Abstract
Background
Studies have linked a lack of dietary fibre, including resistant starch (RS), to disease-associated changes in intestinal bacteria. Healthy people often report abnormal bowel symptoms (ABS), including bloating, constipation, abdominal pain, and diarrhea, however, connections between these symptoms and the gut microbiota are poorly understood. Determining correlations between ABS and taxonomic groups may provide predictive value for using prebiotics to mitigate ABS in combination with stool microbiome testing.
Methods
Post hoc analysis of a three-arm randomized, double-blind, placebo-controlled clinical trial evaluating the effects of 3.5 g and 7 g resistant potato starch (RPS) doses or placebo was conducted. The study population (n = 70) were healthy adults aged 18–69 years old living in and around Guelph, ON. Participants evaluated their stools using the Bristol Stool Chart and also recorded any ABS daily. The presence of ABS was compared between treatment arms at baseline and changes in ABS were compared within treatment arms over 1- and 4-week periods. Pearson correlation analysis was used to identify significant relationships between changes in ABS and changes in bacterial taxa.
Results
Abdominal pain, belching, bloating, constipation, diarrhea, gas, and feeling unwell were reported by participants at low levels at baseline. Neither RPS nor placebo had significant effects on mean ABS scores. However, we identified positive correlations between treatment-dependent changes in symptoms and changes in Granulicatella, Haemophilus, Lachnospira, Olsenella, Papillibacter, Turicibacter, unclassified Enterobacteriaceae, unclassified Fusobacteriaceae, unclassified Pasteurellaceae, and unclassified Gammaproteobacteria. We also identified negative correlations between treatment-dependent changes in symptoms and changes in Anaerotruncus, Dorea, RFN20, Victivallis, unclassified Coriobacteriaceae, and unclassified Oxalobacteraceae. These Pearson correlations were significant after correction for repeated testing. The mean relative abundance of these taxa did not change in response to treatment. Finally, macronutrient intake was unaffected by RPS or placebo treatments.
Conclusion
Changes in ABS can be positively or negatively correlated with changes in specific gut microbiota, creating opportunities for personalized microbiome-targeted interventions to resolve ABS.
Trial Registration
The trial was registered at ClinicalTrials.gov (NCT05242913) on February 16, 2022.
Keywords: Prebiotic, Abdominal pain, Belching, Bloating, Constipation, Potato, Resistant starch, Microbiome, Biomarker
Background
Abnormal bowel symptoms (ABS), including abdominal pain, belching, bloating, constipation, diarrhea, and gas are occasionally experienced by healthy people. As frequency and severity of ABS increase, these symptoms may justify a medical diagnosis, such as Irritable Bowel Syndrome (IBS). Formal IBS diagnosis involves patients meeting some relatively complex criteria [1]. The patient must be experiencing recurrent abdominal pain that began at least 6 months prior to the time of diagnosis. Abdominal pain must occur at least 1 day per week during the most recent 3 months, and the pain must be in association with two or more of the following criteria: The pain is related to defecation, the pain is associated with changes in stool frequency, and/or the pain is associated with a change in stool form [1]. Individuals with IBS-like digestive symptoms who also presented with colonic lesions and inflammation would be diagnosed with inflammatory bowel disease (IBD) [2]. ABS are not a disease unto themselves, but they affect the quality of life of healthy people as well as those with serious diseases, and over time the ABS symptoms may progress and ultimately transition to more serious disease categories.
Recent advances in the ability to identify and characterize unculturable bacteria in the digestive tract have led researchers to characterize the relationship between bacteria in the gut microbiome and ABS, especially in the context of IBS [3–12]. The correlations between microbial taxa in the gut and ABS provide an opportunity to develop personalized stool-based microbiome tests designed to mitigate these symptoms by changing the levels of bacterial taxa in the gut. For example, a person with ABS undergoing microbiome testing may reveal high levels of proteobacteria that are positively correlated with abdominal pain. This correlation would suggest that treatment with a substance known to reduce proteobacteria might efficaciously reduce abdominal pain. However, the complex heterogeneity of the gut microbiome makes it difficult to find microbiome-ABS connections and previous studies have failed to yield generalizable conclusions in diseased states like IBS [13].
In some patients, the presence of pathogenic bacteria or absence of beneficial bacteria may be clearly related with specific disease symptoms, such as enterotoxigenic Escherichia coli, for example [14]. Connections between specific pathogenic bacteria and disease etiology may be straightforward in some conditions. However, we suspect that relationships between ABS and bacteria in the gut microbiome are likely dynamic, with patterns emerging only during specific treatment modalities (e.g. Prebiotic consumption during a clinical trial) where there is correlation between both the makeup of the gut microbiome and the symptom level changes. Relationships between bacterial taxa and symptoms have been previously identified in participants consuming a prebiotic [15, 16].
Resistant starch (RS) is a nutritionally important prebiotic fiber that has essentially disappeared from modern diets [17, 18]. This contrasts with prebiotic oligosaccharides, which are abundant in the diet and contribute more significantly to digestible dietary fiber levels than previously appreciated [19]. Sources of RS include high amylose maize starch, unripe green bananas, and raw potato, including resistant potato starch (RPS) [18]. This scarcity in consumption of RS means that interference from dietary RS sources is minimal in clinical trials where diet is uncontrolled, as was the case in this clinical trial [20]. We previously reported the primary conclusions of the trial, which revealed that consumption of RS lead to significant increases in the relative abundance of Bifidobacterium and Akkermansia compared to placebo, as well as fewer incidences of diarrhea and constipation [20], and metabolomic changes characteristic of enhanced intestinal barrier function [21] and improved free fatty acid metabolism [22]. However, specific evaluations of bacterial changes in relation to bowel symptoms from this clinical study have not been reported.
We hypothesized that shifts in the abundance of some bacterial taxa may be responsible for ABS, and that analyzing changes in both the abundance of bacterial taxa and the magnitude of ABS in participants consuming RPS might reveal important correlations. To this end, we performed post hoc Pearson correlation analysis to detect relationships between changes in ABS and changes in bacterial taxa within the gut microbiome.
Methods
Clinical trial structure, per protocol determination, and sample collection
Clinical trial design, sample size estimations, participant selection, and study procedures have previously been described in detail [20]. Briefly, the trial was a randomized, double-blind, placebo-controlled three-arm parallel group study that evaluated the effects of 7 g per day resistant potato starch (RPS; containing 4.2 g of RS), 3.5 g per day RPS (containing 2.1 g of RS plus 3.5 g of digestible corn starch), and 7 g per day placebo (containing 0 g of RS in 7 g of digestible corn starch) on fecal bacteria composition and bowel movement consistency. The study protocol was approved by Canadian Shield Ethics Review Board (REB Tracking Number: 19-10-001; Burlington, ON, Canada) on October 29, 2019. The investigator or the investigator’s representative explained the nature of the study to the participant or the participant’s legally authorized representative and answered any questions. Participants were informed that their participation was voluntary and that study reports or publications reporting on the study would not disclose the participant’s identity without specific consent. Participants wishing to participate in the study, or their legally authorized representative, signed a statement of informed consent that met the requirements of local regulations, ICH guidelines, and the research ethics board. The authorized person obtaining informed consent also signed the informed consent form and a copy was provided to the participant or the participant’s legally authorized representative. Written informed consent was obtained prior to all study-related procedures. Seventy-five healthy participants (25 participants per arm) from Guelph, ON and the surrounding area aged between 18 and 69 years with a body mass index between 18.0 and 34.9 kg/m2 were recruited and enrolled in the study. The mean age of participants was 38.5 years, and the mean BMI was 25.44 kg/m2. Participants agreed not to use any dietary supplements, including vitamins or minerals, 14 days prior to treatment randomization until completion of the final visit, and were advised to maintain their activity level and habitual diet.
Self-reported abnormal bowel symptom and Bristol Stool Chart scoring
Participants rated their level of abdominal pain, belching, bloating, gas, and overall wellbeing daily throughout the trial on a Likert scale: 0 (none), 1 (some), 2 (moderate), 3 (severe), or 4 (terrible) based on a simplified form of the Gastrointestinal Quality of Life Index [23]. Values were averaged during the run-in period (~ 14 days prior to intervention; Baseline) as well as during the first week (Week 1) and last week (Week 4) of the intervention period. Participants reporting an abnormal bowel symptom or feeling unwell for at least one day during the run-in period were considered to have that symptom or be unwell at baseline.
During the same periods, participants scored their bowel movements using the Bristol Stool Chart (BSC), where Type 1 = constipation with hard, round stools, Type 2 = lumpy and sausage-like, Types 3 and 4 are soft, easily passed ‘normal’ stools, Type 5 = soft blobs with clear-cut edges, Type 6 = mushy consistency with ragged edges, and Type 7 = watery diarrhea [24]. Constipation and diarrhea were scored separately because individuals may suffer from both symptoms, and symptoms could be incorrectly normalized or lost if BSC scores were simply averaged over the study interval. To this end, constipation scores were derived from bowel movements with BSC Types 1–4, where Type 1 was scored 4, Type 2 scored 3, and Types 3 and 4 were each scored 1. Diarrhea scores were derived from bowel movements with BSC Types 3–7, where Type 7 was scored 8, Type 6 scored 7, Type 5 scored 6, and Types 3 and 4 were each scored 1. Diarrhea stools received higher numerical values due to the urgency typically associated with diarrhea. Values were averaged separately for constipation and diarrhea during the run-in period (~ 14 days prior to intervention; Baseline), during the first week (Week 1), and during the last week (Week 4) of the intervention period.
Three-day food records
Participants recorded food consumption using a three-day food record during the three days prior to stool collection at baseline, week 1, and week 4 time points. The food records were entered into ESHA Research Food Processor Nutrition Analysis Software (ESHA Research, Salem, OR) to calculate the macronutrient composition (carbohydrates, protein, fat, sugar, and fiber) for each food record day. The three days of records were then averaged to determine a daily value.
Investigational product
Resistant potato starch (Solnul®; MSP Starch Products Inc., Carberry, MB), an unmodified resistant potato starch (RS type 2) manufactured via a proprietary process yielding a minimum resistant starch (RS) content of 60% (AOAC 2002.02), was used in this study. The placebo used was fully digestible corn starch (Amioca; Ingredion, Brampton, ON) that contained no RS [25, 26].
Microbiome analysis
Microbial analysis was previously described [20]. Briefly, 16s amplicons of the v4 region were obtained from fecal samples collected in OMNIgene-Gut kits (DNA Genotek, Ottawa, ON, Canada) and sequenced on the MiSeq platform (Illumina, San Diego, CA, USA). Fastq files were filtered for quality and clustered into 97% similarity operational taxonomic units using the mothur software package [27] and Greengenes v13.8 database at Microbiome Insights (Vancouver, BC, Canada). Further analysis is underway, and sequence data will be deposited once this work is completed.
Statistical analysis
All analysis was done using Excel (Microsoft, Redmond, WA). Presence versus absence for abnormal bowel symptoms (ABS) and feeling unwell at baseline were compared between treatment groups for each symptom using Chi Square tests. Changes in ABS, feeling unwell, relative abundance of select bacterial taxa, and macronutrients were compared within treatment groups over time using a single factor ANOVA. Chi square and ANOVA comparisons were deemed significant if p < 0.05. For correlations analysis, changes in ABS or feeling unwell were determined by subtracting Baseline values from Week 1 and Week 4 values, and changes in relative abundance for bacterial groups were similarly calculated. Due to the small number of participants, changes at weeks 1 and 4 were pooled [26]. Pearson correlation coefficients were calculated for each symptom-bacterial group interaction, converted to a t statistic, and the p value was determined using a two-tailed distribution. Significant correlations were determined after correcting for multiple testing using the Benjamini-Hochberg method within each symptom-treatment group combination at a false discovery rate of 0.1 [28].
Results
Participant characteristics
Among the participants completing the study, 48 were female and 22 were males. Participants were largely white (89%), non-Hispanic and non-Latino (97%), with small representation of indigenous (1%), Asian (9%), and other (1%) races and Hispanic (3%) ethnicity. Dietary and cultural habits practiced by participants were largely from North America, (80%), with South Asian (4%), East Asian (3%), Mediterranean (3%), Central American (1%), Eastern European (1%), and Middle Eastern (1%) customs being practiced by a minority of participants. Dietary habits practiced by participants that were not associated with geography or ethnicity included vegetarian or vegan diets (5%), and vegetarian diets occasionally including fish or chicken (1%). No participants reported being either gluten or lactose intolerant.
Abnormal bowel symptom prevalence
Abnormal bowel symptoms (ABS), including abdominal pain, belching, bloating, constipation, diarrhea, and gas, as well as feeling unwell, were prevalent in the three treatment groups at baseline (Table 1). However, there were no significant differences between treatment groups for any of these symptoms at baseline.
Table 1.
Baseline presence versus absence of abnormal bowel symptoms (ABS) and feeling unwell at baseline in each treatment group
| Symptoms | Placebo | 3.5 g RPS | 7 g RPS | p value |
|---|---|---|---|---|
| Abdominal pain | 10/24 | 15/24 | 11/22 | 0.348 |
| Belching | 11/24 | 12/24 | 12/22 | 0.84 |
| Bloating | 11/24 | 17/24 | 15/22 | 0.151 |
| Constipation | 15/24 | 14/24 | 14/22 | 0.9259 |
| Diarrhea | 21/24 | 19/24 | 17/22 | 0.6321 |
| Gas | 19/24 | 22/24 | 21/22 | 0.1869 |
| Feeling unwell | 15/24 | 17/24 | 12/22 | 0.5204 |
The proportion of participants reporting ABS or feeling unwell during the run-in period are shown for each treatment group. Differences in symptom presence versus absence were compared between treatment arms using Chi Square test with p < 0.05 considered significant
Changes in abnormal bowel symptoms
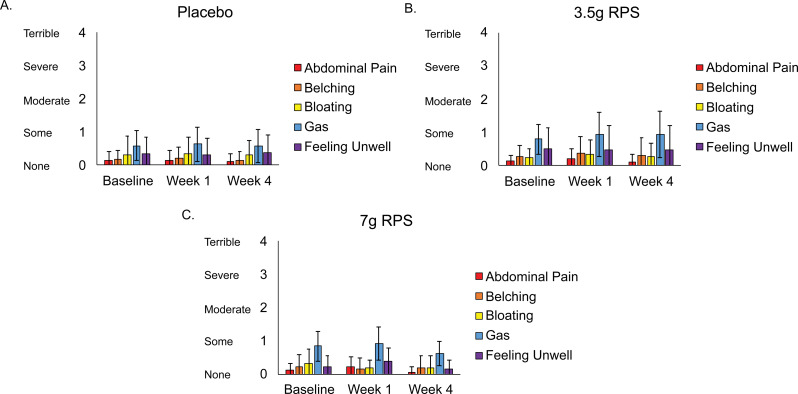
Average scores of participants reporting abdominal pain, belching, bloating, gas, or feeling unwell did not significantly change (p > 0.05) over time in the placebo (Fig. 1A), 3.5 g RPS (Fig. 1B), or 7 g RPS (Fig. 1C) treatment groups.
Fig. 1.
Levels of abdominal pain, belching, bloating, gas, and feeling unwell reported at baseline, week 1, and week 4 in the placebo (A; n = 24), 3.5 g RPS (B; n = 24), and 7 g RPS (C; n = 22) treatment arms. Mean +/- SEM. p > 0.05 for all change over time comparisons (Single-factor ANOVA)
Microbiome-abnormal bowel symptom correlations
After meeting Benjamini-Hochberg criteria for multiple testing correction, 21 significant bacterial taxonomic group-abnormal bowel symptom correlations were categorized by symptom for 3.5 g and 7 g RPS (Table 2, Genus column), and placebo (Table 3, Genus column) interventions.
Table 2.
Correlations between abnormal bowel symptoms and microbiome changes in healthy adults consuming 3.5–7 g of RPS per day
| Phylum | Class | Order | Family | Genus | Pearson r | p value | Treatment |
|---|---|---|---|---|---|---|---|
| Belching | |||||||
| Firmicutes | Clostridia | Eubacteriales | Oscillospiraceae | Papillibacter | 0.51792 | 0.000317 | 7 g RPS |
| Lachnospiraceae | Dorea | -0.53065 | 0.000105 | 3.5 g RPS | |||
| Proteobacteria | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unclassified | 0.50735 | 0.000233 | 3.5 g RPS |
| Actinobacteria | Coriobacteria | Coriobacteriales | Coriobacteriaceae | unclassified | -0.55797 | 0.000038 | 3.5 g RPS |
| Bloating | |||||||
| Firmicutes | Clostridia | Eubacteriales | Lachnospiraceae | Lachnospira | 0.56389 | 0.000007 | 7 g RPS |
| Bacilli | Lactobacillales | Carnobacteriaceae | Granulicatella | 0.53969 | 0.000156 | 7 g RPS | |
| Proteobacteria | Gammaproteobacteria | Pasteurellales | Pasteurellaceae | Haemophilus | 0.56175 | 0.000073 | 7 g RPS |
| unclassified | 0.56214 | 0.000072 | 7 g RPS | ||||
| Enterobacterales | Enterobacteriaceae | unclassified | 0.50612 | 0.000456 | 7 g RPS | ||
| unclassified | unclassified | unclassified | 0.52426 | 0.000259 | 7 g RPS | ||
| Fusobacteria | Fusobacteria | Fusobacteriales | Fusobacteriaceae | unclassified | 0.50095 | 0.000533 | 7 g RPS |
| Constipation | |||||||
| Firmicutes | Bacilli | Lactobacillales | Carnobacteriaceae | Granulicatella | 0.60963 | 0.000011 | 7 g RPS |
| Erysipelotrichia | Erysipelotrichales | Turicibacteraceae | Turicibacter | 0.51073 | 0.000208 | 3.5 g RPS | |
| Proteobacteria | Gammaproteobacteria | unclassified | unclassified | unclassified | 0.50783 | 0.000229 | 3.5 g RPS |
Table 3.
Correlations between abnormal bowel symptoms and microbiome changes in healthy adults consuming placebo
| Phylum | Class | Order | Family | Genus | Pearson r | p value | Treatment |
|---|---|---|---|---|---|---|---|
| Abdominal pain | |||||||
| Lentisphaerota | Lentisphaeria | Victivallales | Victivallaceae | Victivallis | -0.62491 | 0.000002 | Placebo |
| Tenericutes | - | - | - | RFN20 | -0.5244 | 0.00013 | Placebo |
| Actinobacteria | Coriobacteria | Coriobacteriales | Atopobiaceae | Olsenella | 0.52198 | 0.000142 | Placebo |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Oxalobacteraceae | unclassified | -0.49401 | 0.000359 | Placebo |
| Bloating | |||||||
| Lentisphaerota | Lentisphaeria | Victivallales | Victivallaceae | Victivallis | -0.4907 | 0.000399 | Placebo |
| Feeling unwell | |||||||
| Firmicutes | Clostridia | Eubacteriales | Oscillospiraceae | Anaerotruncus | -0.56273 | 0.000031 | Placebo |
| Lentisphaerota | Lentisphaeria | Victivallales | Victivallaceae | Victivallis | -0.49701 | 0.000326 | Placebo |
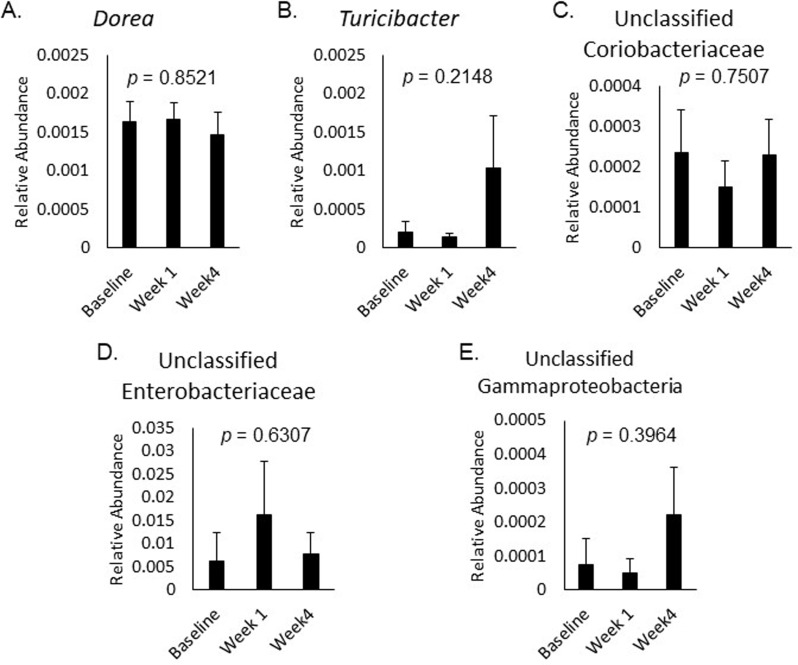
Changes in belching were significantly positively correlated with changes in Papillibacter (p = 0.000317) in the 7 g RPS treatment group and changes in unclassified Enterobacteriaceae (p = 0.000233) in the 3.5 g RPS treatment group (Table 2). Papillibacter was present in only 7% of participants and increases in Papillibacter were associated with increased belching. Unclassified Enterobacteriaceae were present in 79% of participants, and among those with unclassified Enterobacteriaceae, most experienced increased belching with increases in this taxonomic group. Changes in belching were significantly negatively correlated with changes in Dorea (p = 0.000105) and unclassified Coriobacteriaceae (p = 0.000038) in the 3.5 g treatment group (Table 2). Dorea was detected in every participant consuming 3.5 g RPS while unclassified Coriobacteriaceae was found in 83% of the same group. These negative correlations suggest that increases of Dorea and/or unclassified Coriobacteriaceae led to reduced belching in a subset of participants consuming RPS.
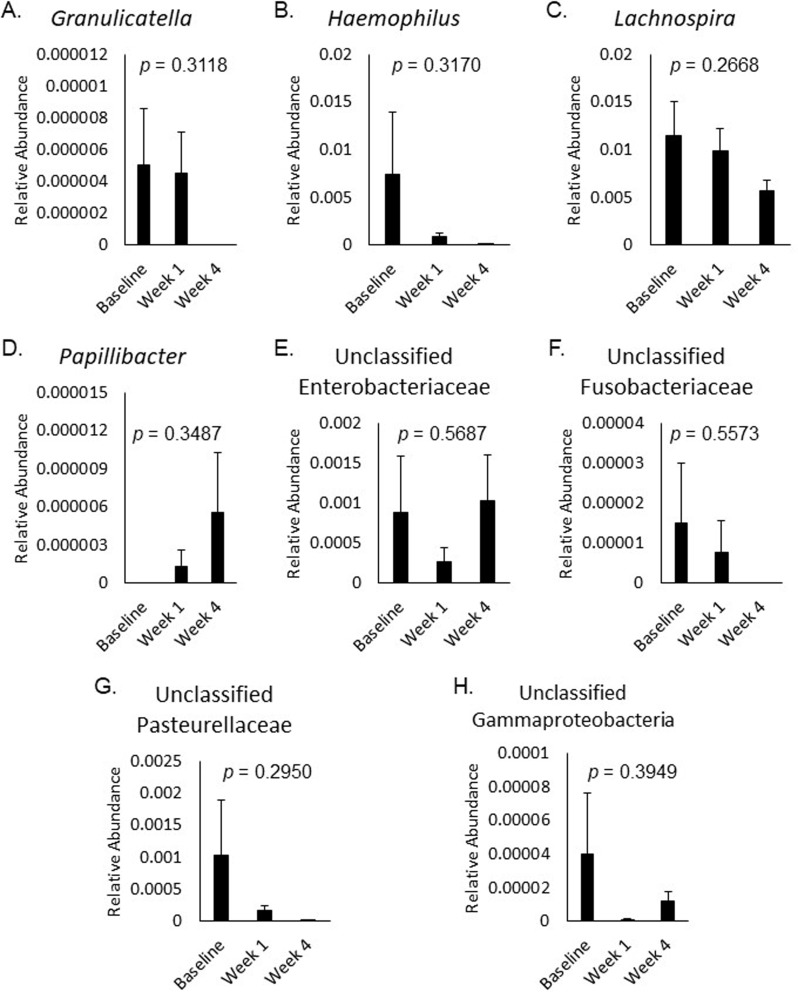
Changes in bloating were significantly positively correlated with changes in Lachnospira (p = 0.000007), Granulicatella (p = 0.000156), Haemophilus (p = 0.000073), unclassified Pasteurellaceae (p = 0.000072), unclassified Enterobacteriaceae (p = 0.000456), unclassified Gammaproteobacteria (p = 0.000259), and unclassified Fusobacteriaceae (p = 0.000533) in the 7 g RPS group (Table 2). Occurrence of these taxa varied, with Lachnospira present in 100%, Granulicatella present in 16%, Haemophilus present in 93%, unclassified Pasteurellaceae present in 75%, unclassified Enterobacteriaceae present in 73%, unclassified Gammaproteobacteria present in 27%, and unclassified Fusobacteriaceae present in 5% of 7 g RPS consuming participants. Except for unclassified Enterobacteriaceae and unclassified Gammaproteobacteria, the positive correlation indicates that participants consuming 7 g RPS tended to experience decreased levels of these taxa with decreased bloating.
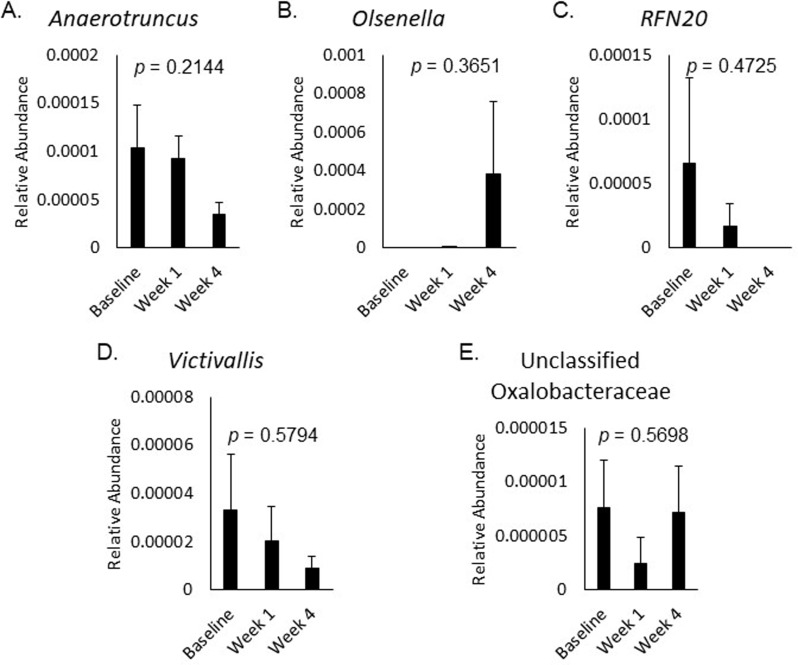
Changes in constipation were significantly positively correlated with changes in Granulicatella (p = 0.000011) in the 7 g RPS group, and with Turicibacter (p = 0.000208) and unclassified Gammaproteobacteria (p = 0.000229) in the 3.5 g RPS group (Table 2). Consumption of 7 g RPS tended to decrease levels of Granulicatella along with decreases in constipation scores despite 7 g RPS not have an overall effect on constipation [20]. Turicibacter was present in 92% of participants in the 3.5 g RPS treatment group and consumption of this dose was previously demonstrated to produce significantly fewer instances of constipation compared to the placebo [20]. In the placebo group, changes in abdominal pain were significantly negatively correlated with changes in Victivallis (p = 0.000002), RFN20 (p = 0.00013), and unclassified Oxalobacteraceae (p = 0.000359; Table 3). These taxonomic groups were uncommon in the placebo consuming group, with Victivallis present in 19%, RFN20 present in 4%, and unclassified Oxalobacteraceae present in 19% or participants. The negative correlation suggests that increasing these taxa could reduce abdominal pain. Conversely, changes in abdominal pain were significantly positively correlated with Olsenella (p = 0.000142; Table 3), another infrequently occurring taxonomic group (19% of placebo-consuming participants). Relative abundance of Olsenella increased in those with elevated pain, indicating that decreasing Olsenella is desirable for abdominal pain mitigation.
Consistent with change in abdominal pain, changes in bloating were significantly negatively correlated with changes in Victivallis (p = 0.000399) as were changes in feeling unwell (p = 0.000326; Table 3). Changes in feeling unwell were also significantly negatively correlated with changes in Anaerotruncus (p = 0.000031; Table 3). Unlike the other taxa that significantly correlated with changes in ABS in the placebo group, Anaerotruncus was present in 81% of participants and the number of participants experiencing increases in Anaerotruncus were roughly equal to those experiencing decreases. This is consistent with heterogenous changes in feeling unwell in the placebo group and suggests that microbiome modulators that can increase Anaerotruncus may decrease unwell feelings.
The relative abundance of bacterial taxa correlated with changes in ABS or feeling unwell did not significantly change over time in response to placebo (Fig. 2A-E), 3.5 g RPS (Fig. 3A-E), or 7 g RPS (Fig. 4A-H; p > 0.05).
Fig. 2.
Relative abundance of Anaerotruncus (A), Olsenella (B), RFN20 (C), Victivallis (D), and unclassified Oxalobacteraceae (E) at baseline, week 1, and week 4 in the placebo group. Mean +/- SEM showed p values > 0.05 for all changes in the five bacterial categories over the three time frames evaluated by Single-factor ANOVA
Fig. 3.
Relative abundance of Dorea (A), Turicibacter (B), unclassified Coriobacteriaceae (C), unclassified Enterobacteriaceae (D), and unclassified Gammaproteobacteria (E) at baseline, week 1, and week 4 in the 3.5 g RPS group. Mean +/- SEM showed p values > 0.05 for all changes in the five bacterial categories over the three time frames evaluated by Single-factor ANOVA
Fig. 4.
Relative abundance of Granulicatella (A), Haemophilus (B), Lachnospira (C), Papillibacter (D), unclassified Enterobacteriaceae (E), unclassified Fusobacteriaceae (F), unclassified Pasteurellaceae (G), and unclassified Gammaproteobacteria (H) at baseline, week 1, and week 4 in the 7 g RPS group. Mean +/- SEM showed p values > 0.05 for all changes in the eight bacterial categories over the three time frames evaluated by Single-factor ANOVA
None of the correlations detected between changes in bacterial taxa and changes in diarrhea or gas met statistical significance in any of the treatment groups (data not shown).
Macronutrient intake
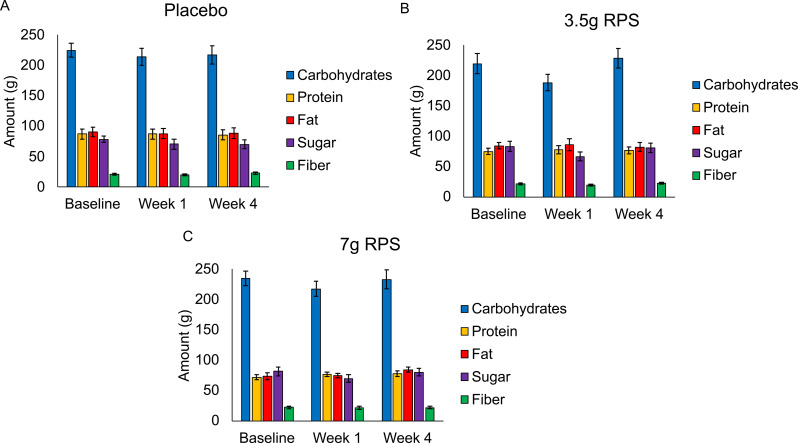
Average daily consumption of carbohydrates, protein, fat, sugar, and fiber did not significantly change (p > 0.05) over time in the placebo (Fig. 5A), 3.5 g RPS (Fig. 5B), or 7 g RPS (Fig. 5C) treatment groups.
Fig. 5.
Consumption of carbohydrates, protein, fat, sugar, and fiber was reported at baseline, week 1, and week 4 in the placebo (A; n = 24), 3.5 g RPS (B; n = 24), and 7 g RPS (C; n = 22) treatment arms. Mean +/- SEM showed ap value > 0.05 for all macronutrient changes in the five macronutrient categories over the three time frames evaluated by Single-factor ANOVA
Discussion
Except for abdominal pain in the placebo and 3.5 g groups, and belching and bloating in the placebo group, all ABS were reported in 50% or more of the participants at baseline, indicating that ABS are prevalent in otherwise healthy individuals. The average levels of abdominal pain, belching, bloating, gas, and feeling unwell did not change over time in response to any treatments. It was previously reported that RPS administration led to fewer instances of constipation and diarrhea in the 3.5 g group, and fewer instances of diarrhea in the 7 g group compared to the placebo [20]. There were 21 bacterial taxa that were significantly correlated with changes in various ABS across the three treatment groups. However, no treatment led to a significant change in the mean levels of these taxa. Additionally, neither the 3.5 g or the 7.0 g RPS treatments affected the baseline macronutrient consumption of the participants, including fiber, which could influence ABS and microbial abundance independent of treatment (i.e. underlying eating habits were unchanged). Collectively, these data suggest that treatment-dependent changes in bacterial taxa are correlated with changes in ABS in a personalized manner. This information may be useful in combination with stool microbiome testing for people seeking to use RPS to alleviate ABS.
The average severity of ABS and feeling unwell was very low in each group, ranging between 0 (none) and 1 (some) for all symptoms. It is therefore unsurprising that treatments had no effect on the average ABS scores in these participants. These observations are important because clinical trials aimed at substantiating structure/function claims on dietary supplements in the United States are required to study healthy people, in whom any symptoms have limited room for improvement [29]. Low levels of ABS at baseline suggest that prebiotic clinical trials evaluating changes in ABS using the Gastrointestinal Quality of Life Index or related self-reported measures in healthy people are unlikely to successfully generate data that substantiates structure/function claims because there are insufficient ABS at baseline to detect a change [23]. Prebiotic studies evaluating changes in stool form may be more successful in healthy populations given that they experience periodic instances of constipation and diarrhea that can be identified using tools such as the BSC [24], and that most prebiotics are types of dietary fiber, which are known to influence bowel habits [20].
The mechanistic linkages between RPS and the ABS-correlated taxa are not clear. Bifidobacterium levels are known to bloom during RPS supplementation in people [20, 25, 30] but changes in this taxon were not significantly correlated with ABS changes. Similarly, RPS stimulated increases in Akkermansia [20] but we detected no significant correlations between changes in abundance and changes in ABS. Previously, we used similar post hoc correlation analysis to detect correlations between changes in Sporacetigenium and blood glucose levels [15], and changes in Parasutterella and low-density lipoprotein in a RPS clinical trial [16]. Neither of these genera are known to be directly influenced by RPS, suggesting that RPS may be acting indirectly, through ecological interactions between primary degraders like Bifidobacterium and secondary or tertiary taxa like those described in the present work. Consistent with this hypothesis, RPS administration did not significantly alter the relative abundance of any ABS-correlated taxa.
RPS modulated belching, bloating, and constipation, largely through positive correlations with changes in several bacterial groups, including Papillibacter, Lachnospira, Granulicatella, Haemophilus, and Turicibacter, as well as unclassified Enterobacteriaceae, unclassified Fusobacteriaceae, and unclassified Gammaproteobacteria. Among these, Papillibacter [31], Lachnospira [12], Granulicatella [12], Haemophilus [10], Turicibacter [32], and members of class Gammaproteobacteria, including family Enterobacteriaceae [33], have previously been associated with ABS symptoms in people or in animal models. For symptom-bacterial taxon relationships like these that bear a positive correlation, RPS administration leading to reductions in the bacterial taxonomic group are expected to reduce levels of the ABS.
Conversely, RPS modulated belching via negative correlations with changes in Dorea and unclassified Coriobacteriaceae. Both Dorea and unclassified members of Coriobacteriaceae (here represented by all bacteria not belonging to the Collinsella genus) are also associated with metabolic health and various other human conditions [34, 35], but have not previously been associated with belching or upper gastrointestinal complaints. In the case of negative symptom-bacterial taxon correlations, RPS supplementation that increases levels of Dorea or unclassified Coriobacteriaceae would be expected to mitigate belching. Gut microbiome testing is an important first step in using these biomarkers given that these bacterial groups were not present in all people.
We also detected significant correlations between changes in gut bacteria abundance and changes in ABS symptoms in the placebo group. Changes in abdominal pain were negatively correlated with changes in Victivallis, RFN20, and unclassified Oxalobacteraceae, and positively correlated with changes in Olsenella in the placebo group. Changes in bloating were negatively correlated with changes in Victivallis, and changes in overall wellbeing were negatively correlated with changes in Victivallis and Anaerotruncus in the placebo group. Interestingly, Victivallis was consistently negatively correlated with changes in abdominal pain, bloating, and overall wellbeing in the placebo group, suggesting that therapies to increase Victivallis might be helpful in mitigating these related symptoms. However, none of the ABS-correlated taxa identified in the placebo group have previously been associated with ABS.
Notably, several of the bacterial groups that were correlated with ABS in this study have previously been correlated with symptom severity in individuals with IBS [10, 12, 33]. Probiotic administration appears to provide symptomatic reduction in IBS patients [36, 37], while prebiotics and synbiotics do not provide significant benefit [37]. Our findings raise the possibility that prebiotics, at least RS, may influence ABS in a personalized manner that is dependent upon the presence of one or more bacterial groups. Therefore, personalized approaches that first characterize the composition of the gut microbiome to identify putative ‘responders’ may be required for RPS, and potentially other prebiotics, to reduce symptoms in patients with IBS.
Finally, the composition of the microbiota in the gut are known to be influenced by dietary patterns, including dietary fiber intake [38]. The present study encouraged participants to consume their habitual diet, and consumption of the test products was meant to mimic how dietary supplement users would normally consume RPS as a prebiotic supplement. Average daily intake of carbohydrates, protein, fat, sugar, and fiber were unaffected by consumption of any of the test products, suggesting that underlying dietary patterns could not explain the microbiome or symptom changes reported here with RPS supplementation or in previous publications from this clinical trial [20, 21].
Further work examining the utility of these symptom-taxon relationships is warranted. For example, one could test the hypothesis implied here that RPS-dependent reductions in Granulicatella will lead to reductions in constipation by screening participants for the presence of both constipation and the constipation-correlated taxon Granulicatella, and then assigning these participants to RPS or placebo controls. If the RPS consuming group experienced both reductions in constipation along with reductions in Granulicatella compared to the placebo group, it would validate the observations made here and provide rationale for conducting microbiome testing ahead of constipation treatment.
There are several limitations to our study. First, gut bacteria exist in a complex ecosystem with substantial functional redundancy among strains, species and even genera, making it difficult to ascertain relationships between bacterial taxa and host symptoms. This trial was not designed to study complex ecological interactions between microbiota in the gut and their consequences on the host. Second, we identified several correlations in the placebo arm, consistent with previous observations that corn starch placebo stimulated the growth of Proteobacteria in the gut [25]. While the placebo corn starch lacked RS [25], it is possible that it has other effects on the gut microbiome. Finally, several correlations were associated with unclassified bacteria taxa, which is a limitation of the microbiome analysis methods used in this study. Future studies using metagenomics and/or more comprehensive taxonomic databases may identify novel taxa responsible for the effects described in this study.
Conclusions
This study reveals that ABS are prevalent in healthy people and that neither the 3.5 g or the 7 g RPS treatments were able to significantly alter the average levels of abdominal pain, belching, bloating, gas, or feeling unwell within groups. However, changes in ABS were correlated with changes in bacterial taxa, suggesting that improvements in ABS following RPS treatment may be personalized and depend upon the presence of specific bacterial taxa. Treatment with RPS did not significantly affect the mean relative abundance of any taxonomic group found to be significantly correlated with ABS or feeling unwell. Treatment also did not affect the consumption of macronutrients, suggesting that underlying dietary changes did not play a role in forming the gut microbiome-ABS correlations. The results of the correlation analysis are consistent with previous studies demonstrating that the composition of the gut microbiome can be used to predict and monitor the efficacy of RPS modulation of host health parameters, such as blood glucose, insulin, and low-density lipoprotein cholesterol levels [15, 16]. The use of biomarkers, such as specific bacteria in the gut microbiome, to personalize strategies for the treatment or mitigation of various health concerns is an emerging opportunity. Personalized nutrition approaches, including the supplementation of the diet with RPS, may help restore a variety of host health parameters, including reduction of ABS.
Acknowledgements
We thank the clinical trial participants, as well as Josh Baisley, Saif Abdulwahhab, Stephanie Recker, Ana Samborski, and Sandra Pacheco (Nutrasource, Guelph, ON), Karl Moran, Pedro Dimitriu, and Khoi Nguyen (Microbiome Insights, Vancouver, BC), and Edward Deehan (University of Nebraska-Lincoln).
Abbreviations
- ABS
Abnormal bowel symptoms
- BSC
Bristol Stool Chart
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- RPS
Resistant potato starch
- RS
Resistant starch
- SEM
Standard error of the mean
Author contributions
JRB designed the study, performed the statistical analysis, generated the tables, and wrote the draft manuscript. MJA contributed to the interpretation and conclusions of the study. Both authors read and approved the final version of the manuscript.
Funding
Funding provided by MSP Starch Products Inc., Carberry, MB, Canada.
Data availability
The data that support the findings of this study are available from MSP Starch Products Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of MSP Starch Products Inc. except for individual patient data, which are unavailable as per the interventional study protocol registration.
Declarations
Ethical approval
The study protocol and other related documents were approved by Canadian Shield Ethics Review Board (Burlington, ON) on 29 Oct 2019. This study was conducted in accordance with the protocol and with the consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable ICH Good Clinical Practice guidelines, and applicable local and federal laws and regulations.
Consent to participate
The investigator or the investigator’s representative explained the nature of the study to the participant or the participant’s legally authorized representative and answered any questions. Participants were informed that their participation was voluntary and that study reports or publications reporting on the study would not disclose the participant’s identity without specific consent. Participants wishing to participate in the study, or their legally authorized representative, signed a statement of informed consent that met the requirements of local regulations, ICH guidelines, and the research ethics board. The authorized person obtaining informed consent also signed the informed consent form and a copy was provided to the participant or the participant’s legally authorized representative. Written informed consent was obtained prior to all study-related procedures.
Consent for publication
Not applicable.
Competing interests
JRB is employed by and MJA provides consulting services for MSP Starch Products Inc., Carberry, MB, Canada. MSP Starch Products Inc. sister company McPharma Biotech holds relevant patents US11058711B2, CA3024201A1, AU2017294806A1, and provisional patent PCT/CA2021/051443 based on the work presented herein.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2016;158:1262–73. [DOI] [PubMed] [Google Scholar]
- 2.Gajula P, Quigley EM. Overlapping irritable bowel syndrome and inflammatory bowel disease. Minerva Gastroenterol Dietol. 2019;65:107–15. [DOI] [PubMed] [Google Scholar]
- 3.Chumpitazi BP, Hollister EB, Oezguen N, Tsai CM, McMeans AR, Luna RA, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynönen U, Rasinkangas P, Satokari R, Paulin L, de Vos WM, Pietilä T, et al. Isolation and whole genome sequencing of a Ruminococcus-like bacterium, associated with irritable bowel syndrome. Anaerobe. 2016;39:60–7. [DOI] [PubMed] [Google Scholar]
- 5.Kerckhoffs APM, Ben-Amor K, Samsom M, van der Rest ME, de Vogel J, Knol J, Akkermans LMA. Molecular analysis of faecal and duodenal samples reveals significantly higher prevalence and numbers of Pseudomonas aeruginosa in irritable bowel syndrome. J Med Microbiol. 2011;60:236–45. [DOI] [PubMed] [Google Scholar]
- 6.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009. 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Yuan X, Li L, Lin L, Zuo X, Cong Y, Li Y. Increased Ileal Immunoglobulin A Production and Immunoglobulin A-Coated Bacteria in Diarrhea-Predominant Irritable Bowel Syndrome. Clin Transl Gastroenterol. 2020;11:e00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyra A, Rinttilä T, Nikkilä J, Krogius-Kurikka L, Kajander K, Malinen E, et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinen E, Krogius-Kurikka L, Lyra A, Nikkilä J, Jääskeläinen A, Rinttilä T, et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saulnier D, Riehle K, Mistretta T, Diaz M, Mandal D, Raza S, Weidler E, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla R, Ghoshal U, Dhole T, Ghoshal U. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: an evidence of Dysbiosis. Dig Dis Sci. 2015;60:2953–62. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S, Liu S, Li H, Zhang Z, Zhang Q, Chen L, et al. Identification of Gut Microbiota and metabolites signature in patients with irritable bowel syndrome. Front Cell Infect Microbiol. 2019. 10.3389/fcimb.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittayanon R, Lau JT, Yuan Y, Leontiadis GE, Tse F, Surette M, Moayyedi P. Gut microbiota in patients with irritable bowel Syndrome-A systematic review. Gastroenterology. 2019;157:97–108. [DOI] [PubMed] [Google Scholar]
- 14.Nair P, Okhuysen P, Jiang Z, Carlin L, Belkind-Gerson J, Flores J, et al. Persistent abdominal symptoms in US adults after short-term stay in Mexico. J Travel Med. 2014;21:153–8. [DOI] [PubMed] [Google Scholar]
- 15.Bush J, Alfa M. Decreasing levels of Sporacetigenium correlate with improved diabetic parameters in healthy adults consuming MSPrebiotic digestion resistant starch. JARCP. 2018;7:176–80. [Google Scholar]
- 16.Bush J, Alfa M. Increasing levels of Parasutterella in the gut microbiome correlate with improving low-density lipoprotein levels in healthy adults consuming resistant potato starch during a randomised trial. BMC Nutr. 2020;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson M, Maiya M, Stewart M. Resistant starch content in Foods commonly consumed in the United States: a narrative review. J Acad Nutr Diet. 2020;120:230–44. [DOI] [PubMed] [Google Scholar]
- 18.Miketinas M, Shankar M, Maiya M, Patterson MA. Usual dietary intake of resistant starch in US adults from NHANES 2015–2016. J Nutr. 2020;150:2738–47. [DOI] [PubMed] [Google Scholar]
- 19.Neyrinck A, Nazare J-A, Rodriguez J, Jottard R, Dib S, Sothier M, et al. Development of a repertoire and a food frequency questionnaire for estimating Dietary Fiber Intake considering Prebiotics: Input from the FiberTAG Project. Nutrients. 2020;12:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush J, Baisley J, Harding S, Alfa M. Consumption of Solnul™ resistant potato starch produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients. 2023;15:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush J, Han J, Deehan E, Harding S, Maiya M, Baisley J, Schibli D, Goodlett D. Resistant potato starch supplementation reduces serum histamine levels in healthy adults with links to attenuated intestinal permeability. J Funct Foods. 2023;108:105740. [Google Scholar]
- 22.Bush J, Iwuamadi I, Han J, Schibli D, Goodlett D, Deehan E. Resistant potato starch supplementation reduces serum free fatty acid levels and influences bile acid metabolism. Metabolites. 2024;14:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eypasch E, Williams J, Wood-Dauphinee S, Ure B, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–22. [DOI] [PubMed] [Google Scholar]
- 24.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 25.Alfa M, Strang D, Tappia P, Graham M, Van Domselaar G, Forbes J, et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin Nutr. 2018;37:797–807. [DOI] [PubMed] [Google Scholar]
- 26.Deehan E, Yang C, Perez-Munoz M, Nguyen N, Cheng C, Triador L, et al. Precision Microbiome Modulation with Discrete Dietary Fiber structures directs short-chain fatty acid production. Cell Host Microbe. 2020;27:389–404. [DOI] [PubMed] [Google Scholar]
- 27.Schloss PD, Westcott SL, Ryabin T, Justine HR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 29.Federal Trade Commission. Health Products Compliance Guidance; 2022. https://www.ftc.gov/system/files/ftc_gov/pdf/Health-Products-Compliance-Guidance.pdf. Accessed 5 Feb 2024.
- 30.Venkataraman A, Sieber JJR, Schmidt A, Waldron C, Theis K, Schmidt T. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Defnoun S, Labat M, Ambrosio M, Garcia J, Patel B. Papillibacter cinnamivorans gen. nov., sp. nov., a cinnamate-transforming bacterium from a shea cake digester. Int J Syst Evol Microbiol. 2000;50:1221–8. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Shen F, Zhang J, Cai H, Pan Y, Sun T, et al. Consumption of wheat peptides improves functional constipation: a translational study in humans and mice. Mol Nutr Food Res. 2022;66:e2200313. [DOI] [PubMed] [Google Scholar]
- 33.Rajilić-Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. [DOI] [PubMed] [Google Scholar]
- 34.Palmnäs-Bédard M, Costabile G, Vetrani C, Åberg S, Hjalmarsson Y, et al. The human gut microbiota and glucose metabolism: a scoping review of key bacteria and the potential role of SCFAs. Am J Clin Nutr. 2022;116:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Yun K, Kim J, Park E, Chang Y, Ryu S, et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020;10:19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umeano L, Iftikhar S, Alhaddad S, Paulsingh C, Riaz M, Garg G, Mohammed L. Effectiveness of Probiotic Use in alleviating symptoms of irritable bowel syndrome: a systematic review. Cureus. 2024;16:e58306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Li Y, Zheng Q, Li L. The efficacy of Probiotics, Prebiotics, Synbiotics, and fecal microbiota transplantation in irritable bowel syndrome: a systematic review and network Meta-analysis. Nutrients. 2024;16:2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. 2019. Cell Host Microbe.25(6):789–802. https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(19)30250-1?returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1931312819302501%3Fshowall%3Dtrue [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from MSP Starch Products Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of MSP Starch Products Inc. except for individual patient data, which are unavailable as per the interventional study protocol registration.