. The clinical effectiveness of Denosumab (Prolia®) for the treatment of osteoporosis in Postmenopausal Women, compared to bisphosphonates, selective estrogen receptor modulators (SERM), and Placebo: a systematic review and network Meta-analysis. Calcif Tissue Int. 2023;112(6):631–46. 10.1007/s00223-023-01078-z. [DOI] [PubMed] [Google Scholar]
- 92.Cosman F. Selective estrogen-receptor modulators. Clin Geriatr Med. 2003;19(2):371–9. 10.1016/s0749-0690(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 93.Hadji P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric. 2012;15(6):513–23. 10.3109/13697137.2012.688079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Zhao Y, Sun X, Qu X. [Prevention and therapeutic effects of sika deer velvet collagen hydrolysate on osteoporosis in rats by retinoic acid]. Zhongguo Zhong Yao Za Zhi. 2010;35(6):759–62. 10.4268/cjcmm20100622. [DOI] [PubMed] [Google Scholar]
- 95.Meng HY, Qu XB, Li N, Yuan S, Lin Z. [Effects of pilose antler and antler glue on osteoporosis of ovariectomized rats]. Zhong Yao cai = Zhongyaocai = J Chin Med Mater. 2009;32(2):179–82. [PubMed] [Google Scholar]
- 96.Yang JH, Cao Y, Wang RL, Fei YR, Zhang H, Feng P, et al. Anti-resorptive effect of pilose antler blood (Cervus nippon Temminck) in ovariectomized rats. Indian J Exp Biol. 2010;48(6):554–8. [PubMed] [Google Scholar]
- 97.Tseng SH, Sung HC, Chen LG, Lai YJ, Wang KT, Sung CH, et al. Effects of velvet antler with blood on bone in ovariectomized rats. Molecules. 2012;17(9):10574–85. 10.3390/molecules170910574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C, Fennessy P. The periosteum: a simple tissue with many faces, with special reference to the antler-lineage periostea. Biol Direct. 2021;16(1):17. 10.1186/s13062-021-00310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.