Highlights
-
•
Methionine restriction promotes the therapeutic effect of PD-L1/PD-1 blockades in gastric cancer.
-
•
Methionine-restricted gastric carcinoma cells exhibited a suppression of macrophage M2 polarization and enhancement of M1 polarization by repressing MIF secretion.
Keywords: Methionine restriction, MIF, Gastric cancer, PD-L1/PD-1 blocker, Macrophage polarization
Abstract
Background
The limited curative effect of PD-L1/PD-1 blockades presents challenges to immunotherapy for advanced gastric cancer. We have found that methionine restriction (MR) enhances the drug resistance of gastric carcinoma cells. We aimed to explore whether MR can enhance the efficacy of PD-L1/PD-1 blockades in gastric cancer.
Methods
To conduct MR, gastric carcinoma cells were transfected with LV-METase in vitro, and 615 mice were injected with MFC cells with stable METase expression in vivo. Flow cytometry was conducted to measure the proportions of M1/M2 macrophages and CD8+ GZMB+/IFN-γ+ T cells. Additionally, the levels of M1/M2 macrophage markers and MIF were also detected.
Results
MR increased M1 and down-regulated M2 macrophages. MR suppressed MIF levels in gastric carcinoma cells, while the addition of anti-MIF neutralizing antibody inhibited the effect of MR on macrophage M1/M2 polarization. MR enhanced the increase of the proportion of CD8+ GZMB+ T cells and CD8+ IFN-γ+ T cells induced by PD-L1/PD-1 blockades. In vivo detection verified the efficacy of the combination of MR and PD-L1/PD-1 blockades on gastric cancer.
Conclusions
MR inhibits the secretion of MIF by gastric carcinoma cells, promotes macrophage M1 polarization, and enhances the therapeutic effect of PD-L1/PD-1 blockades in gastric cancer.
Introduction
Gastric cancer is a prevalent form of malignant tumor that arises persistently in the digestive tract [1]. In recent years, immunotherapy has presented a novel avenue for the treatment of advanced gastric cancer [2]. A multitude of clinical trials have demonstrated that programmed death ligand-1 (PD-L1) represents a perfect target for immune therapy [3]. The Phase II clinical study showed that pembrolizumab (anti-PD-1 antibody) exhibits favorable antitumor activity in individuals with gastric cancer and has been approved for patients with advanced PD-L1-positive gastric cancer [4,5]. However, the blockade of programmed cell death protein-1 (PD-1)/PD-L1 alone has also been demonstrated to be inadequate in numerous clinical studies, while combination with other methods has the potential to enhance efficacy [6].
The amino acid methionine is indispensable and must be obtained through dietary sources. The accelerated proliferation of cancer cells causes their increased consumption of nutrients, which becomes their Achilles heel [7]. MR has been shown to be a cancer intervention measure [8]. Our previous research showed that MR controls chemosensitivity in gastric carcinoma cells [9]. Additionally, MR also enhances anti-tumor immunity and works synergistically with PD-1 blocking to control tumors [10]. However, in gastric cancer, it is unclear whether MR can achieve a synergistic therapeutic effect with PD-L1/PD-1 blockades.
Cancer occurs in a tight link to the tumor microenvironment. Macrophages represent the most dominant components of the tumor microenvironment, which are classified into classically activated M1 macrophages and substitutively activated M2 macrophages. M1 macrophages produce pro-inflammatory cytokines to play a host defense function [11]; M2 macrophages secrete anti-inflammatory cytokines to facilitate tissue regeneration, immunosuppression [12], and tumor immune escape and progression [13,14]. Following co-culture with gastric carcinoma cells, the levels of M1-related genes are down-regulated, while those of M2-related genes are up-regulated [15]. Bioinformatics analysis has shown that M1 macrophages are necessary for anti-PD-L1/PD-1 therapy in gastric cancer [16]. This suggests that combining M1 macrophages with anti-PD-L1/PD-1 immunotherapy may provide superior therapeutic efficacy compared to single-agent anti-tumor strategies. Macrophage migration inhibitory factor (MIF) is essential for the polarization of macrophages towards M2 [17]. Additionally, elevated concentrations of MIF were observed in gastric cancer tissues [18]. Reducing the secretion of MIF has been demonstrated to impair the M2 phenotype and stimulate the M1 polarization of macrophages [17]. It can be thus surmised that MR may inhibit the secretion of MIF by gastric cancer cells, promote the M1 polarization of macrophages, and inhibit the M2 polarization, thereby inhibiting the immune escape of gastric cancer.
Moreover, preliminary experiments showed that MR markedly suppressed the secretion of MIF in gastric carcinoma cells. Given the pivotal role of M1 macrophages in anti-PD-L1 /PD-1 therapy [16], we postulated that MR treatment of gastric carcinoma cells may facilitate the polarization of macrophages towards M1 by inhibiting the secretion of MIF, thereby enhancing the immunotherapy efficacy of PD-L1/PD-1 blockades.
Materials and methods
Cell treatment
The human gastric carcinoma cells, MKN45 and AGS, and the human monocyte THP-1 were obtained from Procell (Wuhan, China). To induce methionine restriction (MR), lentivirus methioninase (LV-METase) or control (LV-NC) vectors were transfected into gastric carcinoma cells. Briefly, the L-methioninase gene (GenBank: L43133.1) carrying HA-tag at the 5′ end was cloned into the lentiviral vector pLVX-Puro. The lentiviral overexpression vector of METase (LV-METase) was constructed together with pSPAX2 and pMD2.G plasmids and then transfected into 293T cells. Following concentration and purification, the venom was used to infect MKN45 and AGS cells [19]. The transfection efficacy of LV-METase was measured using HA antibody via western blot. THP-1 cells (Procell, Wuhan, China) were processed with phorbol‑12‑myristate‑13‑acetate for 24 h to induce differentiation into M0 macrophages [20]. After transfection, cells were incubated for 24 h with (or without) 1 μg/mL of anti-MIF neutralizing mAb (anti-MIF) or isotype control mAb (IgG) [21], or 100 ng/mL of recombinant human MIF (rhMIF) [22], followed by a 48-hour co-culture with M0 macrophages using Transwell device. In this case, gastric carcinoma cells were in the upper chamber, while M0 macrophages were in the bottom [15]. Subsequently, the macrophages were collected for further analysis.
Quantitative real-time PCR (qRT-PCR)
The macrophages and gastric carcinoma cells were made into suspension and added Beyozol (Shanghai, China). After five minutes, the samples were fully lyzed and subsequently treated with chloroform and isopropyl alcohol. The precipitation resulting from centrifugation was identified as the target RNA. Therewith, the RT-PCR reaction mixture (Takara, Japan) was prepared on ice and transferred to the PCR device (Thermo Fisher, USA). The content of CD86, TNF-ɑ, IL-12B, CD206, MIF, CD204, and Arg-1 were measured through the 2–ΔΔCt method. The sequences of the primers employed are presented in Table 1.
Table 1.
The primer sequences.
| Gene name | Primer sequence | Accession |
|---|---|---|
| Homo CD86 | Forward: 5′-TTCCTGCTCTCTGCTAACTTCAG-3′ | NM_001206924.2 |
| Reverse: 5′-TCCCTCTCCATTGTGTTGGT-3′ | ||
| Homo TNF-ɑ | Forward: 5′-GACAAGCCTGTAGCCCATGT-3′ | NM_000594.4 |
| Reverse: 5′-GGAGGTTGACCTTGGTCTGG-3′ | ||
| Homo IL-12B | Forward: 5′-ATGCCCCTGGAGAAATGGTG-3′ | NM_002187.3 |
| Reverse: 5′-GCTGAGGTCTTGTCCGTGAA-3′ | ||
| Homo CD206 | Forward: 5′-GCCTCGTTGTTTTGCGTCTT-3′ | NM_002438.4 |
| Reverse: 5′-GAGAACAGCACCCGGAATGA-3′ | ||
| Homo CD204 | Forward: 5′-AGACGTTGGGGAGATGAGGA-3′ | NM_001363744.1 |
| Reverse: 5′-CTTCAGGAGTTGAGCTGCCA-3′ | ||
| Homo Arg-1 | Forward:5′-ACTTAAAGAACAAGAGTGTGATGTG-3′ | NM_000045.4 |
| Reverse: 5′-GTCCACGTCTCTCAAGCCAA-3′ | ||
| Homo MIF | Forward: 5′-GTGGTGTCCGAGAAGTCAGG-3′ | NM_002415.2 |
| Reverse: 5′-TTGCTGTAGGAGCGGTTCTG-3′ | ||
| Homo β-actin | Forward: 5′-AGAAGGATTCCTATGTGGGCGAC-3′ | NM_001101.5 |
| Reverse: 5′-AGTACTTGCGCTCAGGAGGA-3′ | ||
| Mus MIF | Forward: 5′-TTCCACCTTCGCTTGAGTCC-3′ | NM_010798.3 |
| Reverse: 5′-GCATCGCTACCGGTGGATAA-3′ | ||
| Mus β-actin | Forward: 5′-GCAGGAGTACGATGAGTCCG-3′ | NM_007393.5 |
| Reverse: 5′-ACGCAGCTCAGTAACAGTCC-3′ |
Isolation of CD8+ T cells
CD8+ T cells were isolated using the Stemcell technology Human CD8+ T Cell Isolation Kit. Peripheral blood mononuclear cells (PBMCs) were obtained from whole-blood samples of healthy donors using the Ficoll gradient method. Subsequently, the PBMCs were combined with the Isolation Cocktail in a polystyrene round-bottom tube and incubated for five minutes at room temperature. RapidSpheres™ was added to samples before putting them into the magnet for 3 times. In the final stage, the magnet was picked up and the enriched cells were removed for transfer to new tubes [23].
CD8+ T cells, M0 macrophages, and gastric carcinoma cells transfected with (or without) LV-METase or LV-NC were co-cultured in a C10 medium in a 4:1:1 ratio [16,24]. The 10 μg/mL of anti-PD-1 (PD-L1/PD-1 blockades) or control (IgG) was added to the mixture [25]. After co-culture for 48 h, the co-culture supernatant and CD8+ T cells were collected respectively for flow cytometry and ELISA analysis.
Flow cytometry
The fraction of M1/M2 macrophages, CD8+ T cells, CD8+ GZMB+ T cells, and CD8+ IFN-γ+ T cells, were assessed by flow cytometry. To determine the percentage of M1/M2 macrophages, macrophages were stained with FITC-CD86 and PE-CD206 antibodies (BD Biosciences) avoiding light at 4 °C. For the tumor samples, a single-cell suspension was prepared and F4/80+ cells were isolated using Dynabeads F4/80 (Thermo Fisher). After that, the cells were stained with FITC-CD86 or PE-CD206 antibodies (BD Biosciences). To evaluate the content of CD8+ T cells within the tumor tissues, the samples were made into single-cell suspension and CD45+ cells were isolated using Dynabeads CD45 (Invitrogen). Subsequently, the cells were stained with an anti-CD8 antibody. To evaluate the content of CD8+ GZMB+ T cells and CD8+ IFN-γ+ T cells, the CD8+ T cells were stained with an anti-GZMB antibody or anti-IFN-γ antibody. The labeled cells were detected using a flow cytometer (BD Biosciences, USA).
Immunohistochemical staining
The tumor tissues were obtained and made into frozen slices. After antigen repair, the sections were placed in a 0.3 % hydrogen peroxide solution for 20 min. Slices were then treated with Triton-100 and 5 % BSA, followed by incubation with the anti-CD86 antibody (#SAB5701086, 1:80, Merck), anti-CD206 antibody (#ZRB1440, Merck), or anti-CD8a Antibody (#MABF1983M, Merck). Subsequently, slices were incubated with the secondary antibody (#ab207995 or #ab207997, 1:1000). The prepared avidin-biotin complex reagent and 3, 3′-Diaminobenzidine reagent were then added. Following hematoxylin counterstaining, the sections were observed under a microscope and photographed [26].
Western blot
Cell samples MKN45, MFC, and AGS were harvested and homogenized in RIPA Lysis Buffer supplemented with protease inhibitors. The lysed-cell extracts were processed by centrifugation and the supernatant was collected. The concentration was measured after left for 15 min in the boiling water (Beyotime, CHN). After that, SDS-PAGE and membrane transfer were conducted. The PVDF membrane was trimmed to correspond with the expected molecular weight, after which the blocking buffer (Epizyme, CHN) was added. Subsequently, the membrane was incubated with an anti-MIF antibody (#PA5–27,343, 1:800, Thermo Fisher), anti-HA antibody (ab182009, 1:2000, Abcam), or anti-β-actin antibody (#4967, 1:1000, Cell Signaling Technology) diluted to the appropriate concentration under 4 °C conditions. After overnight, the membranes were incubated with the secondary antibody for 2 h The ECL reagents (Thermo Fisher Scientific, USA) were prepared and incubated with the PVDF membrane for 5 min. The surplus color-developing liquid was then removed with filter paper and the gel imager was used to capture an image.
Enzyme-linked immunosorbent assay (ELISA)
The cells were subjected to centrifugation at 500 g and the resulting supernatant was collected. The specific operational procedure was conducted in accordance with the relevant instructions. The following kits were employed: the Human GzmB ELISA Kit (Sangon Biotech, China), IFN-γ Human ELISA Kit (Thermo Fisher, USA), Human MIF ELISA Kit (Beyotime, China), and Mouse MIF ELISA Kit (Thermo Fisher, USA).
Animal experiment
Mice of the 615 strain, with a body weight between 18 and 22 g, were readily available from the laboratory of Nanchang University. Permission for all experimental procedures carried out was obtained from the Ethics Committee on Animal Use in Research of Nanchang University's Second Affiliated Hospital. MFC cells that had been transfected with LV-METase or LV-NC were injected into 615 mice via the subcutaneous route at a concentration of 2.5 × 105 cells. The mice were subsequently divided into three categories after receiving the injections: LV-NC + IgG, LV-NC + anti-PD-1, and LV-METase + anti-PD-1. On day 7, the mice were administered either IgG or anti-PD-1 (10 mg/kg) via injection. The tumor tissues were collected after 28 days.
Statistical analysis
The statistics were conducted using GraphPad Software and data were presented as mean ± SD. Additionally, the discrepancy between the two groups was assessed by Student's t-test, and the difference among multiple groups was assessed by one-way (or two-way) analysis of variance. The data was deemed statistically meaningful when P < 0.05.
Results
MR promotes the M1 phenotype in macrophages and inhibits the M2 phenotype
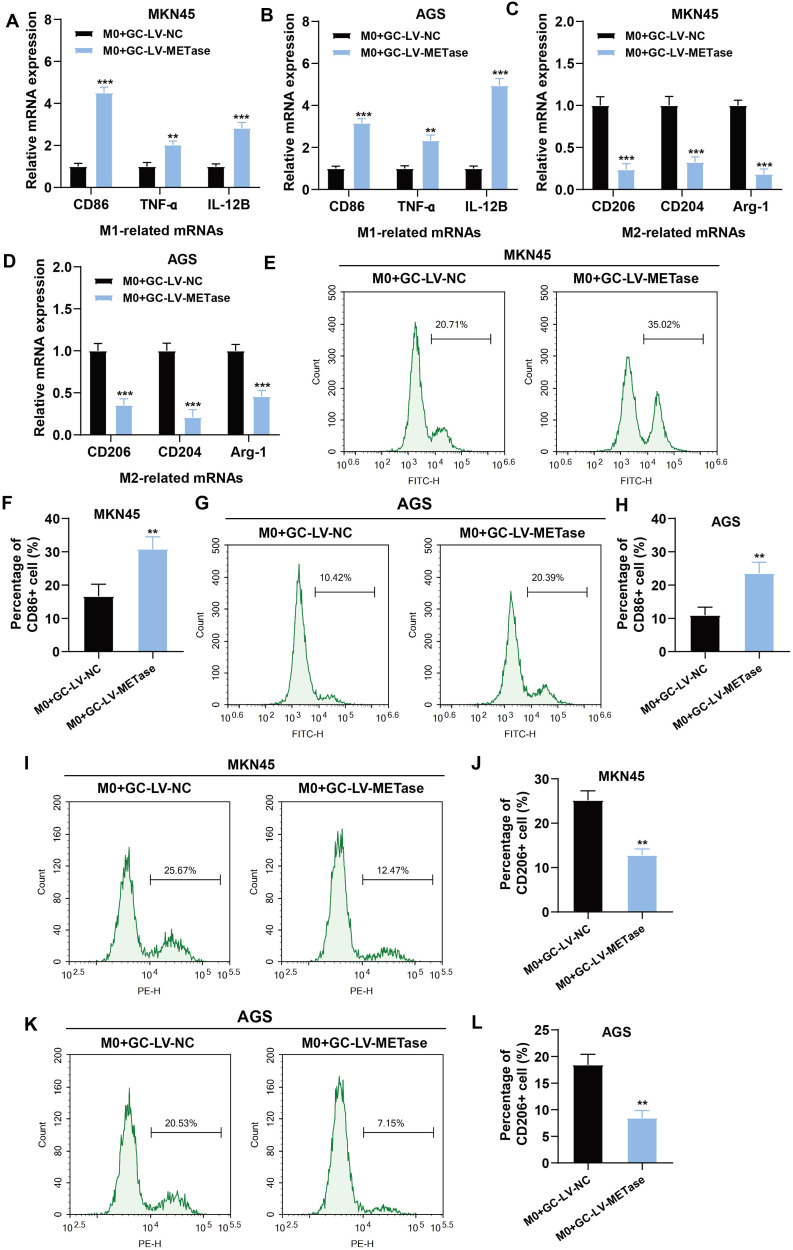
To investigate the effect of MR on macrophage polarization, gastric carcinoma cells transfected with LV-METase were co-cultured with M0 macrophages. Results showed that in the M0 + GC-LV-METase group, the content of M1 macrophage markers (Figs. 1A-B) along with the levels of CD86+ macrophages (Figs. 1E-H) were dramatically elevated, whereas the content of M2 macrophage markers (Figs. 1C-D) and CD206+ macrophages (Figs. 1I-L) were markedly reduced. Data suggested that MR enhanced the differentiation of macrophages into M1 while suppressing their differentiation into M2.
Fig. 1.
Effects of methionine restriction on M1 polarization and M2 polarization of macrophages. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were co-cultured with M0 macrophages. N = 3. (A-B) The expression of M1 macrophage markers (CD86, TNF-ɑ, IL-12B) was measured by qRT-PCR. (C-D) The expression of M2 macrophage markers (CD206, CD204, Arg-1) was measured by qRT-PCR. The content of M1 macrophages (E-H) and M2 macrophages (I-L) was detected by flow cytometry. ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001 vs. M0 + GC-LV-NC. A student's t-test was performed.
MR inhibits the secretion of MIF by gastric carcinoma cells, and then promotes M1 polarization and inhibits M2 polarization
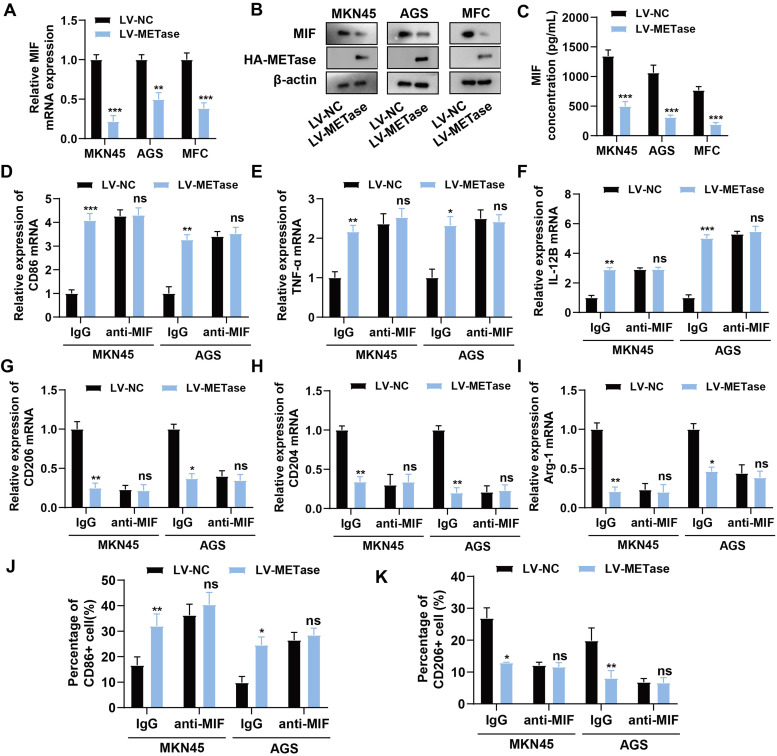
The objective of the subsequent investigation was to ascertain the impact of MR on macrophage polarization. The factor MIF operates as a crucial immunomodulatory factor that promotes cancer progression [27]. It was observed that MIF levels in gastric carcinoma cells transfected with LV-METase were distinctly reduced, while METase expression was elevated (Figs. 2A-B). It is probable that LV-METase also significantly inhibited the secretion of MIF in the supernatant of gastric carcinoma cells (Fig. 2C). After the addition of anti-MIF (1 μg/mL) to neutralize MIF, the promotion of LV-METase on M1 macrophage marker expression (Figs. 2D-F) or the proportion of CD86+ macrophages (Figs. 2J, Supplementary Figure 1A) was inhibited. Additionally, the suppression of LV-METase on M2 macrophage marker expression (Figs. 2G-I) or the content of CD206+ macrophages (Figs. 2K, Supplementary Figure 1B) was also inhibited. Results indicated that MR may affect macrophage polarization by inhibiting the secretion of MIF by gastric carcinoma cells.
Fig. 2.
Effect of methionine restriction on MIF secretion in gastric carcinoma cells. Gastric carcinoma cells (MKN45, MFC, and AGS) were transfected with LV-NC or LV-METase. N = 3. (A) The mRNA levels of MIF were detected by qRT-PCR. (B) The protein levels of MIF and METase (HA-METase) were detected by western blot. (C) The secretion of MIF in the supernatant of gastric carcinoma cells was detected by ELISA. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were added with anti-MIF or IgG, and co-cultured with M0 macrophages. (D-F) The expression of M1 macrophage markers (CD86, TNF-ɑ, IL-12B) was measured by qRT-PCR. (G-I) The expression of M2 macrophage markers (CD206, CD204, Arg-1) was measured by qRT-PCR. The content of M1 macrophages (J) and M2 macrophages (K) was detected by flow cytometry. nsP>0.05, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. A two-way analysis of variance followed by Sidak's multiple comparisons test was performed.
MR inhibits tumor formation and M2 polarization and promotes M1 polarization
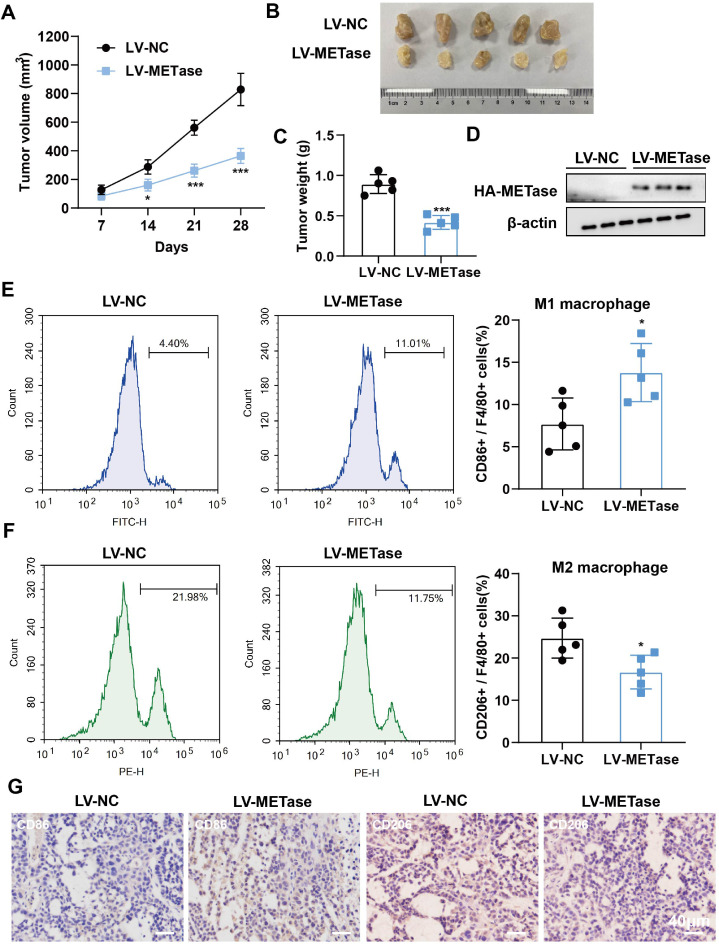
The subsequent investigation sought to ascertain the impact of MR on tumor and macrophage polarization in vivo. Tumor size was significantly reduced in LV-METase mice (Figs. 3A-C). As with its effects on cells, LV-METase promoted METase protein levels and M1 macrophage content, while inhibiting M2 macrophage content (Figs. 3D-G) in tumors. Similarly, in vitro experiments also indicated that the addition of MIF negated the pro-M1 polarization effect of MR (Supplementary Figure 2A-C, G-H) and the anti-M2 polarization effect (Supplementary Figure 2D-F, I-J).
Fig. 3.
. Effect of methionine restriction on tumor formation and M2/M1 polarization in vivo. MFC cells transfected with LV-NC or LV-METase were injected into mice. N = 5. (A-C) The volume and weight of the tumor. (D) The protein levels of METase (HA-METase) were detected by western blot. (E-F) The content of M1 and M2 macrophages was detected by flow cytometry. (G) The immunohistochemical staining of CD86 (M1 macrophage marker) and CD206 (M2 macrophage marker) was performed. Scale bar: 40 μm. *P < 0.05, ⁎⁎⁎P < 0.001 vs LV-NC. (A) A two-way analysis of variance followed by Sidak's multiple comparisons test was performed. (C/E/F) A student's t-test was performed.
MR enhances CD8+T cell toxicity induced by PD-L1/PD-1 blockades
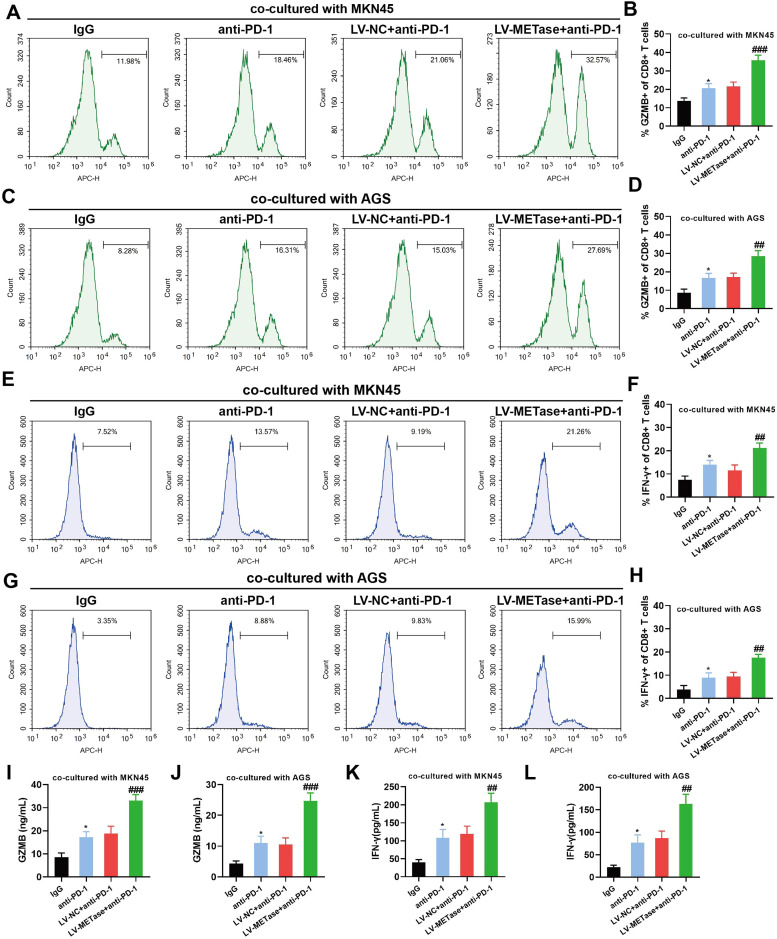
Given the limited immunotherapeutic efficacy of PD-L1/PD-1 blockades in gastric cancer, we sought to investigate the effect of MR in combination with PD-L1/PD-1 blockades on CD8+ T cell toxicity. Results showed that PD-L1/PD-1 blockades augmented the presence of CD8+ GZMB+ T cells (Figs. 4A-D) and CD8+ IFN-γ+ T cells (Figs. 4E-H), which exhibited further enhancement following transfection of LV-METase. In addition, PD-L1/PD-1 blockades enhanced the levels of GZMB (Figs. 4I-J) and IFN-γ (Figs. 4K-L) in the co-culture supernatant, and the gastric carcinoma cells restricted methionine further elevated their levels.
Fig. 4.
Effect of methionine restriction on CD8+ T cell toxicity induced by PD-L1/PD-1 blockades. CD8+ T cells were co-cultured with M0 macrophage and gastric carcinoma cells (MKN45, AGS), with (or without) the addition of anti-PD-1 (10 μg/mL). N = 3. The proportions of CD8+ GZMB+ T cells (A-D) and CD8+ IFN-γ+ T cells (E-H) were detected by flow cytometry. The levels of GZMB (I-J) and IFN-γ (K-L) were detected by ELISA. *P < 0.05 vs. IgG; ##P < 0.01, ###P < 0.001 vs. LV-NC+anti-PD-1. A one-way analysis of variance followed by Tukey's multiple comparisons test was performed.
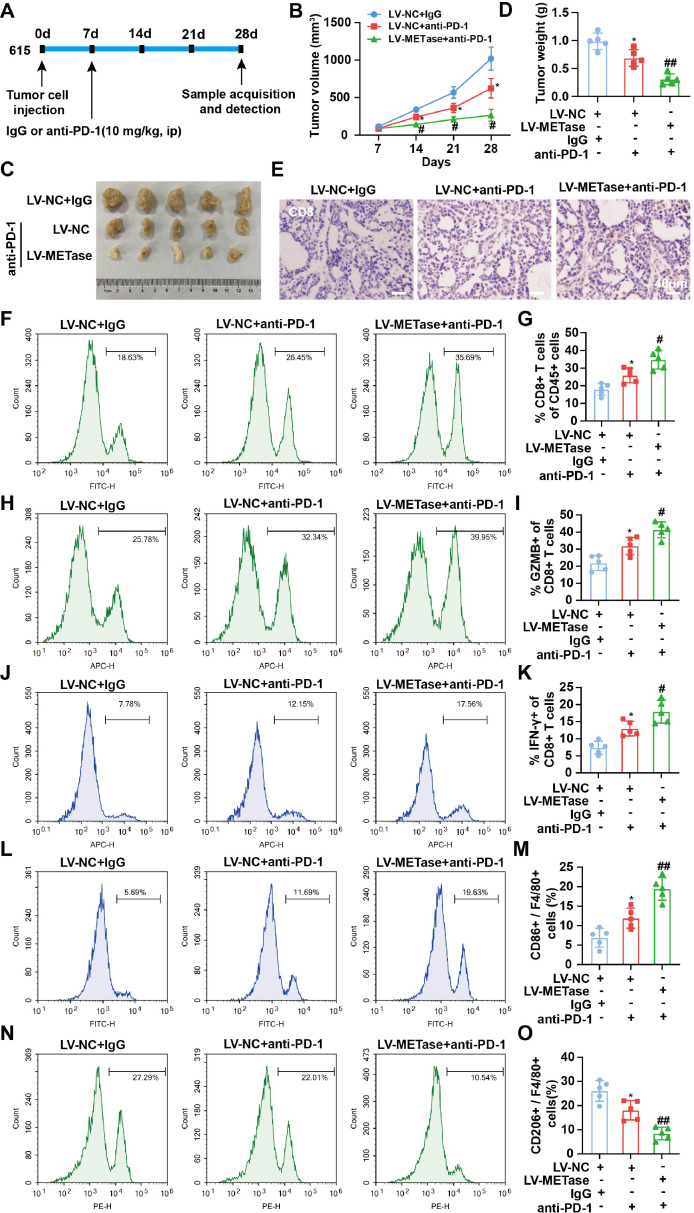
MR promotes the therapeutic effect of PD-L1/PD-1 blockades in gastric cancer
Finally, the effect of combining MR with PD-L1/PD-1 blockade on tumor and macrophage polarization in vivo was validated. Fig. 5A depicts the schedule of the mouse experiments. Furthermore, LV-METase enhanced the inhibitory effect of PD-L1/PD-1 blockades on tumor volume and weight (Figs. 5B-D). Additionally, the number of CD8+ T cells (Figs. 5E-G), CD8+ GZMB+ T cells (Figs. 5H-I), and CD8+ IFN-γ+ T cells (Figs. 5J-K) was increased as a result of PD-L1/PD-1 blockades, and this effect was enhanced after transfection of LV-METase. Moreover, the transfection of LV-METase also augmented the promotion of M1 macrophage content (Figs. 5L-M) and the inhibition of M2 macrophage content (Figs. 5N-O) by PD-L1/PD-1 blockades.
Fig. 5.
Effect of methionine restriction on the treatment of gastric cancer by PD-L1/PD-1 blockades. MFC cells transfected with LV-NC or LV-METase were injected into mice and IgG or anti-PD-1 (10 mg/kg) was injected on day 7. The tumor tissues were collected after 28 days. N = 5. (A) Diagram of the mouse experiments. (B-D) The volume and weight of the tumor. (E) The immunohistochemical staining of CD8 was performed. Scale bar: 40 μm. The proportions of CD8+ T cells (F-G), CD8+ GZMB+ T cells (H-I), CD8+ IFN-γ+ T cells (J-K), M1 macrophages (L-M), and M2 macrophages (N-O) in tumor tissues were detected by flow cytometry. *P < 0.05 vs. LV-NC + IgG; #P < 0.05, ##P < 0.01 vs. LV-NC + anti-PD-1. (B) A two-way analysis of variance followed by Sidak's multiple comparisons test was performed. (D/G/I/K/M/O) A one-way analysis of variance followed by Tukey's multiple comparisons test was performed.
Discussion
Immune therapy, which is currently being employed in the treatment of a range of solid tumors as a novel approach, has yielded promising outcomes for patients with gastric cancer. As a component of immunotherapy, checkpoint inhibition that targets the PD-1/PD-L1 axis has been demonstrated to be more efficacious and to have a lower incidence of serious adverse effects [28]. The interaction mediated by PD-L1 and PD-1 leads to the suppression of immune activity [29]. In particular, the blockade of the PD-1/PD-L1 pathway through the use of antibodies can reactivate depleted immune cells in the tumor microenvironment to destroy cancerous cells. However, in most cancers, PD-L1/PD-1 blockades are only valid in a minority of people and are ineffective in many patients, due to immune evasion and treatment resistance [6,30]. Consequently, clinical practice has prioritized the use of combined therapies with anti-PD-L1/PD-1 monoclonal antibodies over single-dose anti-PD-L1/PD-1 therapy in selected cancers. The combination of MR with PD-L1/PD-1 blockades demonstrated superior therapeutic outcomes in our study. This process may be associated with reduced MIF secretion and enhanced macrophage polarization toward M1.
A substantial body of scientific evidence has demonstrated that the amount of dietary methionine has a profound impact on the methionine metabolism of tumor cells. This tumor-specific metabolic vulnerability, which is influenced by diet intake, has emerged as a promising avenue of investigation in the field of tumor therapy [10]. Among these, MR is regarded as an efficacious method for impeding the growth and development of cancer cells by regulating their metabolism [8]. The data presented here demonstrate that MR promoted M1 polarization and suppressed M2 polarization in gastric carcinoma cells in vitro, and reduced tumor volume and weight in vivo. In addition, MR is believed to bolster anti-tumor immune responses and has been shown to have a synergistic anti-tumor effect when combined with PD-L1 inhibitors in colon cancer [[31], [32], [33]]. Similarly, our data demonstrated that MR could be synergistic with PD-L1/PD-1 blockades in the treatment of gastric cancer. This finding suggested that MR may play an immunotherapeutic role in other cancers besides colon cancer.
The term "tumor microenvironment" (TME) refers to the soil on which cellular and non-cellular elements that are closely related to tumor cells and influence tumor growth, development, and metastasis depend. TAMs typically exhibit an M2-like phenotype and contribute to tumor progression by enhancing angiogenesis, accelerating tumor cell proliferation, and enabling metastasis [34]. We found that methionine-restricted gastric carcinoma cells directly promote the polarization of M1 macrophages in vivo and in vitro. Additionally, they enhance the killing ability of CD8+ T cells induced by PD-L1/PD-1 blockades, a result that aligns with the observations made by Zhao et al [16]. They discovered that M1 macrophages binding to PD-1 blockades greatly increased the tumor-killing effect.
The MIF protein has been linked to a number of biological processes, including immune response, tumorigenesis, and inflammation. As a result, it represents an attractive pharmaceutical target for multiple conditions [27]. In liver cancer, inhibition of MIF expression has been demonstrated to impede the growth and metastasis of tumor cells [35]. Besides that, the absence of MAPK4 in gastric carcinoma cells has been observed to induce the secretion of MIF and to increase the M2 polarization [36]. In our experiments, we observed a reduction in MIF levels in gastric carcinoma cells (AGS and MKN45) following MR, accompanied by an increase in M1 polarization and a decrease in M2 polarization. Moreover, the addition of anti-MIF was observed to negate the impact of MR. Data indicated that MR may affect macrophage polarization by regulating the release and concentration of MIF.
This study is the first to demonstrate that MR promotes the efficacy of PD-L1/PD-1 blockades in gastric cancer, as evidenced by both in vitro and in vivo experiments. Additionally, we have shown that methionine-restricted gastric carcinoma cells suppressed macrophage M2 polarization and promoted M1 polarization by repressing MIF secretion. Our findings suggest a novel strategy for combining PD-L1/PD-1 blockade with other therapeutic modalities in gastric cancer.
CRediT authorship contribution statement
Lin Xin: Writing – original draft, Conceptualization. Jiang Liu: Writing – original draft, Conceptualization. Jun-Yan Lai: Formal analysis. He-Song Xu: Formal analysis. Luo-Jun Fan: Formal analysis. Yong-Hui Zou: Formal analysis. Qi Zhou: Data curation. Zhen- Qi Yue: Data curation. Jin-Heng Gan: Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding information
This work was supported by The National Natural Science Foundation of China (Nos. 82160475, 82360591) and Jiangxi Province Academic and Technical Leaders Training Program for Major Disciplines (Leading Talents Program: 20213BCJ22014).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102181.
Appendix. Supplementary materials
Supplementary Figure 1 Effect of anti-MIF on M1/M2 polarization. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were added with anti-MIF or IgG, and co-cultured with M0 macrophages. The content of M1 macrophages (A) and M2 macrophages (B) was detected by flow cytometry.
Supplementary Figure 2 Effect of MIF supplementation on M1/M2 polarization. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were added with rhMIF (100 ng/mL) and co-cultured with M0 macrophages. N = 3. (A-C) The expression of M1 macrophage markers (CD86, TNF-ɑ, IL-12B) was measured by qRT-PCR. (D-F) The expression of M2 macrophage markers (CD206, CD204, Arg-1) was measured by qRT-PCR. The content of M1 macrophages (G-H) and M2 macrophages (I-J) was detected by flow cytometry. ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; ##P < 0.01, ###P < 0.001. A two-way analysis of variance followed by Sidak's multiple comparisons test was performed.
References
- 1.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 2020;18(3):534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Q, Cao L, Guan L, Bie L, Wang S, Xie B, Chen X, Shen X, Cao F. Immunotherapy for gastric cancer: dilemmas and prospect. Brief. Funct. Genom. 2019;18(2):107–112. doi: 10.1093/bfgp/ely019. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin. Cancer Biol. 2022;86(3):566–582. doi: 10.1016/j.semcancer.2021.12.004. Pt. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5) doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer: official J. Int. Gastric Cancer Assoc. Japanese Gastric Cancer Assoc. 2019;22(4):828–837. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Golbourn BJ, Halbert ME, Halligan K, Varadharajan S, Krug B, Mbah NE, Kabir N, Stanton AJ, Locke AL, Casillo SM, et al. Loss of MAT2A compromises methionine metabolism and represents a vulnerability in H3K27M mutant glioma by modulating the epigenome. Nat. cancer. 2022;3(5):629–648. doi: 10.1038/s43018-022-00348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP, Jr., Ciccarella A, Calcagnotto A, Mikhael PG, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin L, Zhou Q, Yuan YW, Zhou LQ, Liu L, Li SH, Liu C. METase/lncRNA HULC/FoxM1 reduced cisplatin resistance in gastric cancer by suppressing autophagy. J. Cancer Res. Clin. Oncol. 2019;145(10):2507–2517. doi: 10.1007/s00432-019-03015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Tan YT, Chen YX, Zheng XJ, Wang W, Liao K, Mo HY, Lin J, Yang W, Piao HL, et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut. 2023;72(3):501–511. doi: 10.1136/gutjnl-2022-326928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Aktar N, Yueting C, Abbas M, Zafar H, Paiva-Santos AC, Zhang Q, Chen T, Ahmed M, Raza F, Zhou X. Understanding of Immune Escape Mechanisms and Advances in Cancer Immunotherapy. J. Oncol. 2022;2022 doi: 10.1155/2022/8901326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R, Kang T, Chen S. The role of tumor-associated macrophages in tumor immune evasion. J. Cancer Res. Clin. Oncol. 2024;150(5):238. doi: 10.1007/s00432-024-05777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su P, Jiang L, Zhang Y, Yu T, Kang W, Liu Y, Yu J. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 2022;22(1):290. doi: 10.1186/s12935-022-02717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao R, Wan Q, Wang Y, Wu Y, Xiao S, Li Q, Shen X, Zhuang W, Zhou Y, Xia L, et al. M1-like TAMs are required for the efficacy of PD-L1/PD-1 blockades in gastric cancer. Oncoimmunology. 2020;10(1) doi: 10.1080/2162402X.2020.1862520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Li H, Zhao S, Wang E, Zhu J, Feng D, Zhu Y, Dou W, Fan Q, Hu J, et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling. Mol. Cancer. 2021;20(1):46. doi: 10.1186/s12943-021-01343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He LJ, Xie D, Hu PJ, Liao YJ, Deng HX, Kung HF, Zhu SL. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J. Gastroenterol. 2015;21(34):9916–9926. doi: 10.3748/wjg.v21.i34.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L, Yang WF, Zhang HT, Li YF, Liu C. The mechanism study of lentiviral vector carrying methioninase enhances the sensitivity of drug-resistant gastric cancer cells to Cisplatin. Br. J. Cancer. 2018;118(9):1189–1199. doi: 10.1038/s41416-018-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM, Yu ZL, Zhao YF. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol. Rep. 2018;40(5):2558–2572. doi: 10.3892/or.2018.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen Y, Jiang G, Lau C, Yu WC, Bacher M, et al. Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int. J. Cancer. 2003;107(1):22–29. doi: 10.1002/ijc.11287. [DOI] [PubMed] [Google Scholar]
- 22.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33(47):14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 23.Qiu C, Wang J, Zhu L, Cheng X, Xia B, Jin Y, Qin R, Zhang L, Hu H, Yan J, et al. Improving the ex vivo expansion of human tumor-reactive CD8 + T cells by targeting toll-like receptors. Front. Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1027619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YC, Wang X, Yu J, Ma F, Li Z, Zhou Y, Zeng S, Ma X, Li YR, Neal A, et al. Targeting monoamine oxidase A-regulated tumor-associated macrophage polarization for cancer immunotherapy. Nat. Commun. 2021;12(1):3530. doi: 10.1038/s41467-021-23164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CY, Wang Y, Luo GY, Han F, Li YQ, Zhou ZG, Xu GL. Relationship between PD-L1 expression and CD8+ T-cell immune responses in hepatocellular carcinoma. J. Immunother. (Hagerstown, Md: 1997) 2017;40(9):323–333. doi: 10.1097/CJI.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 26.Tandukar G, Flores LC, Allen C, Bai Y, Ikeno Y. Technical development of senescenceassociated β-galactosidase staining in frozen kidney tissue of Sprague Dawley rat. Aging Pathobiol Ther. 2024;6(3):117–124. [Google Scholar]
- 27.Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage migration inhibitory factor (MIF): A multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol. Ther. 2022;233 doi: 10.1016/j.pharmthera.2021.108024. [DOI] [PubMed] [Google Scholar]
- 28.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 29.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L, Hao Y, Yu H, Gu X, Peng Q, Zhuo H, Li Y, Liu Z, Wang J, Chen Y, et al. Methionine restriction promotes cGAS activation and chromatin untethering through demethylation to enhance antitumor immunity. Cancer Cell. 2023;41(6):1118–1133. doi: 10.1016/j.ccell.2023.05.005. e1112. [DOI] [PubMed] [Google Scholar]
- 32.Morehead LC, Garg S, Wallis KF, Siegel ER, Tackett AJ, Miousse IR. Increased response to immune checkpoint inhibitors with dietary methionine restriction. bioRxiv: the Preprint Ser for Biol. 2023 doi: 10.3390/cancers15184467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Qin G, Cui T, Zhao C, Ren J, Qu X. A bimetallic nanoplatform for STING activation and CRISPR/Cas mediated depletion of the methionine transporter in cancer cells restores anti-tumor immune responses. Nat. Commun. 2023;14(1):4647. doi: 10.1038/s41467-023-40345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu GQ, Tang Z, Huang R, Qu WF, Fang Y, Yang R, Tao CY, Gao J, Wu XL, Sun HX, et al. CD36(+) cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell discovery. 2023;9(1):25. doi: 10.1038/s41421-023-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Guo D, Sun Q, Zhang L, Cui Y, Liu M, Ma X, Liu Y, Cui W, Sun L, et al. MAPK4 silencing in gastric cancer drives liver metastasis by positive feedback between cancer cells and macrophages. Exp. Mol. Med. 2023;55(2):457–469. doi: 10.1038/s12276-023-00946-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Effect of anti-MIF on M1/M2 polarization. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were added with anti-MIF or IgG, and co-cultured with M0 macrophages. The content of M1 macrophages (A) and M2 macrophages (B) was detected by flow cytometry.
Supplementary Figure 2 Effect of MIF supplementation on M1/M2 polarization. Gastric carcinoma cells (MKN45 and AGS) transfected with LV-NC or LV-METase were added with rhMIF (100 ng/mL) and co-cultured with M0 macrophages. N = 3. (A-C) The expression of M1 macrophage markers (CD86, TNF-ɑ, IL-12B) was measured by qRT-PCR. (D-F) The expression of M2 macrophage markers (CD206, CD204, Arg-1) was measured by qRT-PCR. The content of M1 macrophages (G-H) and M2 macrophages (I-J) was detected by flow cytometry. ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; ##P < 0.01, ###P < 0.001. A two-way analysis of variance followed by Sidak's multiple comparisons test was performed.