Abstract
Background
Acquiring sufficient knowledge and understanding the importance of intestinal microbiota and probiotics in health and disease, as well as their potential for interactions with concurrently administered drugs, can significantly influence future pharmacotherapeutic practices among health science students.
Objective
This study aimed to assess the knowledge, factors influencing knowledge, attitudes, and practices regarding intestinal microbiota and probiotics and their interactions with drugs among students of the Faculty of Medicine in Novi Sad.
Materials and methods
This cross-sectional study was conducted in the form of an anonymous questionnaire among first- and final-year medical and pharmacy students. Predictors of knowledge scores were analyzed using a negative binomial regression model.
Results
The questionnaire was completed by 263 medical and pharmacy students (44.58% first-year and 55.5% final-year students). Approximately half of the students (53.2%) demonstrated fair knowledge, 34.2% had poor knowledge, and only 12.5% had good knowledge about the intestinal microbiota and probiotics. Study year and self-assessment of knowledge were statistically significant predictors of knowledge scores, while the presence of chronic diseases, previous education, and lifestyle were not. The most common indications for probiotic use among respondents were antibiotic use (75.4%) and gastrointestinal symptoms (69.9%). A large number of respondents reported not paying attention to the concurrent use of probiotics with drugs or food, nor to the choice of specific probiotic strains. Most students expressed that they receive insufficient information on this topic at the university.
Conclusion
Most students demonstrate inadequate knowledge about the gut microbiota and probiotics, which affects their practical use of these supplements. The primary reasons for this are insufficient information and unreliable sources of information. Therefore, enhancing education on this topic could significantly improve the knowledge and pharmacotherapeutic practices of future healthcare professionals.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12909-024-06249-6.
Keywords: intestinal microbiota, probiotics, students, questionnaire, drug interactions, pharmacomicrobiomics
Background
The gastrointestinal tract (GIT) harbors a vast array of microorganisms collectively known as the intestinal microbiota. This dynamic ecosystem profoundly influences host physiology and health. The intestinal microbiota is characterized by its remarkable diversity, with microbial populations varying along the length of the GIT and across individuals. Bacteria predominate in the microbiota, with Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria comprising the major phyla [1–3]. Contrary to the long-held belief that the fetal gut is sterile, studies have unveiled microbial presence within the fetal intestinal tract before birth. Namely, the analysis of meconium samples from newborns revealed the presence of bacterial strains including Staphylococcus and Bifidobacterium, indicating that colonization by microbiota begins in utero [4, 5]. The mode of delivery and breastfeeding exert considerable influence on the development of the intestinal microbiota. Although breast milk is also traditionally considered sterile, recent advances in culture-dependent and sequencing technologies have revealed the presence of a distinct microbial community primarily composed of Streptococcus, Staphylococcus, Bifidobacterium, Lactobacillus, Bacteroides, Acinetobacter, Enterococcus and others [6, 7]. Furthermore, breast milk is rich in bioactive compounds such as fatty acids, antimicrobial peptides, polyamines, oligosaccharides, and immune cells, which collectively contribute to the establishment and modulation of the infant gut microbiota [8]. During the first two years of life, the intestinal microbiota undergoes a period of rapid development and transformation, after which its composition tends to stabilize and remains relatively constant throughout the lifespan [9]. Alterations in the composition and activity of the microbiota, referred to as dysbiosis, can profoundly affect the health and homeostasis of the human organism. Factors that can disrupt the beneficial members of the GIT microbiota include stress, radiation, antibiotic therapy, altered intestinal peristalsis, chronic diseases and changes in eating habits and lifestyle [10]. Dysbiosis has been implicated in the pathogenesis of numerous diseases, including inflammatory bowel disease (IBD), obesity, diabetes, and colorectal cancer [11, 12]. Understanding the mechanisms underlying microbiota-mediated disease pathogenesis is crucial for the development of targeted therapeutic strategies aimed at restoring microbial homeostasis and ameliorating disease symptoms. In this context, there have been attempts to modify the activity and composition of the microbiota, with probiotic use being one of the strategies.
According to the World Health Organization (WHO), probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. Numerous combinations of different bacterial strains are used as commercial probiotics, which are available to consumers in various pharmaceutical forms, including powders, tablets, and capsules [13]. The most commonly used probiotics are Gram-positive bacteria from the genera Lactobacillus (Lactobacillus acidophilus, Lactobacillus case, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus rhamnosus, Lactobacillus GG) and Bifidobacterium (Bifidobacterium infantis, Bifidobacterium longum), which naturally occur as part of the normal intestinal microbiota. Strains of Saccharomyces boulardii, Lactococcus, Bacillus and some strains of Escherichia coli are used less frequently. Probiotic species vary in their metabolic activity, bioavailability and mode of action [1]. They play multiple roles, including maintaining the balance of the intestinal microbiota, inhibiting the growth of pathogenic bacteria, promoting digestion, enhancing immune function, and increasing resistance to infections. Due to their ability to produce organic compounds, such as lactic acid, butyric acid, and acetic acid, probiotics increase the acidity in the intestines, thereby inhibiting the proliferation of various harmful bacteria. Probiotics have been shown to play a significant role in relieving symptoms of diarrhea and managing inflammatory bowel disease. Furthermore, probiotics have favorable effects in addressing allergic reactions to food and atopic diseases, vaginal infections, chronic candidiasis and many other conditions [14–16].
In addition to its role in maintaining intestinal homeostasis, the intestinal microbiota is recognized as a unique biosystem with high potential for interactions with drugs, contributing significantly to interindividual variations in drug response. Interactions between drugs and the intestinal microbiota can lead to changes in drug absorption, metabolism, and bioavailability [1, 17–19]. Therefore, in recent years, the field of pharmacomicrobiomics has undergone intensive development, aiming to investigate the complex interactions between drugs and the microbiota to unravel the underlying mechanisms driving interindividual variability in drug responses [20, 21]. Understanding these interactions is crucial for optimizing drug therapy, minimizing adverse effects, and advancing precision medicine approaches tailored to individual patients.
Probiotics, as supplements containing live microorganisms, also have a considerable impact on the pharmacokinetics of drugs. They influence drug bioavailability through various mechanisms, including: reducing local pH in the GIT by producing short-chain fatty acids, which can consequently affect the absorption of drugs due to a change in the ratio of ionized to non-ionized forms of the drug, prolonging intestinal transit time or increasing the thickness of the adherent mucosa, modulating the expression of intestinal transporters involved in drug transport across the intestinal wall, providing enzymes responsible for drug metabolism, modulating the activity of microbial enzymes through induction or inhibition, and leading to drug bioaccumulation in probiotic bacteria [1, 22–26].
The hesitation among health professionals to recommend probiotics despite the well-established evidence of their health benefits may stem from the overwhelming amount of information about their effects [27]. Furthermore, the variability in effects observed among different species of probiotics adds another layer of complexity, making it difficult for healthcare professionals to navigate the vast array of available options and confidently recommend specific products to their patients. Therefore, it is imperative that future healthcare professionals, including medical and pharmacy students, acquire adequate knowledge on these topics to sift through the available evidence, critically evaluate probiotic products, and provide patients with accurate information and personalized recommendations tailored to their individual health needs. Currently, there is a lack of studies in our country assessing the knowledge, attitudes and practices of health science students regarding intestinal microbiota and probiotic use. Additionally, this study addresses interactions of gut microbiota and probiotics with drugs, a topic that has been lacking in similar studies so far. Conducting such a study could help identify specific knowledge gaps and areas requiring greater emphasis within the educational framework.
Accordingly, the aim of this study was to assess the knowledge, factors influencing knowledge, attitudes, and practices regarding the intestinal microbiota and probiotics and their interactions with drugs among students in the first and final years of integrated academic studies of medicine and pharmacy at the Faculty of Medicine in Novi Sad, Serbia.
Methods
Study design
This research was conducted as a cross-sectional study at the Faculty of Medicine in Novi Sad between December 2023 and January 2024, involving first- and final-year students of integrated academic studies of medicine and pharmacy. The research was approved by the Ethics Committee of the Faculty of Medicine in Novi Sad (approval number 01–39/137). The minimum required sample size (n = 230) was calculated from the total number of first- and final-year students in the selected study programs (N = 590) at the Faculty of Medicine in Novi Sad during the academic year 2023/2024, with a 95% confidence interval, a 5% margin of error, and a 50% distribution of responses. The research was conducted as an anonymous survey in the form of a questionnaire on students’ awareness of the intestinal microbiota, probiotics and their interactions with drugs. The questionnaire was created in an electronic version using the Google Forms and was distributed to students through informal groups on social networks. A total of 263 questionnaires were completed.
Questionnaire
For the purposes of this research, the questionnaire was developed based on a thorough review of the available and related literature on the topic and by combining questions from previously conducted surveys [28–30], with modifications necessary to ensure accuracy of the responses to questions and claims, and to make the content more relevant for prospective doctors and pharmacists, enabling them to provide adequate recommendations to their patients. In addition, questions addressing drug interactions with gut microbiota and probiotics were included as an original component of the questionnaire to raise awareness in this area and highlight knowledge gaps that could be addressed within the current education system.
Detailed information about the survey was provided at the beginning of the questionnaire. Prior to completing each questionnaire, all respondents gave their informed consent. The content, readability, comprehension, and design of the questionnaire were pretested on 30 students who were not included in the final analyses. The questionnaire consisted of 31 questions, organized into three sections, and was intended to be completed in one session. No minimum or maximum time limit was set for completing the questionnaire. Participants were instructed to take as much time as needed, with the average completion time being 10 min. The first part of the questionnaire covered the socio-demographic characteristics of the students and included general questions about gender, age, field of study, year of study, average grade, lifestyle, presence of chronic diseases, as well as previous education related to the intestinal microbiota and probiotics, and the sources of probiotic-related information. Chronic diseases were defined as conditions lasting one year or more that require ongoing medical attention. The term “affected by chronic diseases,” referred to self-reported conditions such as asthma, diabetes, epilepsy, thyroid gland disorders, and some other conditions that require chronic therapy. These individuals may be potential candidates for interactions between probiotics and concurrently administered medications.
The respondents were asked to evaluate their current knowledge about probiotics on a 5-point Likert scale with the following ratings: very poor (1), poor (2), fair (3), good (4), and very good knowledge (5). The second part of the questionnaire was the knowledge section and comprised questions related to the definitions of the gut microbiome, probiotics, prebiotics, synbiotics, factors affecting microbial composition, strains that can be used as probiotics, indications for probiotics, and the use of probiotics during pregnancy and in children. Additionally, it addressed interactions of probiotics with drugs and students’ attitudes related to this topic. The questions from this group were primarily multiple-choice and three questions that had multiple correct answers (questions related to factors affecting the composition of the intestinal microbiota, strains that can be used as probiotics, and interactions of gut microbiota and probiotics with drugs) for which scoring was performed by analyzing the answers to individual items. Based on the answers to the questions related to knowledge, a knowledge score was determined, so that each correct answer was assigned a score of one, and each incorrect answer was assigned a score of zero. The maximum score for the knowledge questions was 44. Students’ knowledge was categorized as good if they answered more than 75% (34–44 correct answers) of the questions correctly, fair if they answered 50–75% (22-33) of the questions correctly, and poor if they answered less than 50% (0–21). A five-point Likert scale was used to assess the attitudes about probiotic use (1 – strongly disagree, 2 - disagree, 3 - neutral, 4 - agree, 5 – strongly agree).
The third part of the questionnaire addressed the respondents’ prior experience with the use of probiotics (for respondents who have used them at any point in their lives). This section included questions regarding whose recommendation influenced their decision to use probiotics, the indications for which they used probiotics, the types of probiotic bacteria they used, how they selected specific probiotic products, and the concurrent use of probiotics with food, antibiotics, and other medications.
Data analysis
The obtained data were exported in CSV format and subsequently processed for statistical analysis using IBM SPSS software (SPSS 22.0 SPSS Inc., Chicago, IL, USA). Descriptive statistics, including the mean, median, minimum, maximum, and standard deviation, were used to summarize the numerical data. Categorical data are presented as percentages and frequencies. The Kolmogorov‒Smirnov test was employed to evaluate the normality of the data distribution. To compare two independent samples, we utilized two different statistical tests. The t-test for independent samples was applied under the assumption of a normal distribution. In cases where this assumption was not met, the Mann‒Whitney test, a nonparametric test equivalent to the independent samples t-test, was used. For more than three samples and variables that did not follow a normal distribution, we used the Kruskal‒Wallis H test. Furthermore, the Chi‒square test of independence was employed to explore the relationships between two categorical variables. The Spearman correlation coefficient was computed to evaluate the correlation between two variables that did not follow a normal distribution. Statistical hypotheses were tested at a significance level (alpha level) of 0.05.
To examine the variability in students’ knowledge scores, we employed a negative binomial regression (NBR) model. This model is advantageous for analyzing count-based data because it arises from the Poisson-gamma mixture distribution. The application of the NBR model was justified by the nature of our dependent variable, which comprises only nonnegative integer values, and the observation that the assumption of equal mean and variance for the dependent variable was not met. Specifically, we encountered instances of overdispersion, where the variance exceeded the mean, likely due to heterogeneity across observations. The model is formulated as follows:
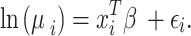 |
In this expression, 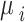 represents the mean value of the NBR distribution, while its variance is given as
represents the mean value of the NBR distribution, while its variance is given as 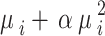 , where
, where 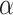 denotes the overdispersion parameter. The results are presented through estimated coefficients (B), standard errors (Std. Error), significance of Chi-square tests (Wald Chi-Square, df, p) and incidence rate ratios (Exp(B)). The incidence rate ratios indicate how a one-unit increase in the predictor variable is expected to multiply the incidence rate of the outcome by the IRR value, assuming all other variables remain constant.
denotes the overdispersion parameter. The results are presented through estimated coefficients (B), standard errors (Std. Error), significance of Chi-square tests (Wald Chi-Square, df, p) and incidence rate ratios (Exp(B)). The incidence rate ratios indicate how a one-unit increase in the predictor variable is expected to multiply the incidence rate of the outcome by the IRR value, assuming all other variables remain constant.
Results
Basic socio-demographic data and previous education on intestinal microbiota and probiotics
Out of a total of 590 first- and final-year medical and pharmacy students, 263 students completed the questionnaire (response rate 44.58%). Table 1 represents basic socio-demographic data of the respondents, alongside with the information about their previous education on intestinal microbiota and probiotics.
Table 1.
Socio-demographic data and previous education on intestinal microbiota and probiotics among respondents
| Field of study | p | Year of study | p | Total n (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Medicine n (%) |
Pharmacy n (%) |
First n (%) |
Final n (%) |
|||||
| Gender | ||||||||
| Male | 35 (24%) | 20 (17.1%) | 0.173 | 20 (17.1%) | 35 (24%) | 0.173 | 55 (20.9%) | |
| Female | 111 (76%) | 97 (82.9%) | 97 (82.9%) | 111 (76%) | 208 (79.1%) | |||
| Presence of chronic diseases | ||||||||
| No | 130 (89%) | 107 (91.5%) | 0.515 | 109 (93.2%) | 128 (87.7%) | 0.138 | 237 (90.1%) | |
| Yes | 16 (11%) | 10 (8.5%) | 8 (6.8%) | 18 (12.3%) | 26 (9.9%) | |||
| Previous education on intestinal microbiota and probiotics | ||||||||
| Not at all | 52 (35.6%) | 53 (45.3%) | 0.115 | 102 (87.2%) | 3 (2.1%) | 0.000 | 105 (39.9%) | |
| Yes, to a small or insignificant extent | 71 (48.6%) | 42 (35.9%) | 13 (11.1%) | 100 (68.5%) | 113 (43%) | |||
| Yes, to a large extent | 23 (15.8%) | 22 (18.8%) | 2 (1.7%) | 43 (29.5%) | 45 (17.1%) | |||
| TOTAL | 146 | 117 | 0.074 | 117 | 146 | 0.074 | 263 | |
Out of the 263 respondents, 146 (55.55%) were medical students, including 56 (38.36%) first-year students and 90 (61.64%) final-year students. Additionally, 117 (44.5%) respondents were pharmacy students, with 61 (52.14%) first-year students and 56 (47.86%) final-year students. There was no statistically significant difference in the distribution of respondents in relation to the field of study or study year (p = 0.074). The average age of the medical students was 19.14 ± 0.84 (18-24) and 23.70 ± 1.21 (23-31) for the first- and final- year students, respectively. For pharmacy students, the average age was 19.15 ± 0.573 (18-21) and 23.38 ± 1.20 (22-27) for the first- and final- year students, respectively. The majority of respondents were female (79.1%), comprising 76% of medical students and 82.9% of pharmacy students. The Chi-square test for gender distribution between fields of study was not statistically significant (χ2 [1] = 1.858, p = 0.173).
A significant difference was found in the average grade distribution between the fields of study (χ2 [3] = 25.011, p = 0.000). Students of medicine (43.8%) had a higher average grade (9.00–10.00) compared to students of pharmacy (18.8%). Regarding the lifestyle assessment that students could rate on a scale from 1 (very poor) to 5 (very good), there were no statistically significant differences between medical and pharmacy students, or between students in the initial and final years of study. The majority of students described their lifestyle as fair (mean score of 3.278 ± 0.738). A total of 90.1% of participants reported no chronic diseases, while 9.9% reported having them, with no difference between medical and pharmacy students (χ2 [1] = 0.424, p = 0.515).
Regarding education on the gut microbiota and probiotics, 39.9% of all participants reported not learning about this topic at all. Among those who did, 43% reported learning to a small or insignificant extent, while 17.1% reported learning to a large extent. The Chi-square test for differences in education on the gut microbiota and probiotics between the fields of study was not statistically significant (χ2 [2] = 4.329, p = 0.115). However, as expected, there was a statistically significant difference in previous education on the gut microbiota and probiotics between first- and final-year students. Specifically, 87.2% of first-year students reported never having been exposed to courses related to the gut microbiota, compared to only 2.1% of final-year students. However, a significant portion (68.5%) of final-year students reported learning about the gut microbiota and probiotics to only a small or insignificant extent. When asked about the courses during their studies in which they encountered this topic, the largest number of students mentioned the subjects Pharmacology and Physiology, with only a small number of students (6.6%) also mentioning Microbiology. Respondents reported that they acquired knowledge about probiotics from various sources including the internet (140 responses given by 53.2% of respondents), doctors (126 responses, 47.9% of respondents), pharmacists (129 responses, 49% of respondents), at the university (118 responses, 44.9% of respondents), and scientific papers and journals (42 responses, 16% of respondents).
Self-assessment of students’ knowledge
Figure 1 shows the results of students’ self-assessment of their knowledge of intestinal microbiota and probiotics, ranked according to a Likert scale from 1 (very poor) to 5 (very good). There were no statistically significant differences between students of medicine and pharmacy regarding their assessment of their knowledge of probiotics (t = 8149,000, p = 0,478). However, in the knowledge assessment category, there was a statistically significant difference between the first-year and final-year students (t = 5217,500, p = 0,000). The mean scores improved from 2.547 in the initial year to 3.110 in the final year, suggesting that students’ assessment scores significantly increased as they progressed in their education.
Fig. 1.
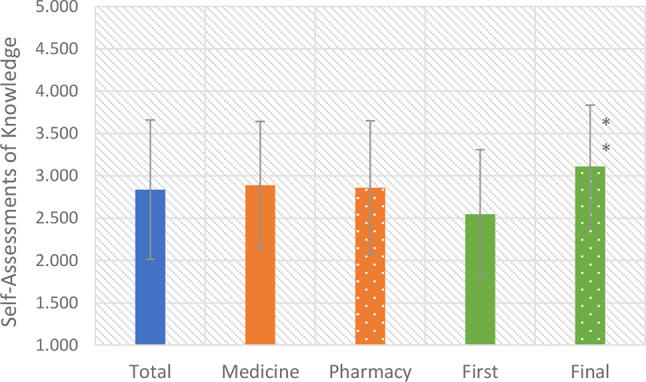
Students’ self-assessment of their knowledge of intestinal microbiota and probiotics
Knowledge score
Figure 2 shows the distribution of knowledge levels about the gut microbiota and probiotics. Among the respondents, 33 (12.5%) provided more than 75% correct answers, indicating good knowledge of the examined topic. Fair knowledge was demonstrated by 140 (53.2%) students who provided 50–75% correct answers, while 90 (34.2%) respondents provided fewer than 50% correct answers, indicating poor knowledge.
Fig. 2.
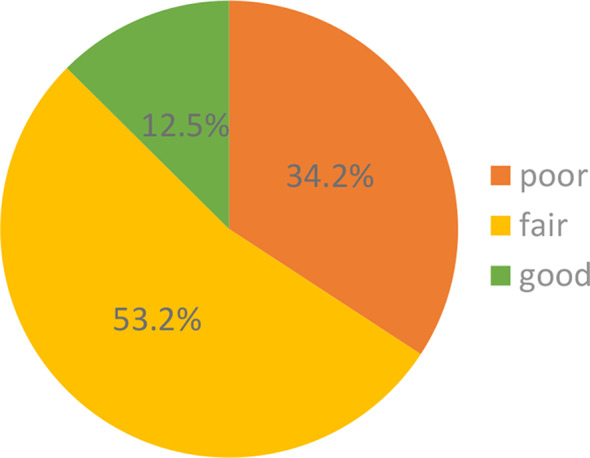
Distribution of students’ knowledge levels about gut microbiota and probiotics
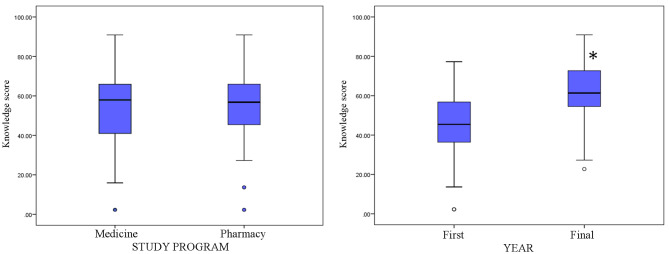
Figure 3 shows the actual knowledge scores of medical and pharmacy students as well as students in the first and final years, which were calculated as the absolute number (0–44) and percentage (0-100%) of correct answers. There was no statistically significant difference between students of medicine and pharmacy in terms of knowledge scores (t=-0.349, df = 261, p = 0.728). However, there was a statistically significant difference in the scores between students in the first and final years of study (t=-8.792, df = 261, p = 0.000). This is further supported by the mean scores, which increased markedly from 20.427 (46.42%) in the first year to 27.425 (62.33%) in the final year (p = 0.000). This substantial increase is supported by an increase in both the median and minimum scores, indicating a general improvement in knowledge scores across the student population.
Fig. 3.
Knowledge scores of medical and pharmacy students, as well as first-year and final-year students, regarding gut microbiota and probiotics
Students’ knowledge about the interactions of intestinal microbiota and probiotics with drugs
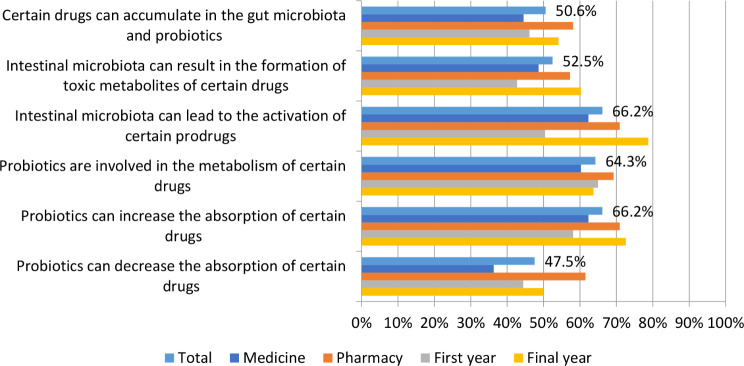
Figure 4 shows the respondents’ knowledge about the interactions of the intestinal microbiota and probiotics with drugs. Approximately half of the respondents (50.6%) knew that certain drugs can accumulate in the intestinal microbiota and probiotics. Regarding the question about the influence of the intestinal microbiota on the formation of toxic metabolites of drugs, 52.5% of the students answered correctly, with the final-year students (33.5%) showing better knowledge compared to first-year students (19%). Additionally, a difference in knowledge between students in the first and final years of studies was observed in the answer to the question of whether the intestinal microbiota can lead to the activation of certain prodrugs, where 43.7% of final year students answered this question correctly, and only 22.4% of first-year students. Of the total number of students, 64.3% knew that probiotics can be involved in the metabolism of certain drugs, and 66.2% knew that probiotics can increase the absorption of some drugs, where a difference in knowledge was again observed between the first-year students (25.9% correct answers) and the final-year students (40.3% correct answers). However, less than half (47.5%) of all respondents knew that probiotics can decrease the absorption of certain drugs. For all questions about drug interactions, the pharmacy students had better knowledge compared to medical students.
Fig. 4.
Respondents’ knowledge about the interactions of intestinal microbiota and probiotics with drugs
Predictive model of students’ knowledge score about probiotics
A negative binomial regression model was used to examine the factors influencing students’ actual knowledge of probiotics. The model included independent variables such as gender, year of study, presence of chronic diseases, course attendance covering the gut microbiota and probiotics topics, lifestyle assessment, and self-evaluated knowledge of probiotics (Table 2).
Table 2.
Negative binomial regression model analysis of predictors for knowledge scores
| Parameter | B | Std. error | Hypothesis test | Exp(B) | ||
|---|---|---|---|---|---|---|
| Wald χ2 | df | p | ||||
| Gender | 0.037 | 0.0381 | 0.960 | 1 | 0.327 | 1.038 |
| Field of study | -0.054 | 0.0320 | 2.897 | 1 | 0.089 | 0.947 |
| Year of study | 0.218 | 0.0627 | 12.135 | 1 | 0.000* | 1.224 |
| Presence of chronic diseases | -0.031 | 0.0512 | 0.365 | 1 | 0.546 | 0.970 |
| Have you had lectures about gut microbiota and probiotics? | 0.039 | 0.0752 | 0.270 | 1 | 0.604 | 1.040 |
| Lifestyle | 0.036 | 0.0217 | 2.700 | 1 | 0.100 | 1.036 |
| Self-assessment score | 0.126 | 0.0232 | 29.695 | 1 | 0.000* | 1.134 |
The goodness-of-fit results indicate a reasonable fit to the data. Both the deviation and Pearson Chi-square values are relatively close to their degrees of freedom (1.176 and 1.043, respectively), suggesting that the model is not significantly over-dispersed. The Omnibus test (likelihood ratio χ2 = 107.964, df = 8, p = 0.000) is highly significant, indicating that the model coefficients are jointly significantly different from zero, which suggests that the model has explanatory power.
Year of study and self-assessment of knowledge significantly influenced the test scores. Assuming that all other variables remain constant, each additional year of study is associated with a 22.4% increase in expected probiotic knowledge scores (IRR = 1.224). Similarly, a one-point progression in self-assessment aligns with a 13.4% increase in expected scores (IRR = 1.134), highlighting the connection between self-assessed knowledge and actual academic proficiency in probiotics. The field of study showed a trend toward significance in favor of medical students. Gender, the presence of chronic diseases, whether students had learned about the gut microbiota and probiotics in any courses, and lifestyle factors were not statistically significant predictors of knowledge scores. The scale parameter for the negative binomial distribution was significant, confirming the appropriateness of this model over a simpler Poisson model, which assumes that the mean of the dependent variable is equal to its variance .
Quantitative analysis of self-assessment of knowledge and actual knowledge of students
Spearman’s rho correlation analysis revealed a moderate positive correlation (ρ = 0.488, p = 0.000) between students’ self-assessment of their knowledge and their actual knowledge scores. The Kruskal-Wallis H test indicated that there was no statistically significant difference in students’ self-assessed knowledge about probiotics across different average grade ranges (H = 2.680, df = 2, p = 0.262), despite slight variations in mean scores. On the other hand, the Kruskal-Wallis H test results showed a statistically significant difference in actual knowledge test scores about probiotics among students with different average grade ranges, with higher scores associated with higher average grades (H = 9.343, df = 2, p = 0.009). The Mann-Whitney U test revealed a statistically significant difference in self-assessed knowledge about probiotics between individuals who used probiotics fewer than twice and those who used them more than twice, with the latter group reporting higher self-assessment scores (U = 1514.500, p < 0.001). Similar results were obtained for actual knowledge test scores about probiotics, with significantly higher scores were associated with students who had used probiotics more than twice (U = 1934.500, p = 0.001).
Knowledge scores and self-assessment scores of knowledge relative to the source of information about probiotics
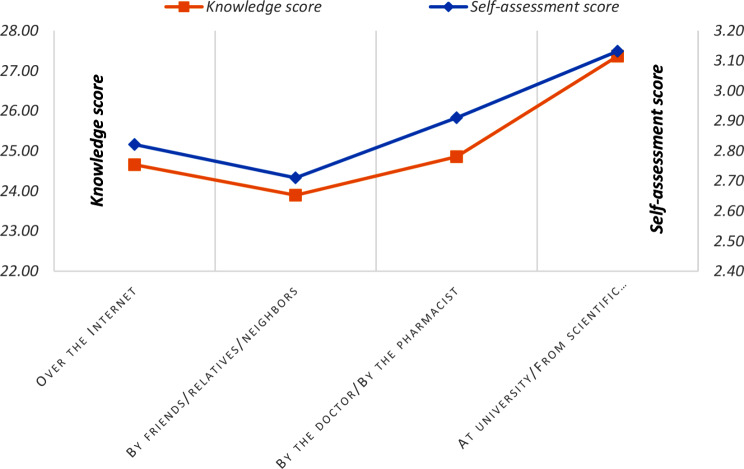
Figure 5 illustrates the correlation between students’ knowledge scores and their self-assessment scores, categorized by the source of information on probiotics. University courses and scientific papers were combined as academic sources, while doctors and pharmacists were grouped as healthcare professionals. The highest knowledge scores, along with higher self-assessment scores, were observed among students who relied on academic sources, particularly scientific papers and university education. In contrast, both knowledge and self-assessment scores were notably lower when the information was obtained from informal sources, such as friends, relatives, or neighbors, suggesting that these channels may be less effective for learning about probiotics.
Fig. 5.
Knowledge scores and self-assessment scores of knowledge relative to the source of information about probiotics
Students’ attitudes toward the use of probiotics
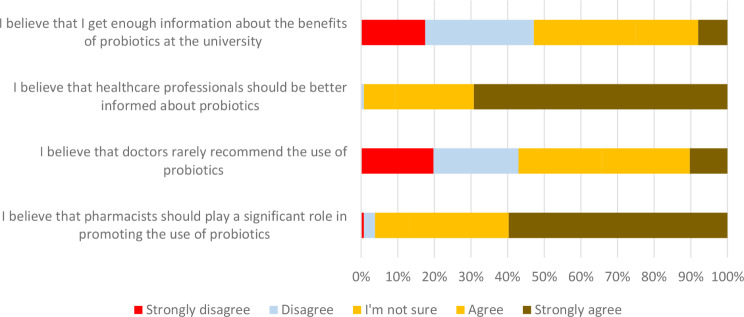
Figure 6 presents students’ attitudes toward the use of probiotics. Among all respondents, 25.1% agreed that they had received sufficient information about the benefits of probiotics during their university education. A significant majority (90.9%) believed that healthcare professionals should be more informed abot the topic. Additionally, most students agreed that pharmacists should play a key role in promoting probiotic use, while opinions were nearly evenly split on whether doctors rarely recommend probiotics.
Fig. 6.
Students’ attitudes toward the use of probiotics
Students’ experience with probiotic use
Table 3 outlines the questions concerning respondents’ previous experience with probiotic use. For questions marked with an asterisk, only students who had reported using probiotics at some point in their lives were required to respond. Notably, only seven students indicated they had never used probiotics. Among those who had used probiotics, the majority (52%) did so based on a doctor’s recommendation, while a substantial proportion (28.9%) used them independently, without consulting a healthcare professional. Concerning the timing of probiotic intake in relation to meals, a significant portion (41.8%) admitted they did not pay attention to this factor. Furthermore, more than half of the respondents (72.7%) reported taking probiotics at different times in relation to their antibiotic use, not at the same time. A smaller percentage (19.9%) stated they avoided probiotics when taking other medications, while 30.5% indicated they did not consider the concurrent use of probiotics with other medications.
Table 3.
Overview of students’ experience with probiotic use
| Total | Field of study | Year of study | |||
|---|---|---|---|---|---|
| n (%) | Medicine n (%) |
Pharmacy N (%) |
First n (%) |
Final n (%) |
|
| I have used probiotics during my lifetime: | |||||
| Never | 7 (2.7%) | 4 (2.7%) | 3 (2.6%) | 4 (3.4%) | 3 (2.1%) |
| Once | 5 (1.9%) | 2 (1.4%) | 3 (2.6%) | 1 (0.9%) | 4 (2.7%) |
| Two times | 13 (4.9%) | 9 (6.2%) | 4 (3.4%) | 8 (6.8%) | 5 (3.4%) |
| More than two times | 238 (90.5%) | 131 (89.7%) | 107 (91.5%) | 104 (88.9%) | 134 (91.8%) |
| Total | 263 (100%) | 146 (55.5%) | 117 (44.5%) | 117 (44.5%) | 146 (55.5%) |
| *I made the decision to use probiotics: | |||||
| Based on a doctor’s recommendation | 133 (52%) | 69 (48.6%) | 64 (56.1%) | 61 (54%) | 72 (50.3%) |
| Based on a pharmacist’s recommendation at the pharmacy | 48 (18.8%) | 38 (26.8%) | 10 (8.8%) | 21 (18.6%) | 27 (18.9%) |
| Self-initiated | 74 (28.9%) | 34 (23.9%) | 40 (35.1%) | 31 (27.4%) | 43 (30.1%) |
| Other | 1 (0.4%) | 1 (0.7%) | - | - | 1 (0.7%) |
| *I have taken probiotics: | |||||
| Before meal | 86 (33.6%) | 56 (39.4%) | 30 (26.3%) | 40 (35.4%) | 46 (32.2%) |
| After meal | 53 (20.7%) | 22 (15.5%) | 31 (27.2%) | 21 (18.6%) | 32 (22.4%) |
| During meal | 10 (3.9%) | 4 (2.8%) | 6 (5.3%) | 4 (3.5%) | 6 (4.2%) |
| I did not pay attention | 107 (41.8%) | 60 (42.3%) | 47 (41.2%) | 48 (42.5%) | 59 (41.3%) |
| *While I was taking antibiotics: | |||||
| I took probiotics at different time from the antibiotic | 186 (72.7%) | 109 (76.8%) | 77 (67.5%) | 86 (76.1%) | 100 (69.9%) |
| I took probiotics at the same time as the antibiotic | 24 (9.4%) | 12 (8.5%) | 12 (10.5%) | 11 (9.7%) | 13 (9.1%) |
| I didn’t pay attention to the time of administration | 27 (10.5%) | 12 (8.5%) | 15 (13.2%) | 11 (9.7%) | 16 (11.2%) |
| I haven’t taken probiotics with antibiotics, only independently | 19 (7.4%) | 9 (6.3%) | 10 (8.8%) | 5 (4.4%) | 14 (9.8%) |
| I have never taken antibiotics | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| *I didn’t use probiotics while taking other medications (other than antibiotics): | |||||
| True | 51 (19.9%) | 23 (16.2%) | 28 (24.6%) | 22 (19.5%) | 29 (20.3%) |
| False | 127 (49.6%) | 73 (51.4%) | 54 (47.4%) | 55 (48.7%) | 72 (50.3%) |
| I am not sure | 78 (30.5%) | 46 (32.4%) | 32 (28.1%) | 36 (31.9%) | 42 (29.4%) |
| Total | 256 (100%) | 142 (55.5%) | 114 (44.5%) | 113 (44.1%) | 143 (55.9%) |
* The questions were answered only by students who declared that they had used probiotics at some point in their lives
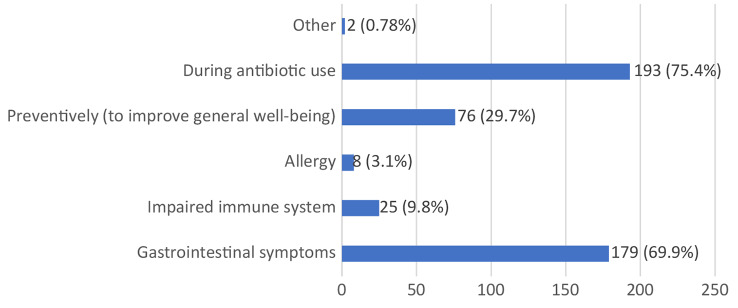
Figure 7 illustrates the most common indications for probiotic use among respondents. The largest proportion of students (75.4%) reported using probiotics during antibiotic therapy, while 69.9% used them to alleviate gastrointestinal symptoms. Additionally, 29.7% of respondents used probiotics preventively to enhance overall health, while much smaller percentages used them for immune system support (9.8%) or to manage allergic reactions (3.1%).
Fig. 7.
The most common indications for probiotic use among respondents
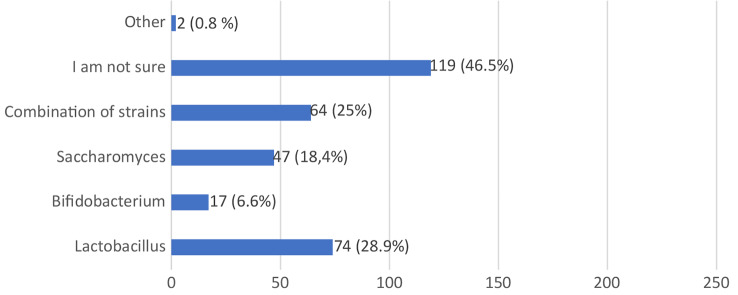
Figure 8 displays the composition of probiotic products used by the respondents. A significant portion (46.5%) were unsure about the specific composition of the probiotics they used. Among those who reported using specific strains, 28.9% mentioned Lactobacillus, 25% indicated a combination of strains, 18.4% referenced Saccharomyces, and 6.6% identified Bifidobacterium.
Fig. 8.
Composition of probiotic products reported to be used by the respondent
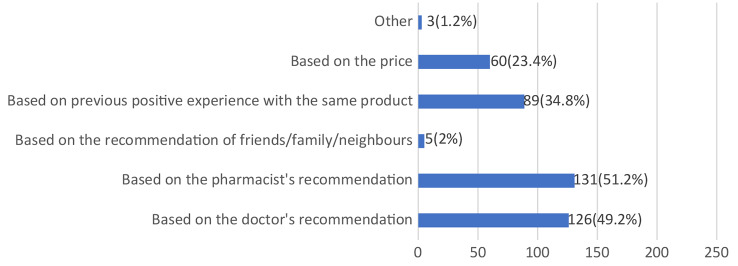
Figure 9 highlights the factors influencing respondents’ choices of specific probiotic supplements. The majority of students based their choices on the advice of healthcare professionals, with 51.2% following the recommendations of pharmacists and 49.2% following those of medical doctors. Additionally, 34.8% of students reported selecting a particular probiotic preparation based on previous positive experiences, while 23.4% considered price as a deciding factor.
Fig. 9.
Factors influencing respondents’ choices of specific probiotic supplements
Discussion
As previously noted, to the best of our knowledge, this is the first study conducted in Serbia that provides insights into the knowledge and factors influencing the knowledge, attitudes, and practices of medical and pharmacy students, as future healthcare professionals, regarding gut microbiota, probiotics, and their interactions with drugs.
In terms of self-assessed knowledge on the subject, only a small percentage of respondents (3.8%) considered themselves to have good knowledge. More than half (55.1%) rated their knowledge as fair, while 27.4% regarded it as quite poor. This pattern was reflected in the actual knowledge scores, where the majority (53.2%) demonstrated fair knowledge. Only 12.5% of students had good knowledge, while 34.2% exhibited generally poor knowledge. In comparison, a significantly higher percentage of students in Jordan (39.1%) rated their knowledge as good, a finding corroborated by their actual knowledge scores [31].
In contrast to students from Indonesia [32] and India [33], where 90.8% and 80.8% of students, respectively, were familiar with the definition of probiotics, a slightly lower percentage of students in our study correctly identified the definition (73.4%). Even fewer were familiar with the definitions of gut microbiota (68.8%) and prebiotics (62%). Most students recognized that factors such as age, diet, antibiotic use, genetics, chronic diseases, alcohol consumption, smoking, and mental health can influence the composition of the gut microbiota [34–36]. However, less than half (47.15%) were aware that the mode of delivery also affects gut microbiota composition. Numerous studies have shown that infants born via cesarean section, compared to those born vaginally, experience a distinct trajectory of gut microbiota development. This difference stems from the lack of exposure to the mother’s vaginal and fecal microbiota, resulting in initial colonization by bacteria from the skin and the surrounding environment [37, 38].
The students in this study exhibited limited knowledge in identifying probiotic species. Respondents were asked to select microorganisms from a list of those they believed included probiotic strains. The species they recognized most frequently were Saccharomyces boulardii (71.48% correct answers) and Lactobacillus acidophilus (70.72%). Conversely, approximately half of the students were uncertain or unaware that species such as Lactobacillus rhamnosus and Bifidobacterium bifidum also contain probiotic strains. Even fewer students recognized that certain strains of Streptococcus thermophilus, Escherichia coli, Bacillus subtilis, and Enterococcus faecium are also classified as probiotic bacteria. In a study conducted in Indonesia, the most recognized species were Saccharomyces boulardii, Lactobacillus acidophilus, and Lactobacillus rhamnosus [32]. Consistent with our findings, only a small percentage of Indonesian students (4.5%) identified Escherichia coli as a probiotic, in contrast to students from Saudi Arabia, where a significant percentage (89.6%) considered it a probiotic [39].
Regarding questions about probiotic use during pregnancy, approximately half of the students indicated that pregnant women can generally consume probiotics throughout pregnancy, while the other half were either unsure or believed they are not recommended during this period. A comprehensive systematic review and meta-analysis conducted by Sheyholislami and Connor [40] have indicated that probiotics and prebiotics are safe for use during pregnancy, postpartum, and during lactation. Probiotic supplementation during pregnancy has been shown to significantly reduce nausea, vomiting, and constipation, improving overall quality of life [41]. Furthermore, more than half of the students were unaware that breast milk contains both probiotics and prebiotics. Breast milk contains several predominant bacterial species, including Staphylococci, Streptococci, Lactobacilli, Enterococci, Lactococci, and Bifidobacteria [6, 7]. The prebiotic effect of breast milk is attributed to its low concentrations of proteins, phosphorus, lactoferrin, lactose, nucleotides, and oligosaccharides [42].
Concerning the general use of probiotics in children, 35% of students were uncertain if and when they could be used, while only 16.7% indicated that probiotics could be used from birth. Although probiotics are generally regarded as safe, specific clinical conditions warrant caution regarding their use. These conditions include prematurity, critical illness, immunocompromised states, the presence of a central venous catheter, cardiac valvular disease, and short-gut syndrome [43]. Some studies indicated that even in prematurely born babies who exhibit an altered gut microbiota composition, with an increased proportion of harmful compared to beneficial bacteria, probiotic use has the potential to restore the balance and normalize the abnormal colonization pattern [5].
In response to questions about the effectiveness of probiotics for various indications, students demonstrated the highest level of familiarity with their efficacy in treating gastrointestinal (GIT) symptoms, with the majority (91.25%) answering correctly. Similar knowledge was observed among students from Saudi Arabia, where 87.3% provided correct answers [39]. However, a significantly smaller percentage of our respondents (44.4%) were aware that probiotics may have a beneficial effect on lipid metabolism. Numerous probiotic strains, including Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Bifidobacterium longum, Enterococcus faecium, and Streptococcus thermophilus, are known to lower blood lipid levels [44]. Likewise, a substantial proportion of students were unaware that probiotics can be effective in preventing vaginal infections (48.3%) [45], reducing allergic reactions (48.3%), or managing eczema (57.8%) [46]. Furthermore, only a small percentage (17.11%) recognized that probiotics may help reduce the risk of dental caries by modulating the oral microbiota [47].
As the relevance of the gut microbiome and probiotics in modulating drug pharmacokinetics continues to grow [1, 19, 23, 24, 48], a section of the questionnaire focused on assessing students’ knowledge of interactions between probiotics, the intestinal microbiota, and concurrently administered drugs. A significant number of respondents were unfamiliar with these potential interactions. The percentage of correct answers ranged from 47.5 to 66.2%, with pharmacy students generally demonstrating better knowledge than medical students. Additionally, final-year students exhibited greater understanding compared to first-year students.
An increasing number of studies have highlighted the implications of microbiota and probiotics for drug efficacy, inactivation, and toxicity [1, 18, 19, 21, 22, 49–52]. One of the earliest studies indicating the role of gut microbiota in drug metabolism was conducted by Peppercorn and Goldman in 1972 who demonstrated that the anti-inflammatory drug, salicylazosulfapyridine, could be degraded in conventional rats and when cultured with human gut bacteria, but not in germ-free rats [53]. The example of drug inactivation by gut microbiota is well illustrated by the case of digoxin. Gut microbes can reduce the lactone ring of the parent drug, digoxin, leading to its conversion into the inactive metabolite, dihydrodigoxin [54]. This may cause the variability in therapeutic outcomes among individuals. It is well-established that patient responses to chemotherapy can vary greatly between individuals, both in terms of treatment effectiveness and the severity of side effects [18]. Emerging research suggests that differences in gut microbiota may play a role in this variability. Beyond their impact on the host immune system, gut microbes can directly modify the chemical structures of cancer drugs and their metabolites, influencing their interaction with host cells. It has been shown that β-glucuronidase produced by gut bacteria, can reactivate the chemotherapeutic agent irinotecan, SN-38, in the intestines. This reactivation leads to severe gastrointestinal toxicity, particularly severe diarrhea, limiting the use of this otherwise effective drug [55]. Recent studies have shown that co-incubation with E. coli or Listeria welshimeri either enhanced or reduced the efficacy of half of a panel of 30 anticancer drugs when tested against cancer cell lines [56]. More recent studies have also pointed out the possibility of the accumulation of certain drugs, such as simvastatin [25], gliclazide [57], duloxetine, rosiglitazone, montelukast, and roflumilast [17], in intestinal and probiotic bacteria, which can significantly alter their pharmacokinetics and therapeutic response. In addition to drug biotransformation and bioaccumulation, it has been demonstrated that probiotic bacteria may affect the absorption of certain drugs. Saputri et al. demonstrated that supplementing rabbits with the probiotic Lactobacillus plantarum resulted in a twofold increase in amlodipine absorption compared to that in control groups that were not pretreated with probiotics [58]. In a study conducted by Al-Salami et al., supplementation of rats with probiotics affected the absorption of gliclazide, increasing it in diabetic animals and reducing it in healthy animals [59]. Matuskova et al. demonstrated that the administration of the probiotic strain Escherichia coli Nissle 1917 led to a 43% increase in the blood levels of amiodarone compared to the control group [60]. In addition to its impact on absorption, an increased level of the metabolite N-desethylamiodarone was also observed in the plasma of these animals, indicating the role of probiotics in both the absorption and metabolism of this drug [60].
The application of the negative binomial regression model revealed important factors influencing respondents’ knowledge scores. The year of study and self-assessment of knowledge emerged as statistically significant predictors of knowledge scores, whereas the presence of chronic diseases, previous education on the gut microbiota and probiotics, and lifestyle factors were not significant predictors. An increase in academic years was associated with higher knowledge scores, underscoring the impact of education. Additionally, better self-assessment of knowledge correlated with higher actual knowledge scores, suggesting that students were generally accurate in evaluating their own knowledge. The study program also showed a trend toward significance, in favor of medical students, indicating its role in knowledge levels.
In response to the question about previous education on the gut microbiota and probiotics, the majority of students (82.9%) reported having received either no education on the topic or only a small amount. Consistent with these findings, most respondents felt that they had not received sufficient information about the gut microbiota and probiotics during their university studies, emphasizing the need for greater awareness and improved education for healthcare professionals in this area. The majority of respondents indicated that they obtained information about probiotics primarily from the internet, followed by doctors and pharmacists. Only 16% reported receiving information at the university, highlighting the need for better integration of this topic into the curriculum. Our findings also showed that students who sought information from credible sources, such as scientific papers and university materials, had significantly better knowledge than those relying on informal sources, such as the internet, friends, and relatives. In comparison, a study conducted among healthcare professionals and students in New Delhi found that 39.41% of respondents received information about probiotics through television and newspaper advertisements, while 22.66% obtained it from websites [61]. Similarly, a large proportion of students from Saudi Arabia (64%) reported acquiring information from the internet and friends (60%), although a significant number (45.3%) emphasized that they obtained information during university lectures [39].
With regard to previous experience with probiotics, the majority of respondents (90.5%) reported having consumed probiotics more than twice in their lifetime. Similarly, students from Indonesia also reported a high prevalence of probiotic use, with 98.8% having used them previously [32]. Notably, students who had used probiotics more than twice scored higher in both self-assessed and actual knowledge, suggesting a better understanding of the efficacy of these supplements.
The most common indications for probiotic use among respondents were antibiotic therapy and gastrointestinal symptoms, similar to findings from students in Saudi Arabia [39]. During antibiotic therapy, 72.7% of students reported taking probiotics within a specific time interval, which is crucial due to the direct effect antibiotics can have on the efficacy of probiotic supplements [62]. A common recommendation is to administer probiotics at least 2 h after taking antibiotics, allowing sufficient time for antibiotic absorption and minimizing its potential impact on probiotic bacteria in the gut. Additionally, it is advised to continue probiotic supplementation for 7–10 days after completing the antibiotic course to aid in restoring the gut microbiota [63].
A significant number of students (42.2%) reported that they did not pay attention to whether they consumed probiotics before, during, or after meals. In a study conducted by Tompkins et al., the highest survival of the tested probiotic strains (Lactobacillus helveticus R0052, Lactobacillus rhamnosus R0011, and Bifidobacterium longum R0175) was observed when taken before or during a meal rich in fats [64]. The majority of respondents reported making the decision to use probiotics based on a doctor’s recommendation, while nearly 30% of the students had taken them on their own initiative. Regarding the selection of specific probiotic supplements, most students reported not paying attention to the composition of the products. This underscores the crucial role of healthcare professionals in guiding patients towards appropriate probiotic choices. For some respondents, previous positive experiences and price were decisive factors in choosing a certain probiotic product. Given that different products contain different probiotic strains and varying bacterial colony counts, which significantly affect the effects of a specific product [65], it is essential for healthcare professionals to have access to evidence-based information to provide appropriate recommendations and guidance to patients. With the increasing number of probiotic products and the growing consideration of probiotics as complementary and alternative therapies alongside vitamins, minerals, and other dietary supplements, it is crucial for healthcare professionals to access scientific and up-to-date sources of information on these supplements. The role of the university, along with education and lectures on intestinal microbiota and probiotics, is significant in this context [66, 67].
This research is significant as it represents the first survey conducted in Serbia to investigate the knowledge, attitudes, and practices of future healthcare professionals concerning intestinal microbiota, probiotics, and their interactions with medications. A notable strength of this study is the high response rate from participants. However, a limitation is that the study was conducted at only one university in Serbia, so the results may not fully reflect the knowledge, attitudes, and practice at the national level. Nonetheless, the findings from this survey can serve as a valuable starting point for enhancing education on the importance of intestinal microbiota and probiotics at the university level, especially since a large number of participants expressed a need for more information on this topic during their studies.
Conclusion
The results of our research indicate a significant knowledge gap among medical and pharmacy students regarding the gut microbiota, probiotics, and their interactions with concurrently administered medications. This deficiency is also evident in their practical use of probiotics. The primary factors contributing to this gap are insufficient awareness and reliance on unreliable sources of information. Most respondents believe they do not receive adequate education on this topic at the university level. Therefore, enhancing healthcare professionals’ understanding of probiotics and their effects could significantly optimize patient care and promote overall health and well-being. This underscores the importance of ongoing education and training initiatives that equip healthcare professionals with the necessary knowledge and skills to navigate the complexities surrounding probiotic use and make informed recommendations based on scientific evidence. Such improvements would undoubtedly have a positive impact on the pharmacotherapeutic practices of future healthcare professionals.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the students of the Faculty of Medicine in Novi Sad for participating in this study.
Author contributions
MĐ: Study concept and design, collection of data, interpretation of data, drafting the manuscript and critical revision of the manuscript. NM: Collection of data, drafting the manuscript. TO: statistics, interpretation of data MK: statistics, interpretation of data. SL: study design, data analysis. MM: supervision. NP: Study concept and design, interpretation of data and critical revision of the manuscript. All authors have read and agreed to the final version of the manuscript.
Funding
This work was supported by Project for Scientific and Technological Development of Vojvodina (142-451-3522/2023-01).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was ethically approved by the Ethics Committee of the Faculty of Medicine in Novi Sad (approval number 01–39/137). At the beginning of the questionnaire, informed consent was obtained after the students were informed about the purpose of the questionnaire. No student’s personal data was collected or archived for the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stojančević M, Bojić G, Al Salami H, Mikov M. The influence of intestinal tract and probiotics on the fate of orally administered drugs. Curr Issues Mol Biol. 2014;16(1):55–68. [PubMed] [Google Scholar]
- 2.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010.90(3):859–904. [DOI] [PubMed]
- 4.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non–culture-based techniques. J Pediatr. 2010;156(1):20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-Tapia E, Sebastiani G, Sailer S, Toledano LA, Serra-Delgado M, García-Algar Ó et al. Probiotic supplementation during the perinatal and infant period: effects on gut dysbiosis and disease. Nutrients. 2020;12(8):2243. 10.3390/nu12082243. [DOI] [PMC free article] [PubMed]
- 6.Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53(4):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundgren SN, Madan JC, Karagas MR, Morrison HG, Hoen AG, Christensen BC. Microbial Communities in Human Milk Relate to Measures of Maternal Weight. Front Microbiol. 2019;10:2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallenborn JT, Vonaesch P. Intestinal microbiota research from a global perspective. Gastroenterol Rep. 2022;10:goac010. 10.1093/gastro/goac010. [DOI] [PMC free article] [PubMed]
- 9.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021;10(3):459. 10.3390/jcm10030459. [DOI] [PMC free article] [PubMed]
- 10.Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004;9(2):180–97. [PubMed] [Google Scholar]
- 11.Mikov MM, Stojančević MP, Bojić GM. Probiotics as a promising treatment for inflammatory bowel disease. Hosp Pharmacology-International Multidisciplinary J. 2014;1(1):52–60. [Google Scholar]
- 12.Jurjus A, Eid A, Al Kattar S, Zeenny MN, Gerges-Geagea A, Haydar H, et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016;5:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017;65(1):117–24. [DOI] [PubMed] [Google Scholar]
- 14.Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004;80(947):516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodke H, Jogdand S. Role of Probiotics in Human Health. Cureus. 2022;14(11):e31313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrariu OA, Barbu IC, Niculescu AG, Constantin M, Grigore GA, Cristian RE, et al. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front Microbiol. 2023;14:1296447. 10.3389/fmicb.2023.1296447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klünemann M, Andrejev S, Blasche S, Mateus A, Phapale P, Devendran S, et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021;597(7877):533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356(6344):eaag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Chen Y, Huang W, Zhou H, Zhang W. Drug-microbiota interactions: an emerging priority for precision medicine. Signal Transduct Target Ther. 2023;8(1):386. 10.1038/s41392-023-01619-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikeocha IJ, Al-Kabsi AM, Miftahussurur M, Alshawsh MA, Pharmacomicrobiomics. Influence of gut microbiota on drug and xenobiotic metabolism. FASEB J. 2022;36(6):e22350. 10.1096/fj.202101986R. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Han Y, Huang W, Jin M, Gao Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm Sin B. 2021;11(7):1789–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purdel C, Ungurianu A, Adam-Dima I, Margină D. Exploring the potential impact of probiotic use on drug metabolism and efficacy. Biomed Pharmacother. 2023;161:114468. 10.1016/j.biopha.2023.114468. [DOI] [PubMed] [Google Scholar]
- 23.Kamath S, Stringer AM, Prestidge CA, Joyce P. Targeting the gut microbiome to control drug pharmacomicrobiomics: the next frontier in oral drug delivery. Expert Opin Drug Deliv. 2023;20(10):1315–31. [DOI] [PubMed] [Google Scholar]
- 24.Đanić M, Pavlović N, Stanimirov B, Lazarević S, Vukmirović S, Al-Salami H, et al. PAMPA model of gliclazide permeability: The impact of probiotic bacteria and bile acids. Eur J Pharm sciences: official J Eur Federation Pharm Sci. 2021;158:105668. 10.1016/j.ejps.2020.105668. [DOI] [PubMed] [Google Scholar]
- 25.Đanić M, Pavlović N, Lazarević S, Stanimirov B, Vukmirović S, Al-Salami H, et al. Bioaccumulation and biotransformation of simvastatin in probiotic bacteria: a step towards better understanding of drug-bile acids-microbiome interactions. Front Pharmacol. 2023;14:1111115. 10.3389/fphar.2023.1111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djanic M, Pavlovic N, Stanimirov B, Stojancevic T, Golocorbin-Kon S, Bojic G, et al. Docking-based preliminary study on the interactions of bile acids with drugs at the transporter level in intestinal bacteria. Eur Rev Med Pharmacol Sci. 2016;20(3):553–60. [PubMed] [Google Scholar]
- 27.Babina K, Salikhova D, Polyakova M, Zaytsev A, Egiazaryan A, Novozhilova N. Knowledge and attitude towards probiotics among dental students and teachers: a cross-sectional survey. Dent J (Basel). 2023;11(5):119. 10.3390/dj11050119. [DOI] [PMC free article] [PubMed]
- 28.Wilson Z, Whitehead K. A cross sectional survey to assess healthcare professionals’ attitudes to and understanding of probiotics. Clin Nutr ESPEN. 2019;34:104–9. [DOI] [PubMed] [Google Scholar]
- 29.Hasosah M, Qurashi M, Balkhair A, Alzahrani Z, Alabbasi A, Alzahrani M, et al. Knowledge, attitudes, and understanding of probiotics among pediatricians in different regions of Saudi Arabia. BMC Med Educ. 2021;21(1):68. 10.1186/s12909-021-02499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver L, Rasmussen H, Gregoire MB, Chen Y. Health Care Provider’s Knowledge, Perceptions, and Use of Probiotics and Prebiotics. Top Clin Nutr. 2014;29(2):139–49. [Google Scholar]
- 31.Abu-Humaidan AHA, Alrawabdeh JA, Theeb LS, Hamadneh YI, Omari MB. Evaluating Knowledge of Human Microbiota among University Students in Jordan, an Online Cross-Sectional Survey. Int J Environ Res Public Health. 2021;18(24):13324. 10.3390/ijerph182413324. [DOI] [PMC free article] [PubMed]
- 32.Rahmah PA, Khairani AF, Atik N, Arisanti N, Fatimah SN. Correlation of Knowledge, Attitude, and Practice Toward Probiotics for the Digestive System Among Health Science Students. J Multidiscip Healthc. 2021;14:1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soni R, Tank K, Jain N. Knowledge, attitude and practice of health professionals about probiotic use in Ahmedabad, India. Nutr Food Sci. 2018;48(1):125–35. [Google Scholar]
- 34.Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795. 10.3390/nu13082795. [DOI] [PMC free article] [PubMed]
- 35.Salazar N, Arboleya S, Fernández-Navarro T, de Los Reyes-Gavilán CG, Gonzalez S, Gueimonde M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11(8):1765. 10.3390/nu11081765. [DOI] [PMC free article] [PubMed]
- 36.Madison AA, Bailey MT. Stressed to the Core: Inflammation and Intestinal Permeability Link Stress-Related Gut Microbiota Shifts to Mental Health Outcomes. Biol Psychiatry. 2024;95(4):339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008;93(4):236–40. [DOI] [PubMed] [Google Scholar]
- 38.Francino MP. Birth Mode-Related Differences in Gut Microbiota Colonization and Immune System Development. Ann Nutr Metab. 2018;73(Suppl 3):12–6. [DOI] [PubMed] [Google Scholar]
- 39.Al Hossan AA, Syed W, Babelghaith SD, Al Arifi MN. Knowledge, attitude, and practice of probiotics among saudi health care students-a cross-sectional study from saudi university in riyadh saudi Arabia. Inquiry. 2024;61:469580231224821. 10.1177/00469580231224821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheyholislami H, Connor KL. Are probiotics and prebiotics safe for use during pregnancy and lactation? a systematic review and meta-analysis. Nutrients. 2021;13(7):2382. 10.3390/nu13072382. [DOI] [PMC free article] [PubMed]
- 41.Liu AT, Chen S, Jena PK, Sheng L, Hu Y, Wan YY. Probiotics improve gastrointestinal function and life quality in pregnancy. Nutrients. 2021;13(11). 10.3390/nu13113931. [DOI] [PMC free article] [PubMed]
- 42.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(Suppl 2):S291–4. [DOI] [PubMed] [Google Scholar]
- 43.Hojsak I, Fabiano V, Pop TL, Goulet O, Zuccotti GV, Çokuğraş FC, et al. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018;107(6):927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Zhou B, Zhou X, Wang Y, Wang H, Jia S, et al. Combined Lowering Effects of Rosuvastatin and L. acidophilus on Cholesterol Levels in Rat. J Microbiol Biotechnol. 2019;29(3):473–81. [DOI] [PubMed] [Google Scholar]
- 45.van de Wijgert J, Verwijs MC. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG. 2020;127(2):287–99. [DOI] [PubMed] [Google Scholar]
- 46.Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Experimental Allergy. 2000;30(11):1605–10. [DOI] [PubMed] [Google Scholar]
- 47.Haukioja A. Probiotics and oral health. Eur J Dent. 2010;4(3):348–55. [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarević S, Đanic M, Al-Salami H, Mooranian A, Mikov M. Gut microbiota metabolism of azathioprine: a new hallmark for personalized drug-targeted therapy of chronic inflammatory bowel disease. Front Pharmacol. 2022;13:879170. 10.3389/fphar.2022.879170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikov M, Đanić M, Pavlović N, Stanimirov B, Goločorbin-Kon S, Stankov K, Al-Salami H. Potential applications of gliclazide in treating type 1 diabetes mellitus: formulation with bile acids and probiotics. Eur J Drug Metab Pharmacokinet. 2018;43(3):269–80. [DOI] [PubMed] [Google Scholar]
- 50.Đanić M, Mikov M. Biotransformation of xenobiotics in living systems—metabolism of drugs: Partnership of liver and gut microflora. Pharmaceutical biocatalysis: Jenny Stanford Publishing; 2020. pp. 129–66. [Google Scholar]
- 51.Dhurjad P, Dhavaliker C, Gupta K, Sonti R. Exploring Drug Metabolism by the Gut Microbiota: Modes of Metabolism and Experimental Approaches. Drug Metab Dispos. 2022;50(3):224–34. [DOI] [PubMed] [Google Scholar]
- 52.Sun C, Chen L, Shen Z. Mechanisms of gastrointestinal microflora on drug metabolism in clinical practice. Saudi Pharm J. 2019;27(8):1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181(3):555–62. [PubMed] [Google Scholar]
- 54.Lindenbaum J, Rund DG, Butler VP Jr., Tse-Eng D, Saha JR. Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. N Engl J Med. 1981;305(14):789–94. [DOI] [PubMed] [Google Scholar]
- 55.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5:14554. 10.1038/srep14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ðanić M, Stanimirov B, Pavlović N, Vukmirović S, Lazić J, Al-Salami H, et al. Transport and biotransformation of gliclazide and the effect of deoxycholic acid in a probiotic bacteria model. Front Pharmacol. 2019;10:1083. 10.3389/fphar.2019.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saputri FA, Kang D, Kusuma ASW, Rusdiana T, Hasanah AN, Mutakin, et al. Lactobacillus plantarum IS-10506 probiotic administration increases amlodipine absorption in a rabbit model. J Int Med Res. 2018;46(12):5004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet. 2008;33(2):101–6. [DOI] [PubMed] [Google Scholar]
- 60.Matuskova Z, Anzenbacherova E, Vecera R, Tlaskalova-Hogenova H, Kolar M, Anzenbacher P. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amiodarone absorption in rats. PLoS ONE. 2014;9(2):e87150. 10.1371/journal.pone.0087150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma R, Gupta S, Gupta D, Kushwaha PK. Awareness and knowledge about probiotics among college students. J Pure Appl Microbiol. 2019;13(4):2201–8. [Google Scholar]
- 62.Boyanova L, Mitov I. Coadministration of probiotics with antibiotics: why, when and for how long? Expert Rev Anti Infect Ther. 2012;10(4):407–9. [DOI] [PubMed] [Google Scholar]
- 63.Kerna N, Brown T. A complementary medicine approach to augmenting antibiotic therapy: current practices in the use of probiotics during antibiotic therapy. Int J Complement Alt Med. 2018;11(2):62–6. [Google Scholar]
- 64.Tompkins TA, Mainville I, Arcand Y. The impact of meals on a probiotic during transit through a model of the human upper gastrointestinal tract. Benef Microbes. 2011;2(4):295–303. [DOI] [PubMed] [Google Scholar]
- 65.Bernatek M, Żukiewicz-Sobczak W, Lachowicz-Wiśniewska S, Piątek J. Factors determining effective probiotic activity: evaluation of survival and antibacterial activity of selected probiotic products using an in vitro study. Nutrients. 2022;14(16):3323. 10.3390/nu14163323. [DOI] [PMC free article] [PubMed]
- 66.Basar-Gunes H, Bayraktar-Ekincioglu A, Karakan T, Demirkan K, Bayraktar-Ekincioglu A. Assessment of knowledge and attitudes of physicians and pharmacists on probiotics: a cross-sectional survey. 2024;21(1):36–41. [DOI] [PMC free article] [PubMed]
- 67.Fijan S, Frauwallner A, Varga L, Langerholc T, Rogelj I, Lorber M et al. Health professionals’ knowledge of probiotics: an international survey. Int J Environ Res Public Health. 2019;16(17):3128. 10.3390/ijerph16173128. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.