Summary
Background
International low back pain guidelines recommend providing education/advice to patients, discouraging routine imaging use, and encouraging judicious prescribing of analgesics. However, practice variation occurs and the effectiveness of implementation strategies to promote guideline-concordant care is unclear. This review aims to comprehensively evaluate the effectiveness of implementation strategies to promote guideline-concordant care for low back pain.
Methods
Five databases (including MEDLINE, Embase, CINAHL, CENTRAL and PEDro were searched from inception until 22nd August 2024. Randomised controlled trials (RCTs) that evaluated strategies to promote guideline-concordant care (providing education/advice, discouraging routine imaging use, and/or reducing analgesic use) among healthcare professionals or organisations were included. Two reviewers independently conducted screening, data extraction, and risk of bias assessments. The primary outcome was guideline-concordant care in the medium-term (>3 months but <12 months). The taxonomy recommended by the Cochrane Effective Practice and Organisation of Care (EPOC) group was used to categorise implementation strategies. Meta-analysis with a random-effects model was conducted where possible. This systematic review was prospectively registered in PROSPERO (registration number: CRD42023452969).
Findings
Twenty-seven RCTs with 32 reports were included. All strategies targeted healthcare professionals (7796 health professionals overseeing 34,890 patients with low back pain), and none targeted organisations. The most commonly used implementation strategies were educational materials (15/27) and educational meetings (14/27), although most studies (24/27) used more than one strategy (‘multifaceted strategies’). In the medium-term, compared to no implementation, implementation strategies probably reduced the use of routine imaging (number of studies [N] = 7, odds ratio [OR] = 1.26, 95% confidence interval [CI]: 1.01–1.58, I2 = 50%, moderate certainty evidence), but made no difference in reducing analgesic use (N = 4, OR = 1.05, 95% CI: 0.96–1.14, I2 = 0%, high certainty evidence). Further, implementation strategies may make no difference to improve the rate of providing education/advice (N = 3, OR = 1.83, 95% CI: 0.87–3.87, I2 = 95%, low certainty evidence), but this finding should be interpreted with caution because the sensitivity analysis showed a weak positive finding indicating unstable results that are likely to change with future research (N = 2, OR = 1.18, 95% CI: 1.04–1.35, I2 = 0%, moderate certainty evidence). No difference was found when comparing one implementation strategy to another in the medium-term.
Interpretation
Implementing guideline recommendations delivered mixed effects in promoting guideline-concordant care for low back pain management.
Funding
There was no funding source for this review.
Keywords: Low back pain, Implementation strategy, Guideline-concordant care
Research in context.
Evidence before this study
It is unclear to what extent implementation strategies can promote guideline-concordant care in low back pain. Previous systematic reviews have provided conflicting results on the effectiveness of strategies to implement recommendations from low back pain guidelines. The latest systematic review on this topic was published in 2016, synthesising studies published up to 2015.
Added value of this study
This review comprehensively evaluated the effectiveness of implementation strategies for three core guideline recommendations in the management of low back pain: providing education/advice, discouraging routine imaging use and reducing analgesic use. Twenty-seven randomized controlled trials were included, of which twenty were not featured in the most recent 2016 systematic review on this topic, and twelve were published after its 2015 search. Through a meta-analysis, our findings suggested that implementation strategies discouraged routine imaging use but did not improve the provision of patient education/advice or reduce analgesic use compared to no implementation in the medium-term (between 3 and 12 months).
Implications of all the available evidence
Implementation strategies may improve healthcare professional adherence to some guideline-concordant practices in the management of low back pain.
Introduction
Globally, low back pain is highly prevalent and a leading cause of disability.1 Clinical practice guidelines serve as important resources to improve the quality of care for low back pain by synthesising and appraising existing evidence, and providing recommendations for evidence-informed management.2, 3, 4 However, a time lag exists in the translation of evidence into practice5 and in many cases, the management of low back pain is inconsistent with guideline-concordant care.6
International guidelines, such as ones from the World Health Organization, Europe, United States of America and Australia, present similar core recommendations for managing low back pain, including providing education/advice to patients, discouraging routine imaging use, and reducing analgesic use, such as opioids.6, 7, 8, 9 Such recommendations are informed by evidence demonstrating that education/advice can provide small, short-term improvements in pain management,10 and routine imaging does not confer clinical benefits yet can prolong recovery in patients with non-specific low back pain.11,12 Further, guidelines are shifting from a focus on analgesic to non-analgesic treatments, due to the limited evidence of efficacy and risk of harm associated with many analgesics.6,13, 14, 15, 16, 17
Despite the consistency of recommendations for the management of low back pain across international guidelines, there is variation in the care provided in practice.18 Guideline implementation involves the use of strategies aimed to facilitate uptake, use or adoption of guideline-concordant care, change healthcare professionals’ behaviours and improve patient outcomes.3,19, 20, 21, 22 Previous research has shown that the use of implementation strategies by guideline developers may increase the uptake of clinical practice guidelines, compared to passive dissemination.19 However, for low back pain, current evidence to support the effectiveness of guideline implementation strategies is limited and findings from systematic reviews on this topic are inconsistent.4,23, 24, 25, 26, 27 Considering that implementation research is a rapidly evolving area, and a number of implementation studies in low back pain have been published28,29 since the last systematic review on this topic in 2016,4,24 an up-to-date systematic review with meta-analysis is warranted.
This systematic review aimed to comprehensively evaluate the effectiveness of implementation strategies for improving the uptake of one or more of three guideline recommendations for the management of low back pain: providing education/advice, discouraging routine imaging use, and/or reducing analgesic use. This review hypothesised that implementing low back pain guideline recommendations would promote guideline-concordant care among healthcare professionals and organisations.
Methods
The systematic review was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022)30 and methods proposed by the EPOC group.31,32 The results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.33 The review was prospectively registered on PROSPERO (CRD42023452969).34
Information sources and search strategy
A comprehensive literature search was performed by a reviewer (S.Z.) in the following electronic databases from inception to the 22nd of August 2024: MEDLINE (Via Ovid), Embase (Via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (Via EBSCO), Cochrane Central Register of Controlled Trials (CENTRAL) and Physiotherapy Evidence Database (PEDro). A University of Sydney librarian was consulted to develop the search strategy containing three aspects: 1) the search filter of randomised controlled trials published by the Cochrane Collaboration,35 2) a combination of index terms and phrases on low back pain developed by the Cochrane Back and Neck Group in January 2013,36 3) and a combination of index terms and phrases on guideline implementation from a Cochrane systematic review.19 The search strategy was initially constructed in the Medline database, and then modified for other databases (Appendix 1). In accordance with EPOC guidance,32 grey literature was retrieved through the OpenGrey and the Grey Literature Report. Incomplete or ongoing trials were additionally searched through the International Clinical Trials Registry Platform (ICTRP). Forward and backward citation searching was performed using the SCOPUS database.37 There were no language or publication restrictions.
Eligibility criteria
All randomised controlled trials (RCTs) were considered eligible if they used any implementation strategy to promote uptake of at least one of the three guideline recommendations of interest: providing education/advice, discouraging routine imaging use, and reducing analgesic use. Studies with implementation strategies targeting healthcare professionals or organisations within any healthcare setting were included. Studies that targeted both healthcare professionals and patients (e.g., mixed population) were eligible, while those targeting only on patients, consumers, the general public, or healthcare professional trainees/students were excluded. Studies did not need to exclusively include patients with non-specific low back pain; studies with mixed patient populations were also included if outcomes of the sub-group of patients with non-specific low back pain were reported. The comparator group of eligible RCTs used either: 1) no implementation strategy (no implementation interventions, or ‘treatment as usual’ defined by each included study), or 2) an alternative implementation strategy (including light touch, single or multifaceted implementation interventions).
Study selection
All retrieved records were imported into Covidence for screening, then duplicates were removed. Each record was screened independently and in duplicate by two of five reviewers (S.Z., A.V.L., M.L., Z.Y., and C.W.C.L.), by title and abstract, then by full text. Disagreements were resolved after discussion between reviewers.
Outcomes
The primary outcome was guideline-concordant care, measured by the rate of patients receiving education/advice, imaging requests (including total imaging requests and requests for X-ray, magnetic resonance imaging (MRI) and computed tomography (CT)), and analgesic use (measured by prescription, dispensing or consumption). Secondary outcomes were healthcare professionals’ knowledge and beliefs (measured by knowledge questionnaires or scales on attitudes and belief, e.g., Neurophysiology of Pain Questionnaire (NPQ)), patient-reported pain intensity (measured by self-reported scales, e.g., Numeric Pain Rating Scale (NPRS)), physical function (measured by self-reported scales, e.g., Oswestry Disability Index (ODI)) and adverse events (measured by proportions (%) of patients reporting an adverse event, e.g., presence of adverse events: yes/no). All data was grouped into three time-points: short-term (≤3 months), medium-term (>3 months but <12 months), and long-term (≥12 months). The medium term was the primary time point of interest. If an included study reported outcomes at multiple time points, we used the time point that was the closest to 3 months and 6 months for short- and medium-term and used the longest available time point for the long-term outcome.
Data extraction
The modified EPOC data extraction form was tailored for data extraction.31 Data were independently extracted by two of five reviewers (S.Z., A.V.L., Q.C., M.L., and C.W.C.L.). When extracting data, all reports from the same study were linked. Discussion among reviewers were used to settle any discrepancies. The following information was extracted: 1) study information: study design (e.g., parallel), first author, year of publication, country of origin, and clinical setting. 2) participants characteristics: population description, sample size, the total number of participants randomised, age, and sex. 3) implementation strategy used in the intervention and comparator group: type (grouped by a taxonomy developed by the EPOC group38 or by consensus between reviewers if the EPOC taxonomy was not applicable. Definitions of each strategy described in Supplementary Table S1), format (e.g., written, electronic or verbal), mode (e.g., internet-based, face-to-face, electronic-based or paper-based), strategy provider and recipient, and duration. 4) outcome data. In addition, data was extracted on how implementation strategies were developed based on feedback from a consumer advisory group. This was added to the protocol after registration.
Risk of bias assessment
Studies were assessed by two independent reviewers (S.Z. and Q.C.) using the Cochrane risk of bias tool.39 Six domains (sequence generation, concealment of allocation, blinded or objective assessment of main outcome(s), incomplete outcome data, selective outcome reporting and other potential sources of bias) were evaluated, with each domain's overall risk of bias being rated as either high, moderate, or low. Disagreements were solved by discussion among the two reviewers, then, if necessary, arbitration by a third, independent reviewer.
Assessment of the certainty of the evidence
The certainty of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.40 Two independent reviewers (S.Z. and Q.C.) rated the certainty of the evidence based on consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias (Appendix 2). The overall certainty of evidence was assessed for all outcomes and categorised the certainty of evidence as high, moderate, low, very low or no evidence. The assessment was performed using GRADEpro.
Statistics
Characteristics and implementation strategies of included studies were reported descriptively. For outcome data, where possible, we conducted a random-effects meta-analysis using the RevMan 5.4 software. Mantel-Haenszel (M−H) method of meta-analysis was used for dichotomous data and Inverse Variance (IV) method was used for continuous data. Heterogeneity among study findings was assessed using I2 statistic (ranging from 0 to 100%). Pooled effects were expressed of dichotomous variables as odds ratios (ORs) and 95% confidence intervals (CIs), or continuous variables as mean differences (MDs) and CIs. If the statistical heterogeneity was large (I2>50%), sensitivity analyses were used to examine the sources of heterogeneity by excluding one study at a time in the primary results at the primary time-point.41 For pain, we converted continuous pain intensity scales to a common 0 to 10 scale,42,43 and physical function scales to a 0 to 100 scale.44,45
Studies were categorised based on the guideline recommendation (providing education/advice, discouraging routine imaging use, reducing analgesic use, or a combination of recommendations if more than one was implemented), and time-points (short-term, medium-term, or long-term). For studies implementing strategies to discourage routine imaging use, subgroup analysis was conducted by the type of imaging (X-ray or MRI/CT scan).
In order to answer whether implementing guideline-concordant care was effective, implementation strategies were compared to no implementation strategy, regardless of the type of implementation strategies used. Additionally, one strategy was compared to another in order to assess the comparative effectiveness of the strategies. In this case, for studies that compared two implementation strategies (either isolated or combinations of them), the group with fewer strategies was designated as the comparator.
Ethics
No ethical approval was required as this review used publicly available data.
Role of funding source
No funding was received for this specific review.
Results
Characteristics of included studies
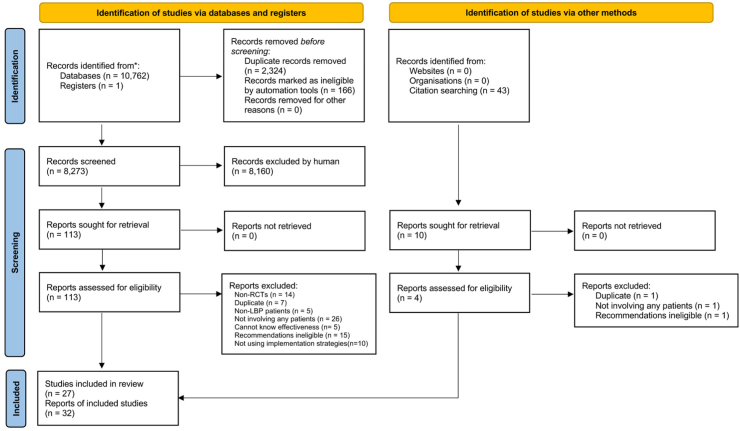
A total of 10,762 records were identified in our search with 8273 records for screening after removing the duplicates. Following screening, 27 studies (including 32 study reports) with implementation strategies targeting 7796 healthcare professionals (overseeing 34,890 patients with low back pain) were included (Fig. 1).28,29,46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 No study investigated strategies targeted at the organisational level. Studies were conducted in 10 countries, and three different settings (e.g., primary care (including community-based and hospital-based), emergency department, and chiropractic and physiotherapy clinics). Of the 27 studies, 17 studies implemented one guideline recommendation (six on providing education/advice, 46,48,54,57,62,74 and 11 on discouraging routine imaging use),28,52,55,58,60,61,63,66,68,71,73 nine implemented two29,49,50,53,56,65,67,69,72 and one implemented all three recommendations.51 Twenty-four studies29,46,48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58,60,61,63,66, 67, 68, 69,71, 72, 73, 74 implemented specific guidelines, published between 1990 and 2018, while three28,62,65 did not specify the guideline but implemented care that was concordant with the recommendations of interest. Characteristics of the included studies are described in Supplementary Table S2.
Fig. 1.
PRISMA flow chart.
Characteristics of implementation strategies
The most commonly used strategies were educational materials (15/27), educational meetings (14/27) and audit and feedback (9/27). Almost all studies (24/27) used more than one strategy (Table 1). Most (81%) of the included studies used implementation strategies delivered in a written format and 44% used verbal formats. The delivery modes of the implementation strategies in 24 studies (89%) were paper-based and in 16 (59%) were face-to-face. The recipients of the implementation strategy were general practitioners in 17 studies (63%). 56% of included studies described how the implementation strategies were developed, of these, the implementation strategies were developed with low back pain patients in two studies,50,58 and healthcare professionals or researchers in 10 studies.29,48,50,52,56, 57, 58,68,69,72 Seven studies29,50,51,55,56,58,69 reported using frameworks or models to inform strategy development. Details of the implementation strategies of included studies are presented in Supplementary Table S2.
Table 1.
Strategies used in the included studies.
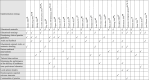 |
The definition of each strategy is provided in Supplementary Table S1.
aThe study included a strategy that cannot be classified by EPOC taxonomy, which was provision of alternatives more easily accessible to healthcare professionals.
aThe study included a strategy that cannot be classified by EPOC taxonomy, which was a facilitation-based approach aimed at optimizing clinical workflows for imaging requests.
Risk of bias and GRADE
Supplementary Figure S1 presents the risk of bias assessment. In general, studies performed well in attrition bias and selection bias, but nine studies had high, and 14 studies had unclear bias for blinding of participants and personnel. The GRADE assessment and summary of findings are shown in Supplementary Table S3. Publication bias analysis using a funnel plot was not performed due to the low number of studies included for each primary outcome analysis.
Primary outcomes on guideline-concordant care
Providing education/advice
Twelve studies29,46, 47, 48, 49,51,53,54,56,57,62,69,74 with 2204 healthcare professionals (overseeing 6971 patients and 7161 cases of consultation) were included. Studies used multifaceted (N = 11)29,46,48,49,51,53,54,56,62,69,74 or single strategies (N = 1)57 compared against no strategy (N = 7)49,51,53,54,57,62,69 or another strategy (N = 5).29,46,48,56,74
Implementation strategy versus no strategy
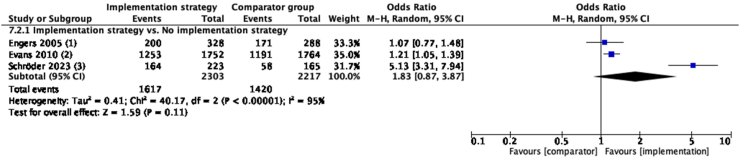
Implementation strategies focused on education (combination of educational materials and educational meetings, etc) did not improve the rate of providing education/advice in the medium-term compared to no implementation strategy (N = 3, OR = 1.83, 95% CI: 0.87–3.87, I2 = 95%, low certainty evidence) (Fig. 2, see Supplementary Table S2 for implementation strategies used by each study). In the short-term, similar results of no improvement were seen. No study investigated long-term outcomes (Supplementary Figure S2).
Fig. 2.
Guideline-concordant care on providing education/advice in medium-term, compared with no implementation strategy. Implementation strategy of included studies: (1): educational materials, educational meetings, distributing clinical practice guidelines, barrier and enabler-based tailored interventions and inter-professional education; (2): educational materials and distributing clinical practice guidelines; (3): educational materials, educational meetings, educational outreach visits, or academic detailing, communities of practice and local opinion leaders. For explanation and details of each implementation strategy, refer to Supplementary Table S2.
Implementation strategy versus alternative strategy
No study compared an implementation strategy to another strategy in the medium-term. Implementation strategy (multifaceted) did not improve the rate of providing education/advice in the short-term compared to single strategy (Supplementary Figure S2). In the long term, one study56 showed that one implementation strategy (educational meetings and barrier and enabler-based tailored interventions) improved the provision of education/advice compared to an alternate strategy (distributing clinical practice guidelines) (N = 1, OR = 3.71, 95% CI: 2.25–6.13). (Supplementary Figure S2).
Discouraging routine imaging use
Nineteen studies28,29,50, 51, 52,55,56,58,60,61,63,65, 66, 67, 68, 69,71, 72, 73 with 5202 healthcare professionals (overseeing 31,852 patients and 56,312 cases of consultation) were included. Studies used multifaceted (N = 17)29,50, 51, 52,55,56,58,60,61,65, 66, 67,69,71,73,76,77 and/or single strategies (N = 3)63,66,72 compared to no strategy (N = 13)28,50,51,58,60,61,63,65,67, 68, 69,71,73 or another strategy (N = 6).29,52,55,56,66,72
Implementation strategy versus no strategy
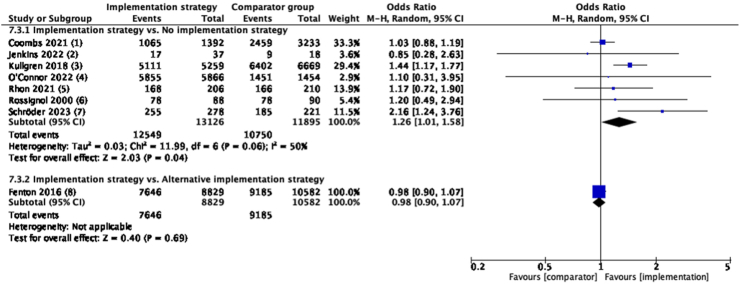
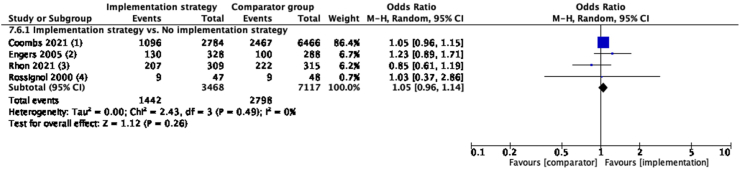
Implementation strategies (combination of educational materials and educational meetings, etc) discouraged routine imaging use from healthcare professionals compared to no strategy in the medium-term. (N = 7, OR = 1.26, 95% CI: 1.01 to 1.58, I2 = 50%, moderate certainty evidence) (Fig. 3, see Supplementary Table S2 for implementation strategies used by each study). Similar results were seen in the short-term, but not in the long-term (Supplementary Figure S3).
Fig. 3.
Guideline-concordant care on discouraging routine imaging use in medium-term, compared with no implementation strategy or another implementation strategy. Implementation strategy of included studies: (1): educational materials, educational meetings, distributing clinical practice guidelines, audit and feedback, educational outreach visits, or academic detailing and patient-mediated interventions; (2): educational materials and educational meetings; (3): educational materials and reminders; (4): educational materials, audit and feedback, reminders and monitoring the performance of the delivery of healthcare; (5): educational materials and patient-mediated interventions; (6): patient-mediated interventions (established a plan of action with the low back pain patients), tailored interventions and routine patient-reported outcome measures; (7): educational materials, educational meetings, educational outreach visits, or academic detailing, communities of practice and local opinion leaders; (8): audit and feedback and patient-mediated interventions (standardized patients). Comparator group for 7.3.2: (8) distributing clinical practice guidelines, and patient-mediated interventions (standardized patients). For explanation and details of each implementation strategy, refer to Supplementary Table S2.
Implementation strategy versus alternative strategy
No between-group difference was found when one implementation strategy (combination of audit and feedback, educational materials and educational meetings, etc) was compared to another strategy (combination of educational materials, distributing clinical practice guidelines and reminders) at any time-point (Fig. 3, and Supplementary Figure S3).
Subgroup analysis by the type of imaging
In a pre-specified subgroup analysis, implementation strategies did not reduce the routine use of X-rays (N = 3, OR = 0.97, 95% CI: 0.57–1.66, I2 = 0%, moderate certainty evidence) or MRI/CT scans (N = 4, OR = 1.03, 95% CI: 0.60–1.76, I2 = 42%, moderate certainty evidence) compared to no implementation strategies in the medium-term (Supplementary Figures S4 and S5). Results in the short- or long-term, and when one implementation strategy was compared to another were presented in Supplementary Figures S4 and S5.
Reducing analgesic use
Seven studies49, 50, 51,53,65,67,72 with a total of 161 healthcare professionals (overseeing 7972 patients and 13,875 cases of consultations) were included. Studies used multifaceted (N = 6)45, 46, 47,58,60,68 strategies or ‘reminders’ as a single strategy (N = 1)72 compared to no strategy (N = 6)49, 50, 51,53,65,67 or another strategy (N = 1).72
Implementation strategy versus no strategy
Implementation strategies (combination of educational materials and educational meetings, etc) were not effective in reducing analgesic use in medium-term (N = 4, OR = 1.05, 95% CI: 0.96–1.14, I2 = 0%, high certainty evidence) compared to no strategy (Fig. 4, see Supplementary Table S2 for implementation strategies used by each study). Similar results were seen in the short-term (Supplementary Figure S6). However, in the long-term, low certainty evidence from one study50 showed that ‘multifaceted strategies’ might reduce analgesic use compared to no strategy (N = 1, OR = 1.69, 95% CI: 1.08–2.63) (Supplementary Figure S6).
Fig. 4.
Guideline-concordant care on reducing analgesic use in medium-term, compared with no implementation strategy. Implementation strategy of included studies: (1): educational materials, educational meetings, distributing clinical practice guidelines, audit and feedback, educational outreach visits, or academic detailing and patient-mediated interventions; (2): educational materials, educational meetings, distributing clinical practice guidelines, tailored interventions and inter-professional education; (3): educational materials and patient-mediated interventions; (4): patient-mediated interventions, tailored interventions and routine patient-reported outcome measures. For explanation and details of each implementation strategy, refer to Supplementary Table S2.
Implementation strategy versus alternative strategy
No between-group difference was found in the short-term (analysis of other time points was not feasible due to insufficient data) when one strategy was compared to another strategy (Supplementary Figure S6).
Secondary outcomes
Knowledge and belief
Eight studies29,51,54,56,62,72,74,75 with 2317 healthcare professionals (overseeing 6802 patients and 861 cases of consultation) were included. Studies used multifaceted (N = 7)29,51,54,56,62,74,75 strategies or ‘reminders’ as a single strategy (N = 1)72 compared against no strategy (N = 4)50,54,62,75 or another strategy (N = 4).29,56,72,74 Three studies targeted implementation of one recommendation (providing education/advice)54,62,74 and five studies targeted implementation of two recommendations (providing education/advice and discouraging routine imaging use,29,56,75 or discouraging routine imaging use and reducing analgesic use).50,72 None of the comparisons demonstrated a between-group difference at any time point, except very low certainty evidence from one study56 showing that in the long-term, educational meetings and barrier and enabler-based tailored interventions improved knowledge and beliefs, compared to distribution of clinical practice guidelines for providing education/advice and discouraging routine imaging use (N = 1, MD = 0.4, 95% CI: 0.1–0.7, I2 = 0%, low certainty evidence) (Supplementary Figure S7).
Pain intensity and physical function
Nine studies29,46,48,50,62,65,67,69,71 involving 883 healthcare professionals (overseeing 9191 patients and 7250 cases of consultation) assessed patients’ pain intensity and/or physical function. All studies used multifaceted strategies compared against no strategy (N = 6)50,62,65,67,69,71 or another strategy (N = 3).29,46,48 Four studies targeted implementation of one recommendation (providing education/advice46,48,62 or discouraging routine imaging use,71 separately). Five studies targeted implementation of two recommendations (providing education/advice and discouraging routine imaging use,29,69 or discouraging routine imaging use and reducing analgesic use).50,65,67 No comparison found that an implementation strategy was effective, except that, compared to no strategy, multifaceted implementation strategies reduced pain intensity in the short-term when implementing all three recommendations (N = 4, MD = −0.19, 95% CI: −0.33 to −0.06, I2 = 0%, moderate certainty evidence) (Supplementary Figures S8 and S9).
Adverse events
No adverse events were reported in any of the included studies.
Sensitivity analysis
In the meta-analysis of the outcome of providing education/advice in the medium-term, high heterogeneity (I2 = 95%) was observed. Excluding Schröder et al., 202369 in the sensitivity analysis reduced heterogeneity to 0% and demonstrated that implementation strategies were effective in improving the rate of providing education/advice compared to no strategy (N = 2, OR = 1.18, 95% CI: 1.04–1.35, I2 = 0%, moderate certainty evidence) (Supplementary Figure S10), in contrast to the primary finding of no effect (Fig. 2). This discrepancy indicated that the primary finding should be interpreted with caution.
Discussion
This systematic review and meta-analysis included 27 studies investigating the effects of implementation strategies for low back pain guideline recommendations, targeting 7796 healthcare professionals overseeing 34,890 patients. Multifaceted strategies were most commonly evaluated, primarily targeting primary care professionals. Across studies, implementation strategies discouraged routine imaging use but did not improve the provision of patient education/advice or reduce analgesic use compared to no implementation strategy at the primary time point (low to moderate certainty evidence). Moreover, no evidence demonstrated that implementation strategies improved healthcare professionals' knowledge and beliefs, decreased patients’ pain, or improved function at the primary time point.
This review found that implementation strategies were not consistently effective across recommendations and time points. This may reflect the range of barriers that healthcare professionals face when attempting to implement guideline recommendations for low back pain.78 Such barriers (e.g., lack of funding support to access to guideline-recommended treatment alternatives) are multiple and varied, arising from various sources, including patients and healthcare systems.78, 79, 80 Additionally, most included studies targeted strategies at healthcare professionals only, and did not address patients or health systems, likely affecting the success of the strategies. For example, previous literature has identified that patient's demands are a key barrier to reducing routine imagine use for low back pain.81,82 Although strategies did not target patients or their behaviours, it is possible that the implementation strategies supported healthcare professionals to educate patients about the potential harms of imaging, subsequently decreasing inappropriate imaging.83 But for other recommendations like reducing analgesic use, barriers may be more complicated, including a lack of alternative treatment options to manage patient's pain and workload pressures that prevent healthcare professionals from being able to engage in comprehensive conversations about deprescribing.84 As such, even if strategies intend to change healthcare professional's behaviour, without adequate support from healthcare systems, or buy-in from patients, guideline concordant care may not be able to be enacted.80 This may explain why the effectiveness of strategies was not observed in the medium-term.
This review focused on the effectiveness of implementation strategies; one limitation was that studies using different implementation strategies (either in isolation or in combination) were lumped together, so the review is unable to answer whether a specific implementation strategy (e.g., audit and feedback) is effective compared to no implementation. The diversity, multifaceted, and combined nature of strategies makes it difficult to identify which components of the strategy may have led to the desired outcomes. Some of the pooled data exhibited high heterogeneity. For instance, the sensitivity analysis indicated that the study by Schröder et al., 202369 contributed to the heterogeneity observed in the medium-term outcome of providing education/advice. This variation may stem from differences in the intensity of interventions, as Schröder et al.69 delivered a 13-h educational workshop for healthcare professionals, while the other two studies included in this analysis delivered workshops lasting less than 2 h.53,54 Implementation strategies for specific guideline recommendations should be tailored to the needs of end-users (e.g., healthcare professionals and patients) and the contexts in which they are implemented, taking into account the interaction between the recommendation itself, implementation strategies, and healthcare settings.85, 86, 87 Additionally, we found that there was significant variability in how strategies were developed and implemented. Most strategies were developed by healthcare professionals or researchers, with no patient/public input, and were rarely informed by a theoretical model or guiding framework. Poor theoretical underpinning and insufficient consultation with key stakeholders might diminish the likelihood of implementation success.78,87,88 Some implementation frameworks identify key determinants (e.g., influential contextual barriers) that need to be considered during implementation.89 But the lack of consideration for those matching determinants during development might lead to the failure of the implementation strategy in practice.90 For example, in primary care settings, using a framework to select potential components of strategies to overcome prominent barriers (barrier-strategy matching process) may increase the uptake of the recommendation.91
This review provided some different findings compared to other previous studies. Unlike previous reviews that found implementation strategies to be effective in enhancing healthcare professionals' knowledge and beliefs,26 our study did not observe such effects. This may be due to many healthcare professionals already being familiar with guideline recommendations for low back pain,92 preventing the implementation strategies from conferring significantly greater improvements in care. This highlights that the multifaceted barriers faced by healthcare professionals hinder the promotion of guideline-consistent care, even when they possess sufficient knowledge and beliefs. Thus, future research should focus on comprehensive implementation strategies addressing patients' and healthcare professionals’ behaviours and knowledge.93 Additionally, none of the included studies reported adverse events related to the implementation strategies, an outcome that has not been examined in previous reviews on this topic.24 The absence of adverse effects was likely due to a lack of data collection for this outcome in primary studies, therefore uncertainty about their occurrence remains.
In summary, with low to moderate certainty evidence, the review showed that implementation strategies discouraged routine imaging use but did not improve the provision of patient education/advice or reduce analgesic use compared to no implementation strategy between 3 and 12 months. Systematically developed implementation strategies addressing multifaceted barriers are needed for achieving and sustaining effectiveness of strategies to improve guideline-concordant care for low back pain.
Contributors
Siya Zhao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final version of the manuscript.
Concept and design: Chung-Wei Christine Lin, Aili V. Langford, Simon D. French, Christopher M. Williams, and Siya Zhao.
Verification of underlying data: Siya Zhao, Qiuzhe Chen, and Meng Lyu.
Acquisition, analysis, or interpretation of data: Siya Zhao, Chung-Wei Christine Lin, Aili V. Langford, Qiuzhe Chen, Meng Lyu, and Zhiwei Yang.
Drafting of the manuscript: Siya Zhao.
Critical revision of the manuscript for important intellectual content: Chung-Wei Christine Lin, Aili V. Langford, Simon D. French, Christopher M. Williams, Qiuzhe Chen, Meng Lyu, and Zhiwei Yang.
Statistical analysis: Siya Zhao, and Qiuzhe Chen.
Supervision: Chung-Wei Christine Lin, and Aili V. Langford.
The decision for the manuscript submission and publication: Chung-Wei Christine Lin.
Data sharing statement
The data analysed during the current systematic review and meta-analysis are available from the corresponding author on reasonable request.
Declaration of interests
Siya Zhao is funded by a tuition fee and stipend scholarship from the University of Sydney and the Faculty of Medicine and Health throughout her PhD candidature. Chung-Wei Christine Lin, Aili V. Langford and Christopher M. Williams are funded by National Health and Medical Research Council (NHMRC) Investigator Grants (1193939, 2025289 and 1177226 respectively). Aili V. Langford serves as an unpaid volunteer on the Executive Committee of the Australian Deprescribing Network (ADeN). The remaining authors have no conflicts of interest to declare.
Acknowledgements
We thank the ANZBACK Consumer Advisory Group for advice on the protocol, data interpretation and reporting.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102916.
Appendix A. Supplementary data
References
- 1.Wang L., Ye H., Li Z., et al. Epidemiological trends of low back pain at the global, regional, and national levels. Eur Spine J. 2022;31(4):953–962. doi: 10.1007/s00586-022-07133-x. [DOI] [PubMed] [Google Scholar]
- 2.Dimitra P., Helena L.-Q., Christoph R., Günter O., Corinna S., Reinhard B. In: Improving healthcare quality in Europe: characteristics, effectiveness and implementation of different strategies-9 Clinical Practice Guidelines as a quality strategy. Busse R., Klazinga N., Panteli D., Quentin W., editors. 2019. European observatory health policy series. Copenhagen (Denmark) [PubMed] [Google Scholar]
- 3.Wilson R., Pryymachenko Y., Abbott J.H., et al. A guideline-implementation intervention to improve the management of low back pain in primary care: a difference-in-difference-in-differences analysis. Appl Health Econ Health Pol. 2023;21(2):253–262. doi: 10.1007/s40258-022-00776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesner S.A., Foster N.E., French S.D. Implementation interventions to improve the management of non-specific low back pain: a systematic review. BMC Musculoskelet Disord. 2016;17:258. doi: 10.1186/s12891-016-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster N.E., Anema J.R., Cherkin D., et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368–2383. doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira C.B., Maher C.G., Pinto R.Z., et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 8.Corp N., Mansell G., Stynes S., et al. Evidence-based treatment recommendations for neck and low back pain across Europe: a systematic review of guidelines. Eur J Pain. 2021;25(2):275–295. doi: 10.1002/ejp.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . World Health Organization; 2023. WHO guideline for non-surgical management of chronic primary low back pain in adults in primary and community care settings.https://www.who.int/publications/i/item/9789240081789 [PubMed] [Google Scholar]
- 10.Jones C.M., Shaheed C.A., Ferreira G.E., Kharel P., Christine Lin C.-W., Maher C.G. Advice and education provide small short-term improvements in pain and disability in people with non-specific spinal pain: a systematic review. J Physiother. 2021;67(4):263–270. doi: 10.1016/j.jphys.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Müskens J.L.J.M., Kool R.B., van Dulmen S.A., Westert G.P. Overuse of diagnostic testing in healthcare: a systematic review. BMJ Qual Saf. 2022;31(1):54–63. doi: 10.1136/bmjqs-2020-012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall A.M., Aubrey-Bassler K., Thorne B., Maher C.G. Do not routinely offer imaging for uncomplicated low back pain. BMJ. 2021;372 doi: 10.1136/bmj.n291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreijenberg M., Koes B.W., Lin C.-W.C. Guideline recommendations on the pharmacological management of non-specific low back pain in primary care - is there a need to change? Expet Rev Clin Pharmacol. 2019;12(2):145–157. doi: 10.1080/17512433.2019.1565992. [DOI] [PubMed] [Google Scholar]
- 14.Schiphorst Preuper H.R., Geertzen J.H.B., van Wijhe M., et al. Do analgesics improve functioning in patients with chronic low back pain? An explorative triple-blinded RCT. Eur Spine J. 2014;23(4):800–806. doi: 10.1007/s00586-014-3229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cashin A.G., Wand B.M., O'Connell N.E., et al. Pharmacological treatments for low back pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2023;4(4) doi: 10.1002/14651858.CD013815.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel Shaheed C., Maher C.G., Williams K.A., Day R., McLachlan A.J. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958–968. doi: 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- 17.Jones C.M.P., Day R.O., Koes B.W., et al. Opioid analgesia for acute low back pain and neck pain (the OPAL trial): a randomised placebo-controlled trial. Lancet. 2023;402(10398):304–312. doi: 10.1016/S0140-6736(23)00404-X. [DOI] [PubMed] [Google Scholar]
- 18.Williams C.M., Maher C.G., Hancock M.J., et al. Low back pain and best practice care: a survey of general practice physicians. Arch Intern Med. 2010;170(3):271–277. doi: 10.1001/archinternmed.2009.507. [DOI] [PubMed] [Google Scholar]
- 19.Flodgren G., Hall A.M., Goulding L., et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD010669.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimlichman E., Meilik-Weiss A. Clinical guidelines as a tool for ensuring good clinical practice. Isr Med Assoc J. 2004;6(10):626–627. [PubMed] [Google Scholar]
- 21.Straus S.E., Tetroe J., Graham I.D. 2nd ed. 2009. Knowledge translation in health care: moving from evidence to practice. [Google Scholar]
- 22.Pereira V.C., Silva S.N., Carvalho V.K.S., Zanghelini F., Barreto J.O.M. Strategies for the implementation of clinical practice guidelines in public health: an overview of systematic reviews. Health Res Policy Syst. 2022;20(1):13. doi: 10.1186/s12961-022-00815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Wees P.J., Jamtvedt G., Rebbeck T., de Bie R.A., Dekker J., Hendriks E.J.M. Multifaceted strategies may increase implementation of physiotherapy clinical guidelines: a systematic review. Aust J Physiother. 2008;54(4):233–241. doi: 10.1016/s0004-9514(08)70002-3. [DOI] [PubMed] [Google Scholar]
- 24.Suman A., Dikkers M.F., Schaafsma F.G., van Tulder M.W., Anema J.R. Effectiveness of multifaceted implementation strategies for the implementation of back and neck pain guidelines in health care: a systematic review. Implement Sci. 2016;11(1):126. doi: 10.1186/s13012-016-0482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins H.J., Hancock M.J., French S.D., Maher C.G., Engel R.M., Magnussen J.S. Effectiveness of interventions designed to reduce the use of imaging for low-back pain: a systematic review. Can Med Assoc J. 2015;187(6):401–408. doi: 10.1503/cmaj.141183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Zoubi F.M., Menon A., Mayo N.E., Bussières A.E. The effectiveness of interventions designed to increase the uptake of clinical practice guidelines and best practices among musculoskeletal professionals: a systematic review. BMC Health Serv Res. 2018;18(1):435. doi: 10.1186/s12913-018-3253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fillipo R., Pruka K., Carvalho M., et al. Does the implementation of clinical practice guidelines for low back and neck pain by physical therapists improve patient outcomes? A systematic review. Implement Sci Commun. 2022;3(1):57. doi: 10.1186/s43058-022-00305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor D.A., Glasziou P., Maher C.G., et al. Effect of an individualized audit and feedback intervention on rates of musculoskeletal diagnostic imaging requests by Australian general practitioners: a randomized clinical trial. 2022;328(9):850–860. doi: 10.1001/jama.2022.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.French S.D., O'Connor D.A., Green S.E., et al. Improving adherence to acute low back pain guideline recommendations with chiropractors and physiotherapists: the ALIGN cluster randomised controlled trial. Trials. 2022;23(1):142. doi: 10.1186/s13063-022-06053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J., Thomas J., Chandler J., et al. Cochrane; 2022. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022) [Google Scholar]
- 31.EPOC . 2017. Cochrane effective practice and organization of care, screening, data extraction and management.https://epoccochraneorg/sites/epoccochraneorg/files/public/uploads/Resources-for-authors2017/screening_data_extraction_and_managementpdf [Google Scholar]
- 32.EPOC Cochrane effective practice and organization of care, how to develop a search strategy for an intervention review. 2019. https://zenodoorg/record/5106292#ZEVOtOxBzMI
- 33.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.-W.C., Langford A.V., French S.D., Williams C.M., Yang Z., Zhao S. PROSPERO; 2023. Effectiveness of strategies for implementing evidence-based recommendations for the management of low back pain: a systematic review and meta-analysis.https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023452969 Available from: [Google Scholar]
- 35.Collaboration TC, Technical Supplement to Chapter 4: Searching for and selecting studies . 2022. Cochrane Handbook for Systematic Reviews of Interventions Version 63. [Google Scholar]
- 36.Group CBR . 2013. Updated search strategies for CBRG Jan 2013.https://backcochraneorg/intervention-reviews Available from: [Google Scholar]
- 37.Scopus . 2023. Scopus - wikipedia.https://enwikipediaorg/wiki/Scopus [Google Scholar]
- 38.EPOC . Cochrane; 2015. Cochrane effective practice and organisation of care (EPOC) taxonomy. [Google Scholar]
- 39.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE handbook for Grading quality of evidence and strength of recommendations. Version 2014. guidelinedevelopmentorg/handbook. 2014. [Google Scholar]
- 41.Patsopoulos N.A., Evangelou E., Ioannidis J.P.A. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopec J.A. Measuring functional outcomes in persons with back pain: a review of back-specific questionnaires. Spine. 2000;25(24):3110–3114. doi: 10.1097/00007632-200012150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Shafshak T.S., Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. 2021;27(7):282–285. doi: 10.1097/RHU.0000000000001320. [DOI] [PubMed] [Google Scholar]
- 44.Tang X., Schalet B.D., Hung M., Brodke D.S., Saltzman C.L., Cella D. Linking Oswestry Disability Index to the PROMIS pain interference CAT with equipercentile methods. Spine J. 2021;21(7):1185–1192. doi: 10.1016/j.spinee.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saragiotto B.T., Maher C.G., Yamato T.P., et al. Motor control exercise for nonspecific low back pain: a Cochrane review. Spine. 2016;41(16) doi: 10.1097/BRS.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 46.Becker A., Leonhardt C., Kochen M.M., et al. Effects of two guideline implementation strategies on patient outcomes in primary care: a cluster randomized controlled trial [with consumer summary] Spine. 2008;33(5):473–480. doi: 10.1097/BRS.0b013e3181657e0d. [DOI] [PubMed] [Google Scholar]
- 47.Bekkering G.E., Hendriks H.J., van Tulder M.W., et al. Effect on the process of care of an active strategy to implement clinical guidelines on physiotherapy for low back pain: a cluster randomised controlled trial [with consumer summary] Qual Health Care. 2005;14(2):107–112. doi: 10.1136/qshc.2003.009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bekkering G.E., van Tulder M.W., Hendriks E.J., et al. Implementation of clinical guidelines on physical therapy for patients with low back pain: randomized trial comparing patient outcomes after a standard and active implementation strategy. 2005;85(6):544–555. [PubMed] [Google Scholar]
- 49.Bishop P.B., Wing P.C. Knowledge transfer in family physicians managing patients with acute low back pain: a prospective randomized control trial. 2006;6(3):282–288. doi: 10.1016/j.spinee.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Coombs D.M., Machado G.C., Richards B., et al. Effectiveness of a multifaceted intervention to improve emergency department care of low back pain: a stepped-wedge, cluster-randomised trial. 2021;30(10):825–835. doi: 10.1136/bmjqs-2020-012337. [DOI] [PubMed] [Google Scholar]
- 51.Dey P., Simpson C.W., Collins S.I., et al. Implementation of RCGP guidelines for acute low back pain: a cluster randomised controlled trial [with consumer summary] Br J Gen Pract. 2004;54(498):33–37. [PMC free article] [PubMed] [Google Scholar]
- 52.Eccles M., Steen N., Grimshaw J., et al. Effect of audit and feedback, and reminder messages on primary-care radiology referrals: a randomised trial. 2001;357(9266):1406–1409. doi: 10.1016/S0140-6736(00)04564-5. [DOI] [PubMed] [Google Scholar]
- 53.Engers A.J., Wensing M., van Tulder M.W., et al. Implementation of the Dutch low back pain guideline for general practitioners: a cluster randomized controlled trial. Spine. 2005;30(6):559–600. doi: 10.1097/01.brs.0000155406.79479.3a. [DOI] [PubMed] [Google Scholar]
- 54.Evans D.W., Breen A.C., Pincus T., et al. The effectiveness of a posted information package on the beliefs and behavior of musculoskeletal practitioners: the UK Chiropractors, Osteopaths, and Musculoskeletal Physiotherapists Low Back Pain ManagemENT (COMPLeMENT) randomized trial. Spine. 2010;35(8):858–866. doi: 10.1097/BRS.0b013e3181d4e04b. [DOI] [PubMed] [Google Scholar]
- 55.Fenton J.J., Kravitz R.L., Jerant A., et al. Promoting patient-centered counseling to reduce use of low-value diagnostic tests: a randomized clinical trial. JAMA Intern Med. 2016;176(2):191–197. doi: 10.1001/jamainternmed.2015.6840. [DOI] [PubMed] [Google Scholar]
- 56.French S.D., McKenzie J.E., O'Connor D.A., et al. Evaluation of a theory-informed implementation intervention for the management of acute low back pain in general medical practice: the IMPLEMENT cluster randomised trial. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrotin Y., Moyse D., Bazin T., et al. Study of the information delivery by general practitioners and rheumatologists to patients with acute low back pain. Eur Spine J. 2011;20(5):720–730. doi: 10.1007/s00586-010-1612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkins H.J., French S.D., Young A., et al. Feasibility of testing the effectiveness of a theory-informed intervention to reduce imaging for low back pain: a pilot cluster randomised controlled trial. 2022;8(1):249. doi: 10.1186/s40814-022-01216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones C.M., Coombs D., Lin C.C., et al. Implementation of a model of care for low back pain produces sustained reduction in opioid use in emergency departments. Emerg Med J. 2023;40(5):359–360. doi: 10.1136/emermed-2022-212874. [DOI] [PubMed] [Google Scholar]
- 60.Kerry S., Oakeshott P., Dundas D., Williams J. Influence of postal distribution of the Royal College of Radiologists' guidelines, together with feedback on radiological referral rates, on X-ray referrals from general practice: a randomized controlled trial. Fam Pract. 2000;17(1):46–52. doi: 10.1093/fampra/17.1.46. [DOI] [PubMed] [Google Scholar]
- 61.Kullgren J.T., Krupka E., Schachter A., et al. Precommitting to choose wisely about low-value services: a stepped wedge cluster randomised trial. BMJ Qual Saf. 2018;27(5):355–364. doi: 10.1136/bmjqs-2017-006699. [DOI] [PubMed] [Google Scholar]
- 62.Lane E., Magel J.S., Thackeray A., et al. Effectiveness of training physical therapists in pain neuroscience education for patients with chronic spine pain: a cluster-randomized trial. Pain. 2022;163(5):852–860. doi: 10.1097/j.pain.0000000000002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oakeshott P., Kerry S.M., Williams J.E. Randomized controlled trial of the effect of the Royal College of Radiologists' guidelines on general practitioners' referrals for radiographic examination. Br J Gen Pract. 1994;44(382):197–200. [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsay C.R., Eccles M., Grimshaw J.M., Steen N. Assessing the long-term effect of educational reminder messages on primary care radiology referrals. Clin Radiol. 2003;58(4):319–321. doi: 10.1016/s0009-9260(02)00524-x. [DOI] [PubMed] [Google Scholar]
- 65.Rhon D.I., Mayhew R.J., Greenlee T.A., Fritz J.M. The influence of a MOBile-based video Instruction for Low back pain (MOBIL) on initial care decisions made by primary care providers: a randomized controlled trial. BMC Fam Pract. 2021;22(1):200. doi: 10.1186/s12875-021-01549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robling M.R., Houston H.L., Kinnersley P., et al. General practitioners' use of magnetic resonance imaging: an open randomized trial comparing telephone and written requests and an open randomized controlled trial of different methods of local guideline dissemination. Clin Radiol. 2002;57(5):402–407. doi: 10.1053/crad.2001.0864. [DOI] [PubMed] [Google Scholar]
- 67.Rossignol M., Abenhaim L., Séguin P., et al. Coordination of primary health care for back pain. A randomized controlled trial. Spine. 2000;25(2):251–258. doi: 10.1097/00007632-200001150-00018. [DOI] [PubMed] [Google Scholar]
- 68.Schectman J.M., Schroth W.S., Verme D., Voss J.D. Randomized controlled trial of education and feedback for implementation of guidelines for acute low back pain. J Gen Intern Med. 2003;18(10):773–780. doi: 10.1046/j.1525-1497.2003.10205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schroder K., Oberg B., Enthoven P., Hedevik H., Abbott A. Improved adherence to clinical guidelines for low back pain after implementation of the BetterBack model of care: a stepped cluster randomized controlled trial within a hybrid type 2 trial. Physiother Theory Pract. 2023;39(7):1376–1390. doi: 10.1080/09593985.2022.2040669. [DOI] [PubMed] [Google Scholar]
- 70.Schroder K., Oberg B., Enthoven P., Hedevik H., Fors M., Abbott A. Effectiveness and quality of implementing a best practice model of care for low back pain (Betterback) compared with routine care in physiotherapy: a hybrid type 2 trial. J Clin Med. 2021;10(6):1–17. doi: 10.3390/jcm10061230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simula A.S., Jenkins H.J., Hancock M.J., Malmivaara A., Booth N., Karppinen J. Patient education booklet to support evidence-based low back pain care in primary care - a cluster randomized controlled trial. BMC Fam Pract. 2021;22(1):178. doi: 10.1186/s12875-021-01529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soon J., Traeger A.C., Elshaug A.G., et al. Effect of two behavioural 'nudging' interventions on management decisions for low back pain: a randomised vignette-based study in general practitioners. BMJ Qual Saf. 2019;28(7):547–555. doi: 10.1136/bmjqs-2018-008659. [DOI] [PubMed] [Google Scholar]
- 73.Suman A., Schaafsma F.G., van de Ven P.M., et al. Effectiveness of a multifaceted implementation strategy compared to usual care on low back pain guideline adherence among general practitioners. BMC Health Serv Res. 2018;18(1):358. doi: 10.1186/s12913-018-3166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Dulmen S.A., Maas M., Staal J.B., et al. Effectiveness of peer assessment for implementing a Dutch physical therapy low back pain guideline: cluster randomized controlled trial. 2014;94(10):1396–1409. doi: 10.2522/ptj.20130286. [DOI] [PubMed] [Google Scholar]
- 75.Schroder K., Oberg B., Enthoven P., Kongsted A., Abbott A. Confidence, attitudes, beliefs and determinants of implementation behaviours among physiotherapists towards clinical management of low back pain before and after implementation of the BetterBack model of care. BMC Health Serv Res. 2020;20(1):443. doi: 10.1186/s12913-020-05197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cross A.J., Buchbinder R., Mathieson S., et al. Barriers and enablers to monitoring and deprescribing opioid analgesics for chronic non-cancer pain: a systematic review with qualitative evidence synthesis using the Theoretical Domains Framework. BMJ Qual Saf. 2022;31(5):387–400. doi: 10.1136/bmjqs-2021-014186. [DOI] [PubMed] [Google Scholar]
- 77.Schectman J.M., Schroth W.S., Verme D., Voss J.D. Randomized controlled trial of education and feedback for implementation of guidelines for acute low back pain. J Gen Intern Med. 2003;18(10):773–780. doi: 10.1046/j.1525-1497.2003.10205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fischer F., Lange K., Klose K., Greiner W., Kraemer A. Barriers and strategies in guideline implementation-A scoping review. Healthcare (Basel) 2016;4(3) doi: 10.3390/healthcare4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grimshaw J.M., Patey A.M., Kirkham K.R., et al. De-implementing wisely: developing the evidence base to reduce low-value care. BMJ Qual Saf. 2020;29(5):409–417. doi: 10.1136/bmjqs-2019-010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang T., Tan J.-Y., Liu X.-L., Zhao I. Barriers and enablers to implementing clinical practice guidelines in primary care: an overview of systematic reviews. BMJ Open. 2023;13(1) doi: 10.1136/bmjopen-2022-062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall A.M., Scurrey S.R., Pike A.E., et al. Physician-reported barriers to using evidence-based recommendations for low back pain in clinical practice: a systematic review and synthesis of qualitative studies using the Theoretical Domains Framework. Implement Sci. 2019;14(1):49. doi: 10.1186/s13012-019-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pike A., Patey A., Lawrence R., et al. Barriers to following imaging guidelines for the treatment and management of patients with low-back pain in primary care: a qualitative assessment guided by the Theoretical Domains Framework. BMC Prim Care. 2022;23(1):143. doi: 10.1186/s12875-022-01751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ludwig R.L., Turner L.W. Effective patient education in medical imaging: public perceptions of radiation exposure risk. J Allied Health. 2002;31(3):159–164. [PubMed] [Google Scholar]
- 84.Langford A.V., Gnjidic D., Lin C.-W.C., et al. Challenges of opioid deprescribing and factors to be considered in the development of opioid deprescribing guidelines: a qualitative analysis. BMJ Qual Saf. 2021;30(2):133–140. doi: 10.1136/bmjqs-2020-010881. [DOI] [PubMed] [Google Scholar]
- 85.Li S.-A., Jeffs L., Barwick M., Stevens B. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: a systematic integrative review. Syst Rev. 2018;7(1):72. doi: 10.1186/s13643-018-0734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haines E.R., Dopp A., Lyon A.R., et al. Harmonizing evidence-based practice, implementation context, and implementation strategies with user-centered design: a case example in young adult cancer care. Implement Sci Commun. 2021;2(1):45. doi: 10.1186/s43058-021-00147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa N., Blyth F.M., Amorim A.B., Parambath S., Shanmuganathan S., Huckel Schneider C. Implementation initiatives to improve low back pain care in Australia: a scoping review. Pain Med. 2022;23(12):1979–2009. doi: 10.1093/pm/pnac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laur C., Ball L., Keller H., Ivers N. Building on what we know: moving beyond effectiveness to consider how to implement, sustain and spread successful health interventions. BMJ Nutr Prev Health. 2020;3(2):123–125. doi: 10.1136/bmjnph-2020-000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damschroder L.J., Reardon C.M., Widerquist M.A.O., Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75. doi: 10.1186/s13012-022-01245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balis L.E., Houghtaling B. Matching barriers and facilitators to implementation strategies: recommendations for community settings. Implement Sci Commun. 2023;4(1):144. doi: 10.1186/s43058-023-00532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahern M., Dean C.M., Dear B.F., Willcock S.M., Hush J.M. Management of acute low back pain: the practices and perspectives of primary care clinicians in Australia. Aust J Prim Health. 2020;26(3):256–264. doi: 10.1071/py19152. [DOI] [PubMed] [Google Scholar]
- 93.Slade S.C., Kent P., Patel S., Bucknall T., Buchbinder R. Barriers to primary care clinician adherence to clinical guidelines for the management of low back pain: a systematic review and metasynthesis of qualitative studies. Clin J Pain. 2016;32(9):800–816. doi: 10.1097/ajp.0000000000000324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.