Abstract
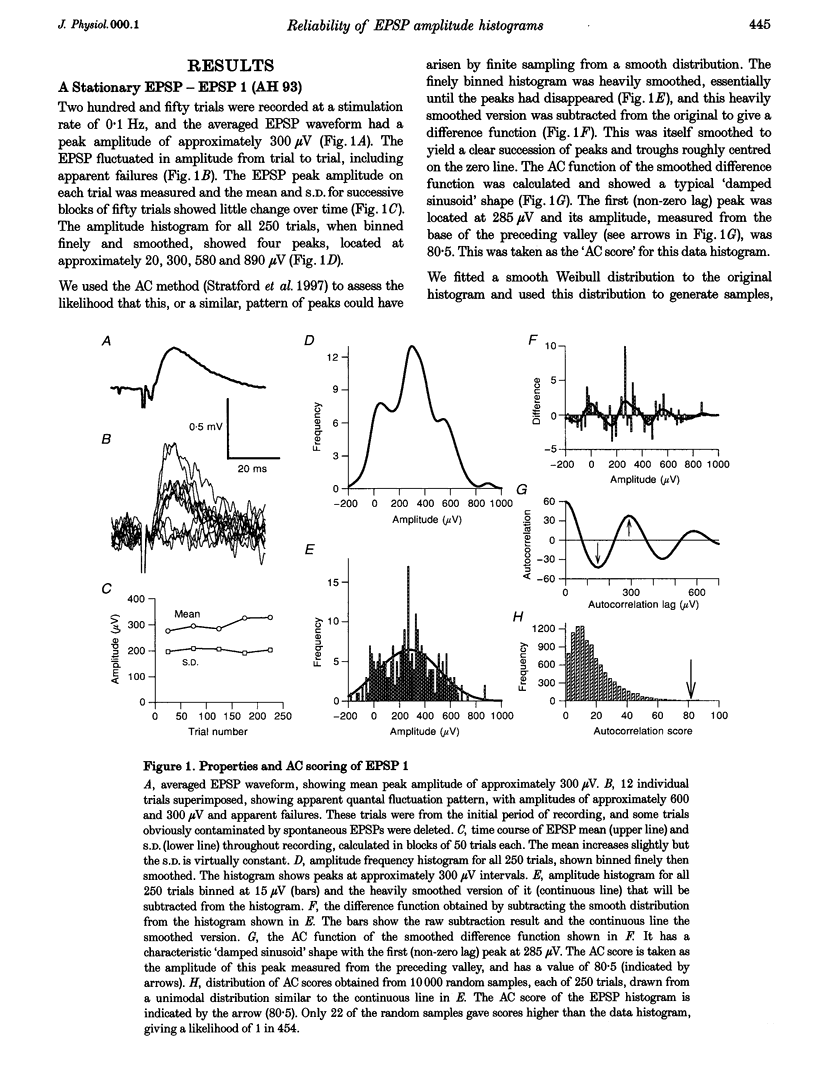
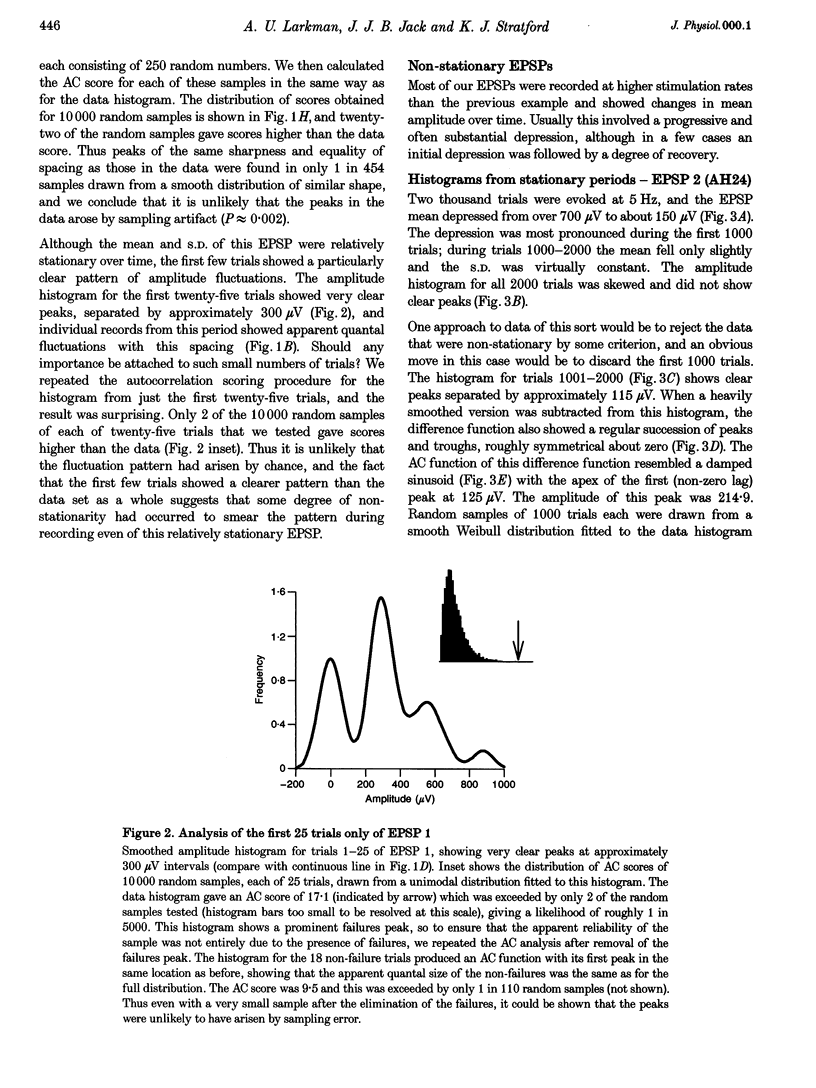
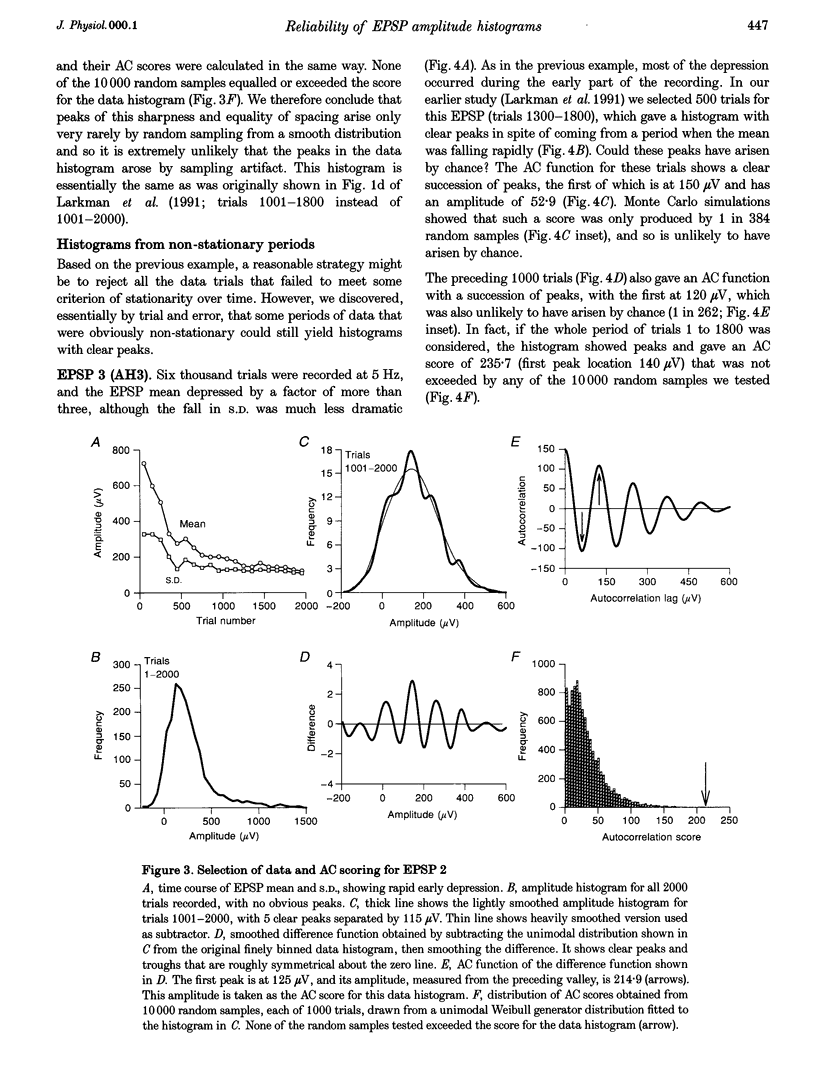
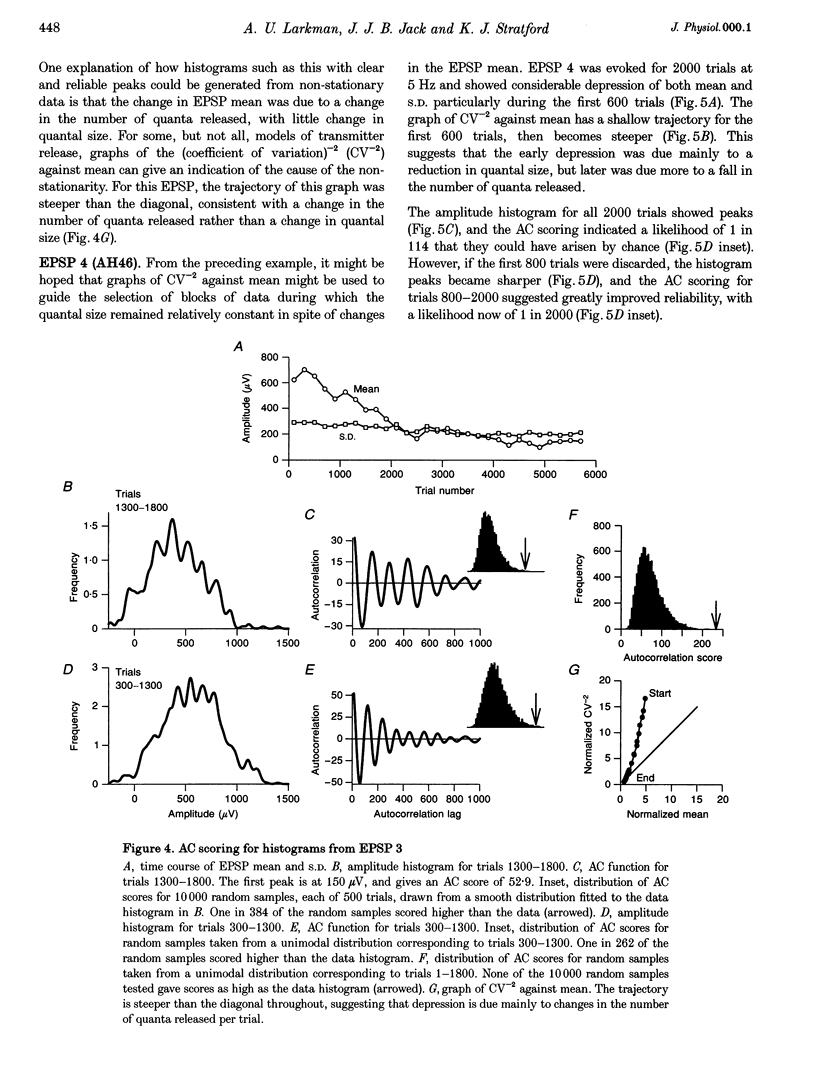
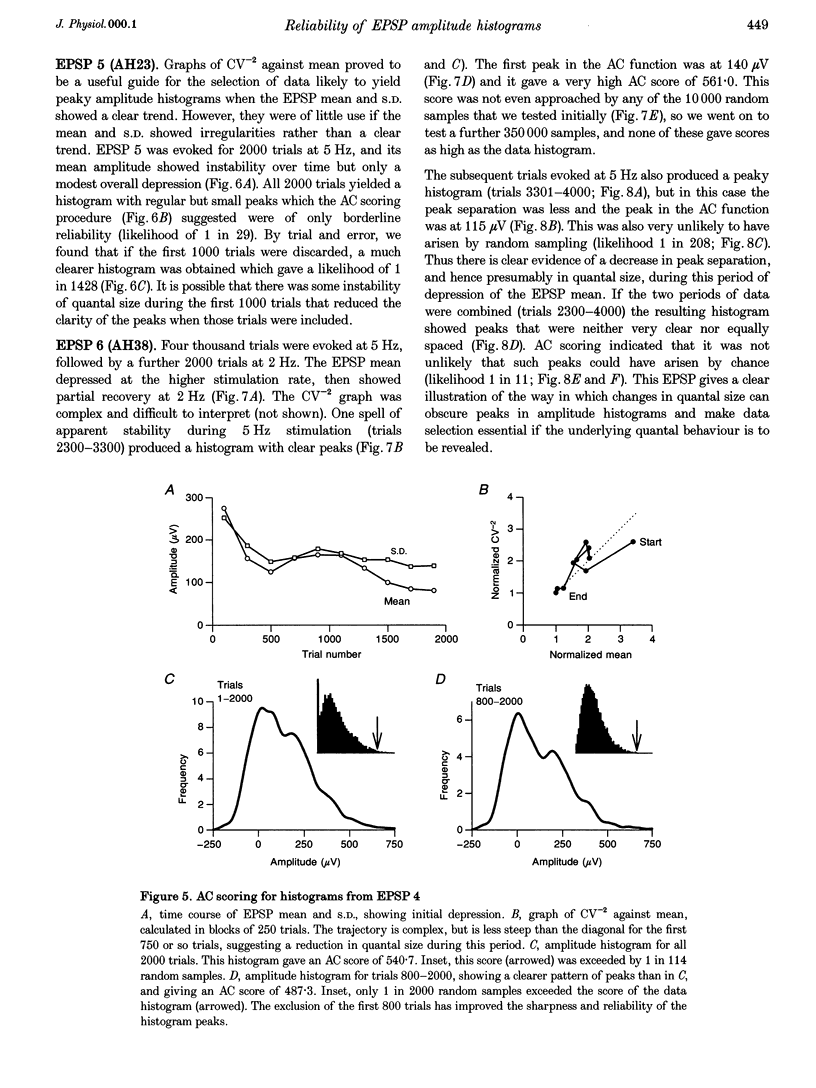
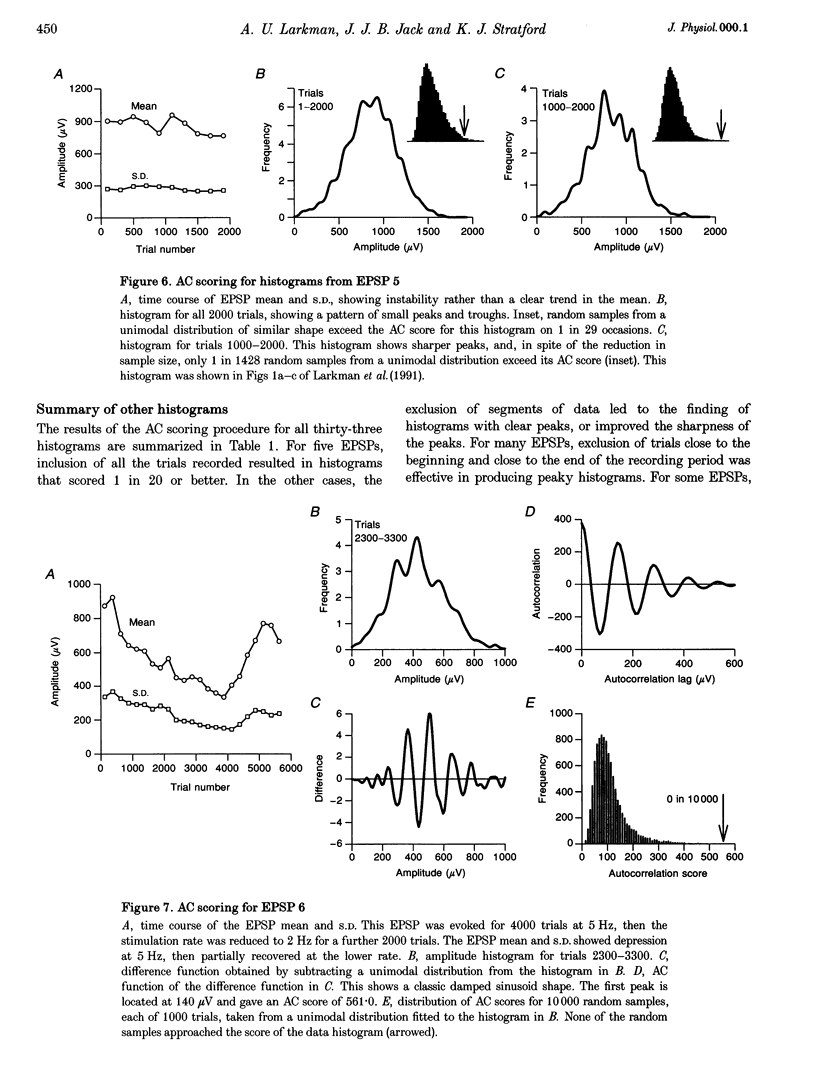
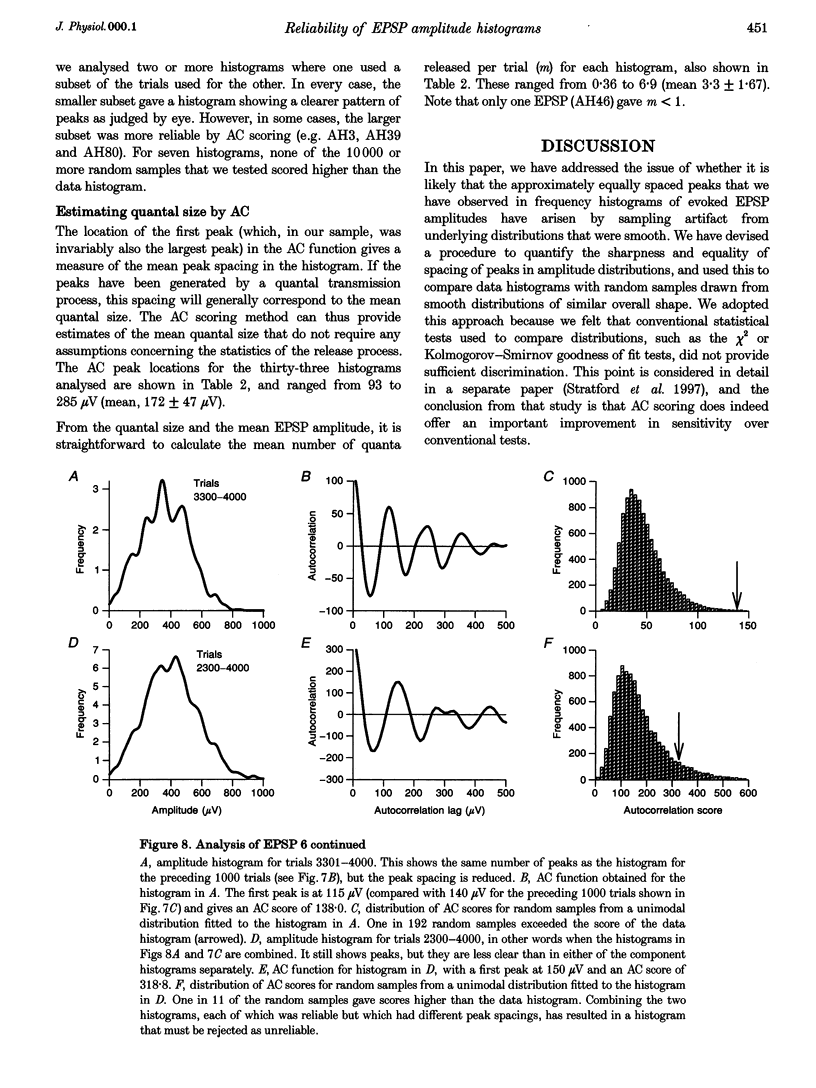
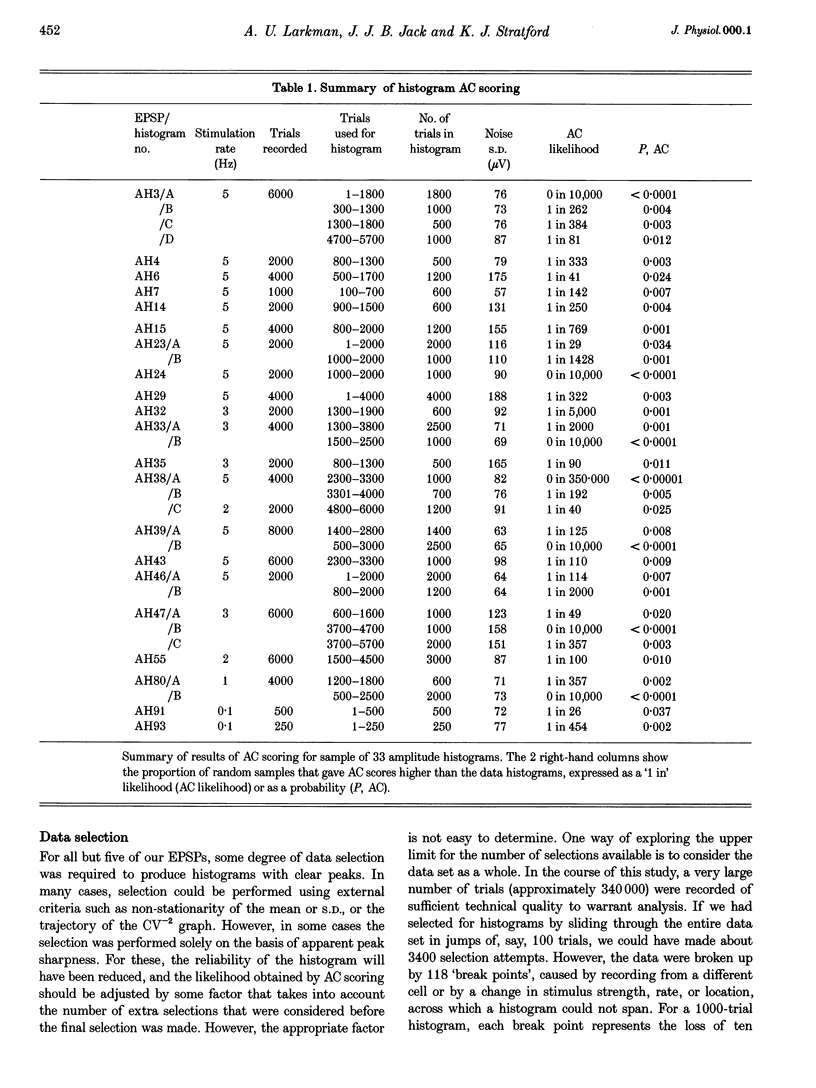
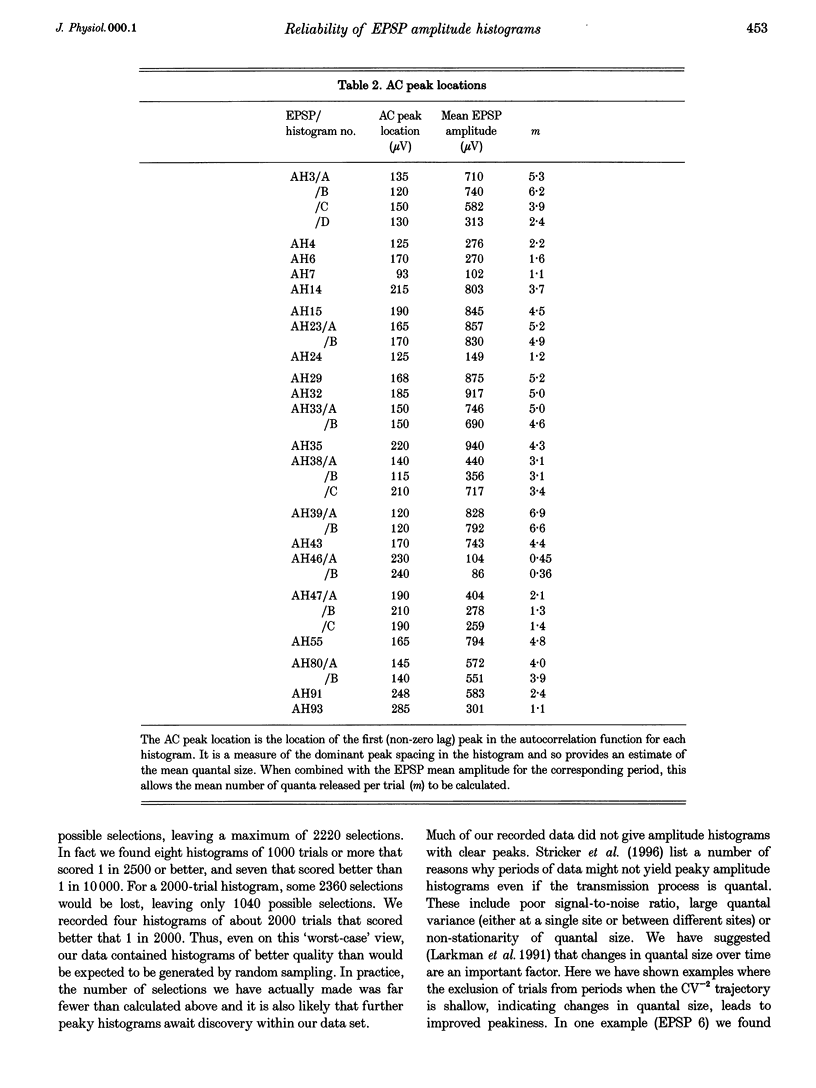
1. Excitatory postsynaptic potentials (EPSPs) were evoked using minimal extracellular stimulation and recorded from pyramidal cells from the CA1 region of slices taken from adult rats and maintained in vitro. 2. Segments of data were selected that gave EPSP amplitude frequency histograms that showed approximately equally spaced peaks. Selection was performed either on the basis of stationarity of the EPSP mean and standard deviation, or on the trajectory of a graph of the (coefficient of variation)-2 against mean for the EPSP, or, in some cases, by trial and error. 3. For each histogram, we determined the likelihood that peaks of similar sharpness and equality of spacing could have arisen by sampling artifact from a smooth distribution, using a method based on autocorrelation and Monte Carlo simulation. 4. Thirty-three histograms were analysed. For twenty-six of these, the likelihood of sampling artifact was estimated at 1 in 100 or less, and for eleven histograms the likelihood was less than 1 in 1000. For the histogram with the clearest peaks, the likelihood was less than 1 in 350,000. Histograms judged to be reliable by this method could occur when the EPSP mean amplitude was changing. 5. We conclude that random sampling artifact is very unlikely to be the explanation for the peaks in our data histograms. It seems more likely that they are due to a quantal synaptic transmission mechanism with low quantal variability. 6. The autocorrelation method also gives a measure of the mean peak spacing, and hence the mean quantal size, for each histogram. Quantal sizes ranged from 93 to 285 microV, with a mean +/- S.D. of 172 +/- 47 microV. 7. From these quantal sizes and the EPSP mean amplitudes we calculated the mean number of quanta released per trial for each histogram. This ranged from 0.36 to 6.9, with a mean of 3.3 +/- 1.67.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Stevens C. F. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J Neurophysiol. 1995 Mar;73(3):1145–1156. doi: 10.1152/jn.1995.73.3.1145. [DOI] [PubMed] [Google Scholar]
- Bolshakov V. Y., Siegelbaum S. A. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995 Sep 22;269(5231):1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Clements J. D. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996 May;19(5):163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Clements J. Quantal synaptic transmission? Nature. 1991 Oct 3;353(6343):396–396. doi: 10.1038/353396a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Frerking M., Wilson M. Saturation of postsynaptic receptors at central synapses? Curr Opin Neurobiol. 1996 Jun;6(3):395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- Goda Y., Stevens C. F. Two components of transmitter release at a central synapse. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995 Oct;15(4):875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Kullmann D. M., Larkman A. U., Major G., Stratford K. J. Quantal analysis of excitatory synaptic mechanisms in the mammalian central nervous system. Cold Spring Harb Symp Quant Biol. 1990;55:57–67. doi: 10.1101/sqb.1990.055.01.008. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Larkman A. U., Major G., Stratford K. J. Quantal analysis of the synaptic excitation of CA1 hippocampal pyramidal cells. Adv Second Messenger Phosphoprotein Res. 1994;29:275–299. doi: 10.1016/s1040-7952(06)80021-2. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992 Sep;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M. Quantal variability of excitatory transmission in the hippocampus: implications for the opening probability of fast glutamate-gated channels. Proc Biol Sci. 1993 Jul 22;253(1336):107–116. doi: 10.1098/rspb.1993.0088. [DOI] [PubMed] [Google Scholar]
- Larkman A. U., Jack J. J., Stratford K. J. Quantal analysis of excitatory synapses in rat hippocampal CA1 in vitro during low-frequency depression. J Physiol. 1997 Dec 1;505(Pt 2):457–471. doi: 10.1111/j.1469-7793.1997.457bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Liu G., Tsien R. W. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995 Jun 1;375(6530):404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Malenka R. C., Nicoll R. A. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996 Mar 1;271(5253):1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- Raastad Morten, Storm Johan F., Andersen Per. Putative Single Quantum and Single Fibre Excitatory Postsynaptic Currents Show Similar Amplitude Range and Variability in Rat Hippocampal Slices. Eur J Neurosci. 1992 Oct;4(1):113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994 Oct 20;371(6499):704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Stratford K. J., Jack J. J., Larkman A. U. Calibration of an autocorrelation-based method for determining amplitude histogram reliability and quantal size. J Physiol. 1997 Dec 1;505(Pt 2):425–442. doi: 10.1111/j.1469-7793.1997.425bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford K. J., Tarczy-Hornoch K., Martin K. A., Bannister N. J., Jack J. J. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996 Jul 18;382(6588):258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Stricker C., Field A. C., Redman S. J. Statistical analysis of amplitude fluctuations in EPSCs evoked in rat CA1 pyramidal neurones in vitro. J Physiol. 1996 Jan 15;490(Pt 2):419–441. doi: 10.1113/jphysiol.1996.sp021155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker C., Redman S., Daley D. Statistical analysis of synaptic transmission: model discrimination and confidence limits. Biophys J. 1994 Aug;67(2):532–547. doi: 10.1016/S0006-3495(94)80513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Margulis M., Shi Q. Y., Fielding A. Saturation of postsynaptic glutamate receptors after quantal release of transmitter. Neuron. 1994 Dec;13(6):1385–1393. doi: 10.1016/0896-6273(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Volgushev M., Voronin L. L., Chistiakova M., Artola A., Singer W. All-or-none excitatory postsynaptic potentials in the rat visual cortex. Eur J Neurosci. 1995 Aug 1;7(8):1751–1760. doi: 10.1111/j.1460-9568.1995.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Voronin L. L., Kuhnt U., Hess G., Gusev A. G., Roschin V. Quantal parameters of "minimal" excitatory postsynaptic potentials in guinea pig hippocampal slices: binomial approach. Exp Brain Res. 1992;89(2):248–264. doi: 10.1007/BF00228242. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Edwards F. R., Tracey D. J. Nonuniform release probabilities underlie quantal synaptic transmission at a mammalian excitatory central synapse. J Neurophysiol. 1988 Sep;60(3):889–908. doi: 10.1152/jn.1988.60.3.889. [DOI] [PubMed] [Google Scholar]


