Abstract
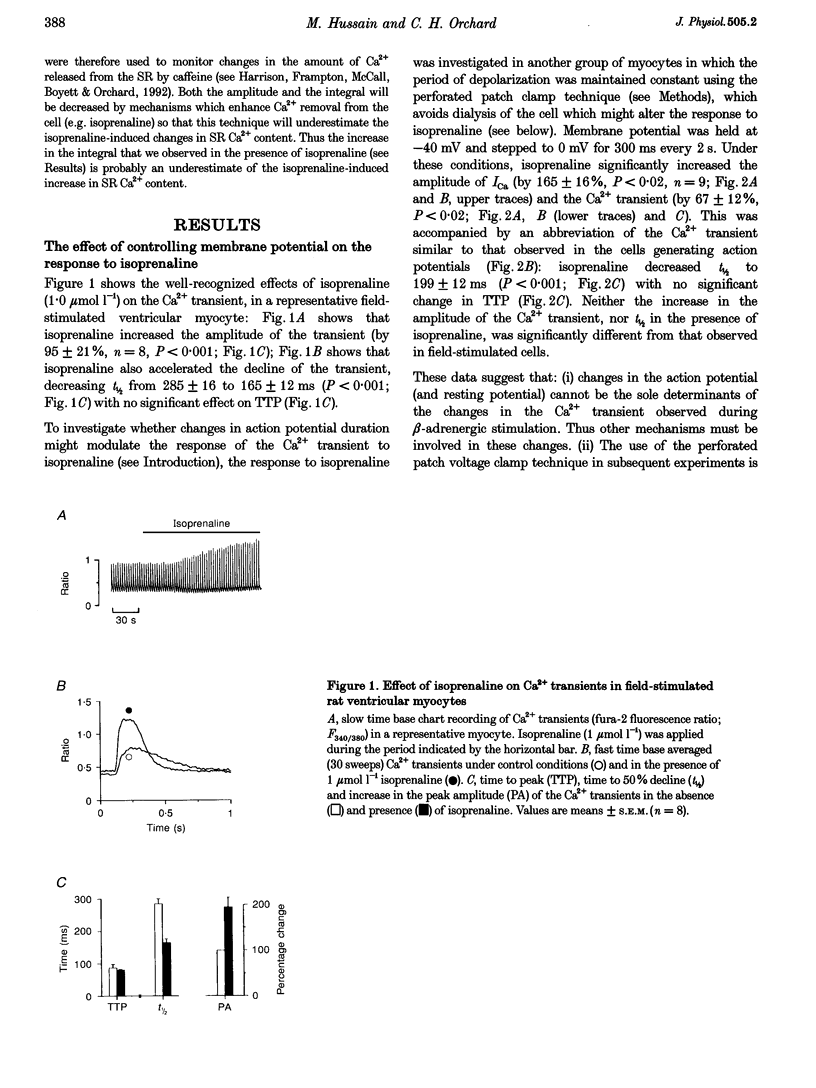
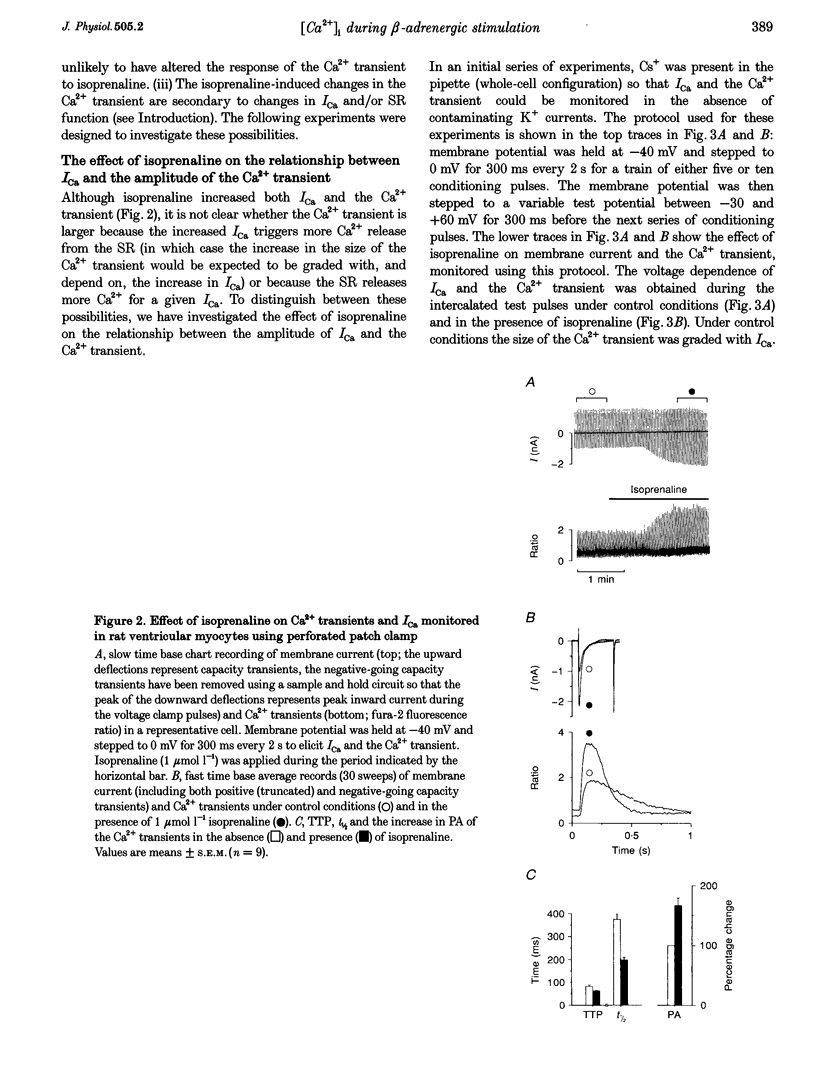
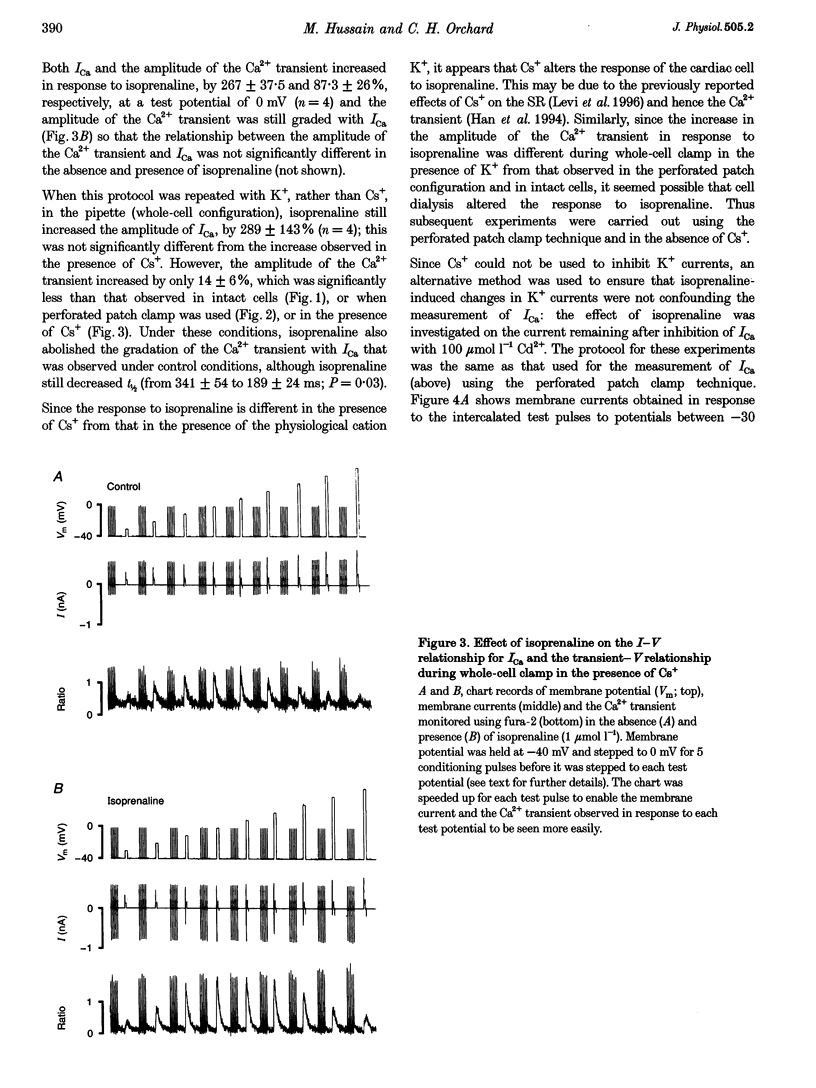
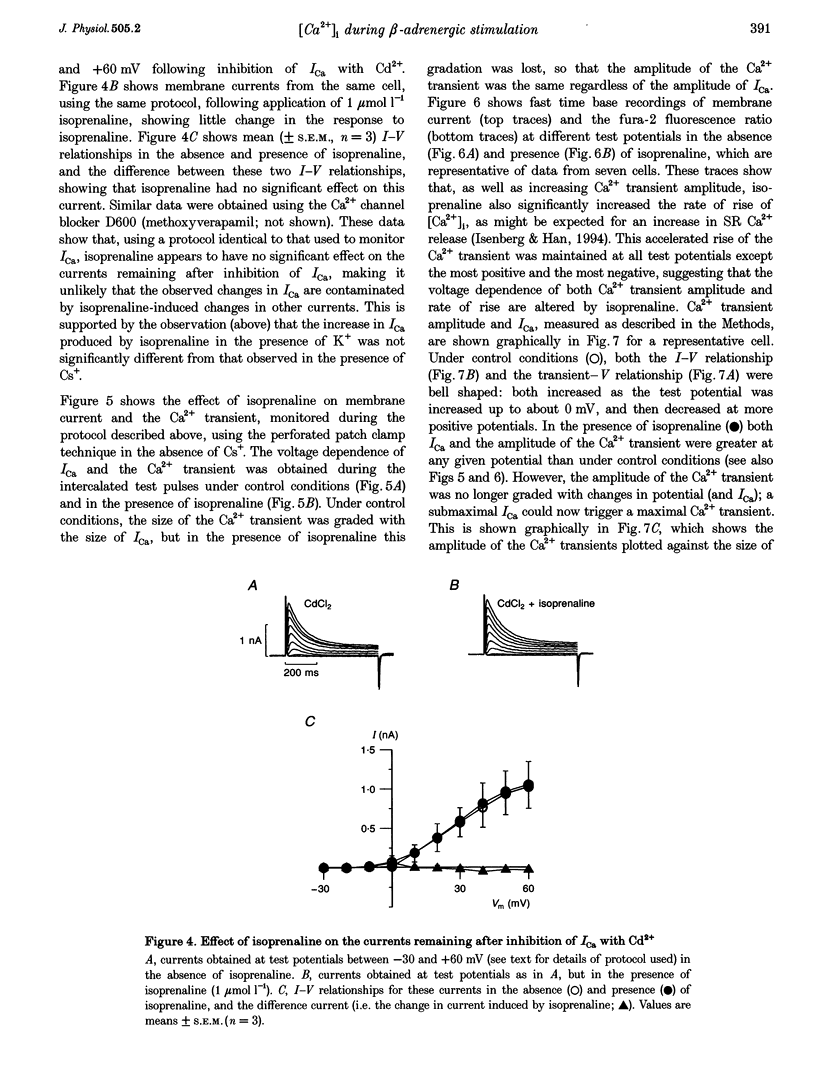
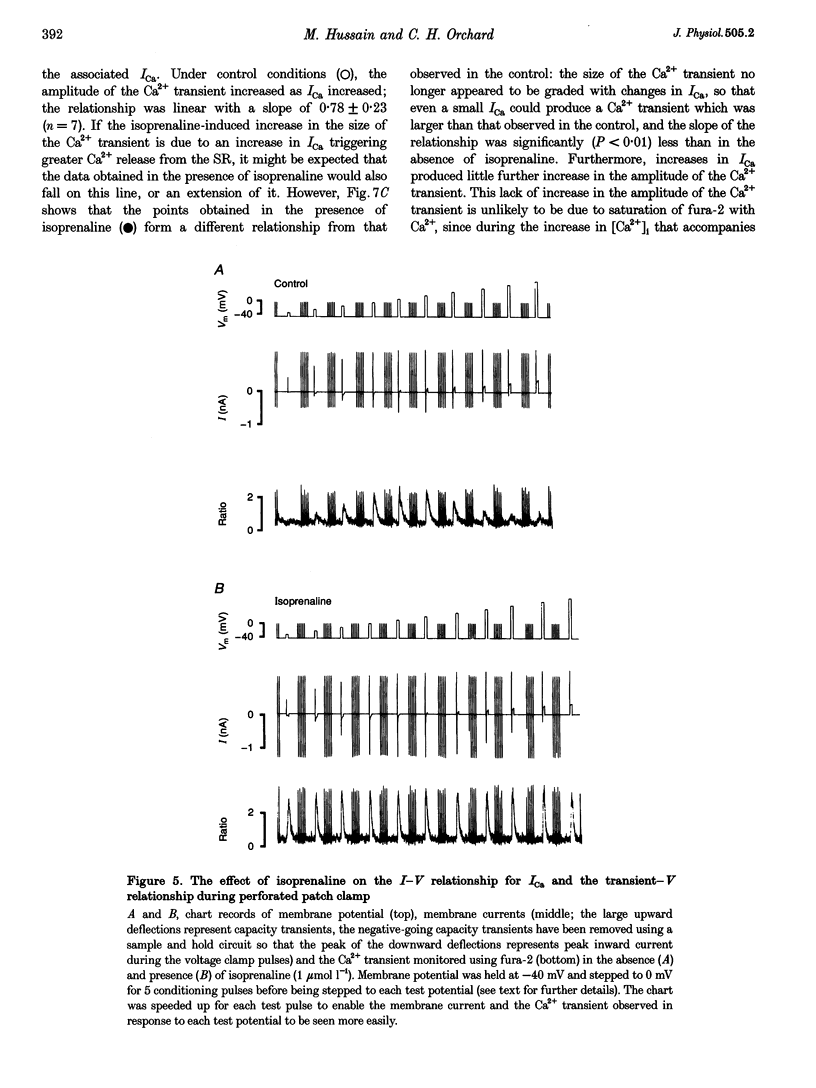
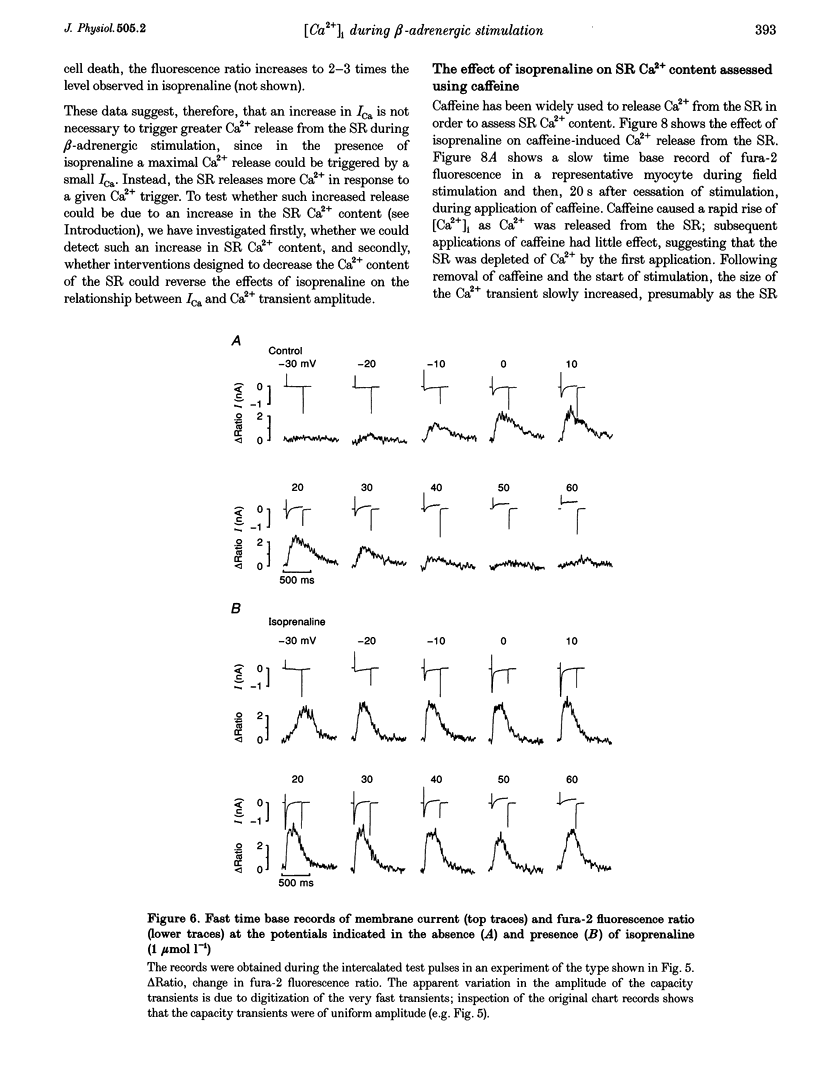
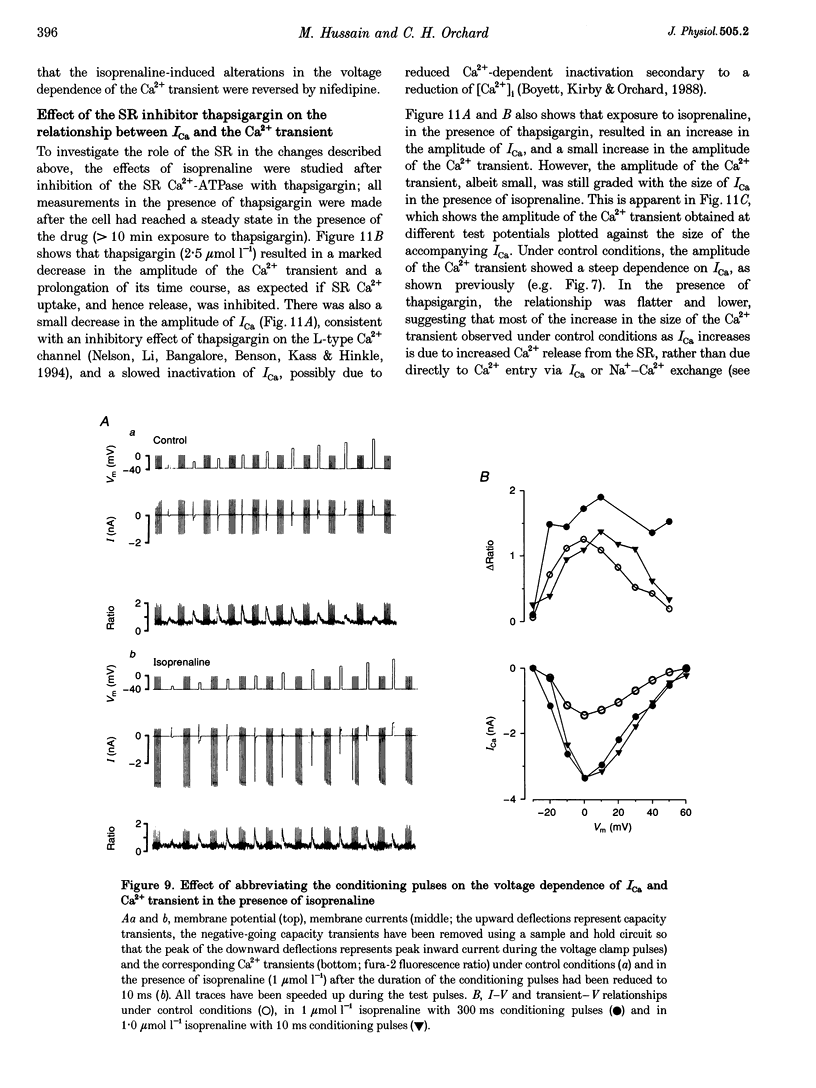
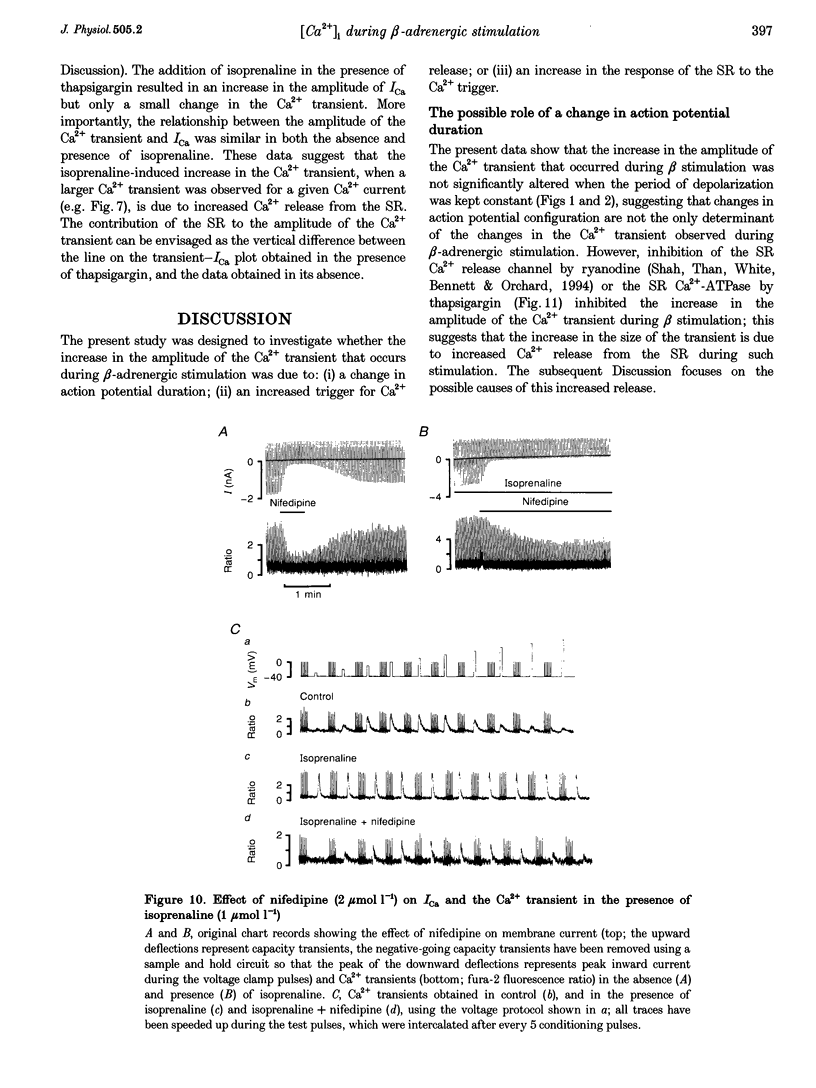
1. The effect of beta-adrenergic stimulation on the relationship between the intracellular Ca2+ transient and the amplitude of the L-type Ca2+ current (ICa) has been investigated in ventricular myocytes isolated from rat hearts. Intracellular [Ca2+] was monitored using fura-2 during field stimulation and while membrane potential was controlled using voltage clamp techniques. 2. The increase in the amplitude, and the rate of decline, of the Ca2+ transient produced by isoprenaline (1.0 mumol l-1) was not significantly different in myocytes generating action potentials and in those voltage clamped with pulses of constant duration and amplitude. 3. Under control conditions, the current-voltage (I-V) relationship for ICa was bell shaped. The amplitude of the Ca2+ transient also showed a bell-shaped voltage dependence. In the presence of isoprenaline, the amplitude of both ICa and the Ca2+ transient was greater at all test potentials and the I-V relationship maintained its bell-shaped voltage dependence. However, the size of the Ca2+ transient was no longer graded with changes in the amplitude of ICa: a small ICa could now elicit a maximal Ca2+ transient. 4. Rapid application of caffeine (10 mmol l-1) was used to elicit Ca2+ release from the sarcoplasmic reticulum (SR). Isoprenaline increased the integral of the subsequent rise in cytoplasmic [Ca2+] to 175 +/- 13% of control. 5. Abbreviation of conditioning pulse duration in the presence of isoprenaline was used to reduce the amplitude of the Ca2+ transient to control levels. Under these conditions, the amplitude of the Ca2+ transient was again graded with the amplitude of ICa in the same way as under control conditions. 6. Nifedipine (2 mumol l-1) was also used to decrease Ca2+ transient amplitude in the presence of isoprenaline. In the presence of isoprenaline and nifedipine, the amplitude of the Ca2+ transient again showed a bell-shaped voltage dependence. 7. The SR Ca(2+)-ATPase inhibitor thapsigargin (2.5 mumol l-1) reduced the effect of isoprenaline on the amplitude of the Ca2+ transient. In the presence of thapsigargin, the size of the Ca2+ transient increased as ICa increased in response to isoprenaline. 8. These data suggest that the increase in the amplitude of the Ca2+ transient produced by beta-adrenergic stimulation in cardiac muscle is due to an increase in the gain of the SR Ca2+ release process, due principally to an increase in the Ca2+ content of the SR.
Full text
PDF


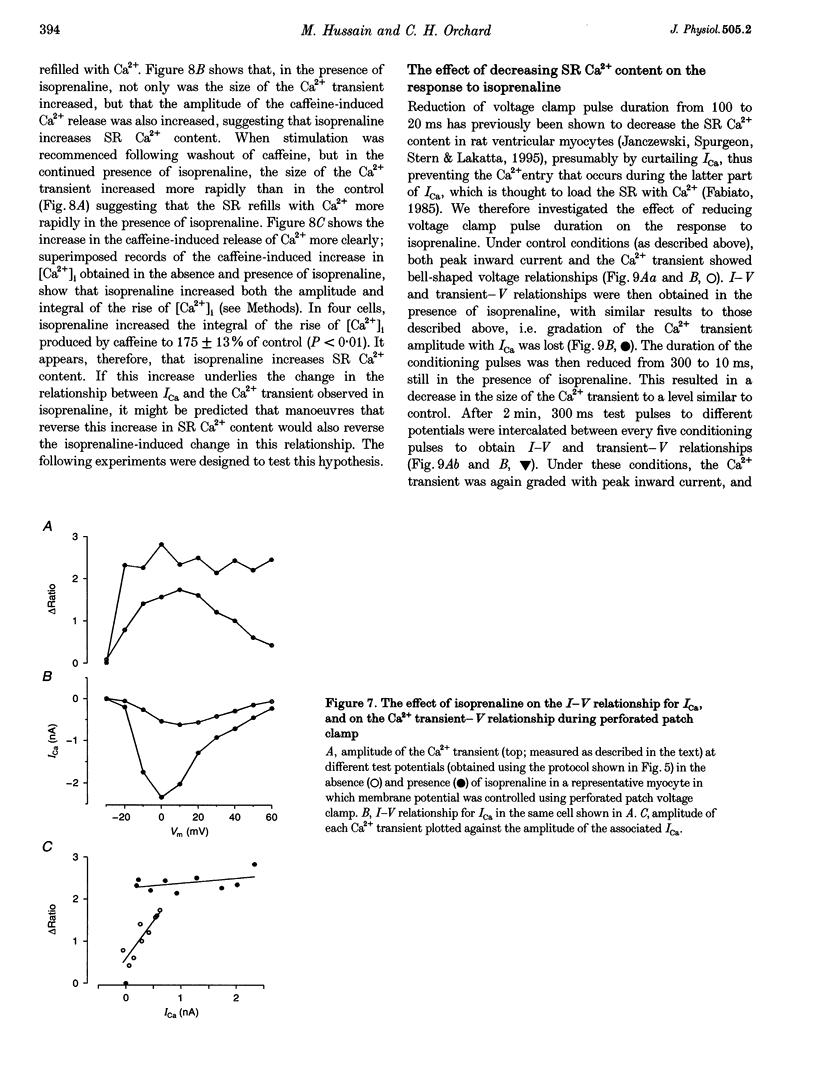
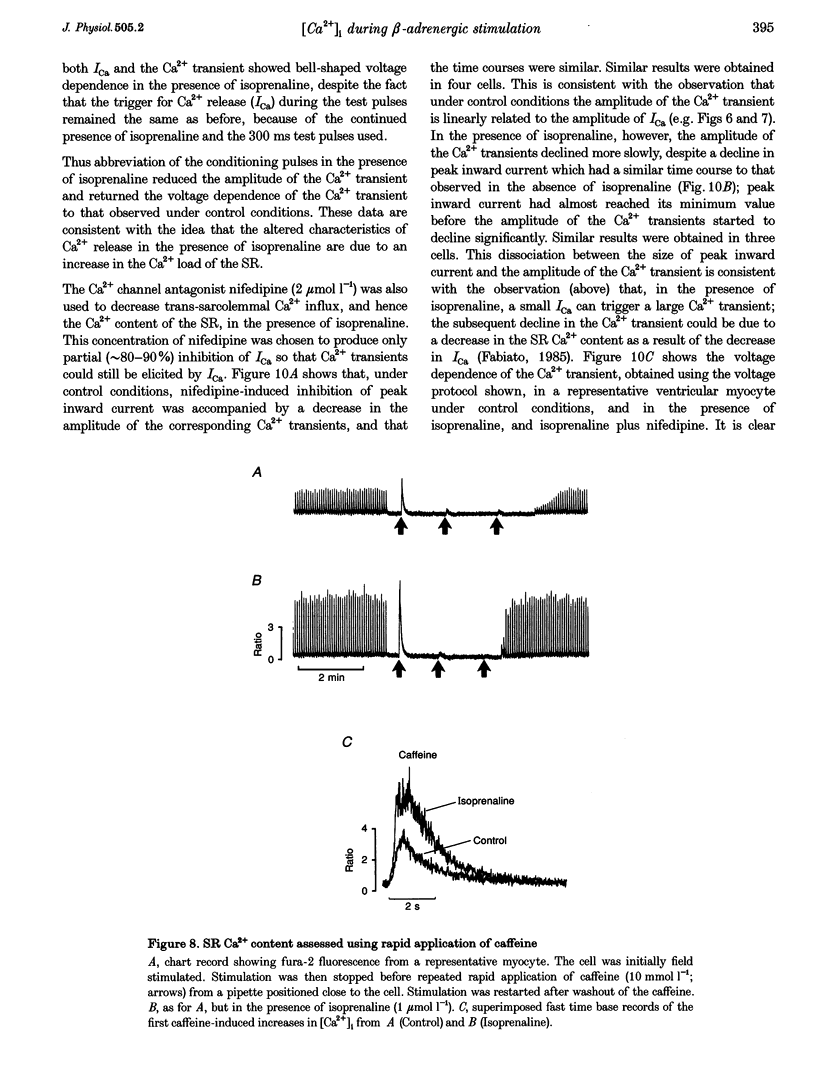





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H. Rapid regulation of the 'second inward current' by intracellular calcium in isolated rat and ferret ventricular myocytes. J Physiol. 1988 Dec;407:77–102. doi: 10.1113/jphysiol.1988.sp017404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Frampton J. E., Orchard C. H., Boyett M. R. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J Physiol. 1991 Jun;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J., Onoue H., Mayrleitner M., Fleischer S., Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995 Feb 3;270(5):2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Han S., Schiefer A., Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994 Nov 1;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Frampton J. E., McCall E., Boyett M. R., Orchard C. H. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992 Feb;262(2 Pt 1):C348–C357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski A. M., Spurgeon H. A., Stern M. D., Lakatta E. G. Effects of sarcoplasmic reticulum Ca2+ load on the gain function of Ca2+ release by Ca2+ current in cardiac cells. Am J Physiol. 1995 Feb;268(2 Pt 2):H916–H920. doi: 10.1152/ajpheart.1995.268.2.H916. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Spitzer K. W., Kohmoto O., Bridge J. H. Depolarization-induced Ca entry via Na-Ca exchange triggers SR release in guinea pig cardiac myocytes. Am J Physiol. 1994 Apr;266(4 Pt 2):H1422–H1433. doi: 10.1152/ajpheart.1994.266.4.H1422. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Lokuta A. J., Rogers T. B., Lederer W. J., Valdivia H. H. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J Physiol. 1995 Sep 15;487(Pt 3):609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Grupp I. L., Harrer J., Ponniah S., Grupp G., Duffy J. J., Doetschman T., Kranias E. G. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994 Sep;75(3):401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. J., Li C. C., Bangalore R., Benson T., Kass R. S., Hinkle P. M. Inhibition of L-type calcium-channel activity by thapsigargin and 2,5-t-butylhydroquinone, but not by cyclopiazonic acid. Biochem J. 1994 Aug 15;302(Pt 1):147–154. doi: 10.1042/bj3020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Phosphorylation restores activity of L-type calcium channels after rundown in inside-out patches from rabbit cardiac cells. J Physiol. 1992 Aug;454:673–688. doi: 10.1113/jphysiol.1992.sp019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana L. F., Cheng H., Gómez A. M., Cannell M. B., Lederer W. J. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996 Jan;78(1):166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Shah N., Than N., White E., Bennett K. L., Orchard C. H. The role of the sarcoplasmic reticulum in the response of isolated ferret cardiac muscle to beta-adrenergic stimulation. Exp Physiol. 1994 Nov;79(6):929–941. doi: 10.1113/expphysiol.1994.sp003818. [DOI] [PubMed] [Google Scholar]
- Sham J. S., Jones L. R., Morad M. Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1344–H1349. doi: 10.1152/ajpheart.1991.261.4.H1344. [DOI] [PubMed] [Google Scholar]
- Steele D. S., Miller D. J. Effects of cAMP and forskolin on caffeine-induced contractures and myofilament Ca-sensitivity in saponin-treated rat ventricular trabeculae. J Muscle Res Cell Motil. 1992 Apr;13(2):146–152. doi: 10.1007/BF01874151. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Vornanen M., Shepherd N., Isenberg G. Tension-voltage relations of single myocytes reflect Ca release triggered by Na/Ca exchange at 35 degrees C but not 23 degrees C. Am J Physiol. 1994 Aug;267(2 Pt 1):C623–C632. doi: 10.1152/ajpcell.1994.267.2.C623. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell W. H., Lederer W. J., Rogers T. B. Dynamic modulation of excitation-contraction coupling by protein phosphatases in rat ventricular myocytes. J Physiol. 1996 Jun 15;493(Pt 3):793–800. doi: 10.1113/jphysiol.1996.sp021423. [DOI] [PMC free article] [PubMed] [Google Scholar]


