Abstract
Objective
To build and validate a periprostatic fat magnetic resonance imaging (MRI) based radiomics nomogram for prediction of biochemical recurrence-free survival (bRFS) of patients with non-metastatic prostate cancer (PCa) receiving radical prostatectomy (RP).
Methods
A retrospective study was conducted on 356 patients with non-metastatic PCa who underwent preoperative mpMRI followed by RP treatment at our institution. Radiomic features were extracted from both intratumoral region and the periprostatic fat region, which were segmented on images obtained through T2-weighted imaging (T2WI) and apparent-diffusion coefficient (ADC) imaging. Three radiomics models were developed by applying the Least absolute shrinkage and selection operator (LASSO) Cox regression, followed by Cox risk regression to construct a combined radiomics-clinical model by integrating the optimal radiomics score and clinicopathological risk factors to draw a nomogram. The predictive performance was evaluated using receiver operating characteristic (ROC) curves, Kaplan-Meier analysis and calibration curves.
Results
One hundred and twenty-one patients (33.98%) experienced biochemical recurrence. ROC analyses showed that the Area Under the Curve (AUC) of the periprostatic fat-intratumoral radiomics model demonstrated the highest AUC at 0.921 (95%CI, 0.857–0.981), 0.875 (95%CI, 0.763–0.950), 0.854 (95%CI, 0.706–0.923) for 1-year, 3-years and 5-years bRFS. Multivariate Cox regression analysis revealed that Pathological T stage, ISUP grading group and Positive surgical margin were independent prognostic factors for predicting bRFS. A radiomics-clinical nomogram based on these clinical predictors and periprostatic fat-intratumoral radiomics score was constructed. Kaplan-Meier analyses showed that radiomics-clinical nomogram was significantly related with survival of PCa (P < 0.001); and calibration curves revealed the predicted and observed survival probability of 1-year, 3-year and 5-year bRFS had high degree of consistency in the training and validation group. The radiomics-clinical nomogram showed a significant improvement than the clinical model for 1-year (AUC, 0.944; 95%CI, 0.912–0.990 vs. AUC, 0.839; 95%CI, 0.661–0.928; P = 0.009), 3-year (AUC, 0.864; 95%CI, 0.772–0.969 vs. AUC, 0.776; 95%CI, 0.602–0.872; P = 0.008), and 5-year bRFS (AUC, 0.907; 95%CI, 0.836–0.982 vs. AUC, 0.819; 95%CI, 0.687–0.915; P = 0.027).
Conclusions
This study developed and validated the radiomics-clinical nomogram for the prediction of bRFS in non-metastatic PCa patients underwent RP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13207-4.
Keywords: Prostate cancer, Radiomics, Periprostatic fat, Biochemical recurrence-free survival, Nomogram
Introduction
Prostate cancer (PCa) is globally recognized as one of the most prevalent malignancies of the genitourinary system in males, with its morbidity in men rising to second place, being the sixth leading cause of death [1]. Radical prostatectomy (RP) is regarded as the primary treatment option for non-metastatic PCa [2]. Nevertheless, studies have shown that approximately 27–53% of patients receiving RP experienced biochemical recurrence (BCR) [3]. Patients who experience BCR are at an increased likelihood of developing clinical metastases and facing a higher risk of poor prognosis [4]. Therefore, it is crucial to accurately recognize BCR for subsequent decision-making regarding PCa patients.
Radiomics is an extensively studied method in various tumors, involving the extraction of quantitative features from images to estimate clinical events [5, 6]. Previous researches have demonstrated the great value of radiomic features obtained from multiparametric magnetic resonance imaging (mpMRI) in PCa detection, Gleason Score discrimination, and treatment response assessment [7, 8]. Woźnicki et al. [9]. found that ensemble models combining radiomics, Prostate Imaging-Reporting and Data System, prostate-specific antigen density, and digital rectal examination outperformed experienced radiologist assessments for PCa characterization and clinically significant PCa detection. Wang et al. [10]. revealed that mpMRI radiomics features were crucial for predicting bone metastases in PCa patients, and combining clinical characteristics with MRI-based radiomics features could significantly enhance the accuracy of prediction [10].
Previous radiomics studies of PCa have primarily concentrated on the intratumoral region [9–12], leaving the role of radiomics features derived from periprostatic fat tissue relatively unexplored. The prostate gland is surrounded by periprostatic fat tissue, and several studies have shown the tight relationship between periprostatic fat and PCa progression [13–15]. A series of metabolic changes and bidirectional crosstalk of many molecules exist between tumor cells and adipocytes [16]. It has been reported that pro-inflammatory adipokines, tumor necrosis factor (TNF)-α, and C-C motif chemokine ligand 7 (CCL7) secreted by periprostatic fat tissue can promote PCa cell proliferation, migration, and invasion. Coy et al. [17]. discovered that patients with high-risk PCa exhibited alterations in the lipid composition of periprostatic fat, which influenced tumor cell processes by affecting PCa cell lipid metabolism. The interactions between PCa cells and periprostatic fat cells can induce phenotypical changes in adipose tissue, which can manifest as subtle alterations on MRI that may not be discernible to the naked eye. These alterations may contain key features associated with the drug resistance, and progress of the disease.
However, to our knowledge, there was no study exploring the correlation between MRI radiomics features derived from periprostatic fat region and the BCR or biochemical recurrence-free survival (bRFS) of PCa patients receiving RP treatment. Therefore, this study retrospectively collected clinical information and MRI imaging data from 356 PCa patients receiving RP treatment, and extracted radiomics features from periprostatic fat and intratumoral region to assess the value of periprostatic fat radiomics features in predicting bRFS.
Materials and methods
Patients and data collection
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University and written informed consent was provided by all patients. We conducted a retrospective analysis of 356 non-metastatic PCa patients who underwent pre-operative prostate mpMRI and RP in the First Affiliated Hospital of Fujian Medical University between January 2013 and August 2020. Tumors were staged following the American Joint Committee on Cancer/ Union for International Cancer Control (AJCC/UICC) Tumor, Node, Metastasis (TNM) classification system 2017 Edition. The absence of distant metastasis was confirmed through pre-treatment mpMRI, Computed Tomography (CT), bone scanning, or Prostate-specific membrane antigen ligand positron emission tomography/CT (PSMA PET/CT).
The inclusion criteria were as follows: (1) pathologically diagnosed with PCa; (2) prostate mpMRI was performed before RP. The exclusion criteria were as follows: (1) were without T2 weighted images (T2WI) or Apparent Diffusion Coefficient (ADC) images [n = 63]; (2) received prior additional therapies (radiotherapy, chemotherapy, endocrine) before RP [n = 126]; (3) were with prostate specific antigen (PSA) persistence[n = 28]; (4) incomplete clinicopathologic data [n = 17]; (5) MRI images were of insufficient quality [n = 34]; (6) the follow up information did not include postoperative serum PSA measurements [n = 19]. Eventually, a total of 356 non-metastatic PCa patients with complete clinicopathologic data were enrolled and randomly divided into training/validation sets at a ratio of 7:3 (treated between 2013 and 2016; 118 cases in the training group and 53 cases in the validation group, respectively), and testing group (treated between 2017 and 2020; n = 185). The comparison of clinicopathological characteristics between included and excluded patients is shown in Supplementary Table 1.
The clinicopathological date of patients were collected for analysis, including age, body mass index (BMI), International Society of Urological Pathology (ISUP) Gleason grading group, pathological T stage, initial Prostate-specific antigen (PSA) at diagnosis, lymph nodes metastasis, peri-prostatic fat involvement, perineal invasion, lymphovascular invasion and positive surgical margin.
Definition of biochemical recurrence, post-operative PSA follow-up protocol
According to the European Association of Urology (EAU) Guidelines of 2021 edition, BCR is determined by the presence of two consecutive serum prostate-specific antigen (PSA) measurements exceeding 0.2 ng/mL, bRFS refers to the period between the date of RP and the point of BCR. Undetectable PSA is defined as a serum PSA level of less than 0.1 ng/mL within 4 to 8 weeks of surgery [18]. Patients underwent serum PSA testing at 6–8 weeks post-surgery, followed by every 3 months for the first year, every 6 months for the second year, and annually thereafter. Routine follow-up for patients was conducted through various means, including In-person visits, telephone communication.
Examination procedure of mpMRI
All patients were imaged on 3 Tesla (3T) MRI scanners (Siemens Healthcare, Erlangen, Germany, Verio 3.0T) with pelvic phased-array coil within two weeks before RP. A supine position was adopted for the patient, and a comprehensive scan of the entire prostate was executed with the scanning range centered on the central part of the prostate. The patient was asked to have a bowel movement before the examination and ensure moderate bladder filling. The scanning sequences included transverse, sagittal, and frontal T2WI images, diffusion-weighted imaging (DWI) images, and corresponding ADC maps. The detailed mpMRI sequences parameters were presented in Supplementary Table 2. The gadopentetate dimeglumine (Gd-DTPA) was administered via a pressure injector into the dorsal hand vein at a flow rate of 3 mL/s. A total of 18 sequential scans were performed, with each individual scan lasting for 11 s.
Delineation of regions of interest
Two experienced radiologists with more than 8 years of expertise in prostate mpMRI interpretation, blinded to each other’s delineations and clinical data, independently outlined the regions of interest (ROI) for intratumoral and periprostatic fat areas in T2WI and ADC images using ITK-SNAP software version 3.6.0. (Yushkevich P and Gerig G). The periprostatic fat region includes the neurovascular bundles, where the seminal vesicle and metastatic lymph nodes excluded. The intratumoral and periprostatic fat ROI were showed in Supplementary Fig. 1 and Supplementary Fig. 2. To select robust features, fifty patients were randomly selected to conduct a test-retest study. The inter-observer repeatability of the extracted features between two radiologists was assessed, and the intra-observer repeatability was assessed by comparing the extracted features of the same radiologist (twice, one week apart).
Radiomics features extraction and selection
Radiomics features were extracted using Python (version 3.7.3) package PyRadiomics version 3.0. Radiomic features were extracted from original images as well as two basic image filters, namely wavelet and Laplacian of Gaussian (LoG) images. The extracted features included: (1) shape features; (2) first-order statistical features; (3) gray-level run length matrix (GLRLM) features; (4) gray-level co-occurrence matrix (GLCM) features; (5) gray-level dependence matrix (GLDM) features; (6) gray-level size zone matrix (GLSZM) features; (7) neighboring gray tone difference matrix (NGTDM) features.
To remove scale variations and ensure comparability, all features underwent normalization using the Z-Score transform. Afterward, characteristics displaying limited repeatability were eliminated from subsequent examination. Inter-observer and intra-observer repeatability were quantified by employing the infraclass correlation coefficient (ICC). Features with ICC greater than 0.8 were chosen for further analysis. In order to select the features with the highest correlation and minimum redundancy, radiomics features from intratumoral and periprostatic fat ROIs were ranked by using the maximum relevance minimum redundancy (mRMR) algorithm respectively.
Radiomics scores, clinical model, nomogram construction and validation
In the training group, the Least absolute shrinkage and selection operator (LASSO) Cox regression was used to determine three radiomics scores based on the selected radiomics features: (1) periprostatic fat radiomics score; (2) intratumoral radiomics score; (3) combined periprostatic fat-intratumoral radiomics score. The radiomics score with the highest area under the receiver operating characteristic (ROC) was selected for further exploration. Significant clinical features were selected through univariable and multivariable Cox risk regression analyses for clinical model building. And the combined radiomics-clinical model was constructed with both significant clinical parameters and optimal radiomics score using multivariable Cox risk regression analysis. Finally, according to the combined radiomics-clinical model, the nomogram for predicting bRFS was constructed to achieve a more intuitive purpose of predicting survival.
Kaplan-Meier analysis was conducted to assess the survival prediction performance of the radiomics scores, clinical model, and nomogram in both the training and validation groups. ROC curves were plotted to evaluate the diagnostic performance of these models. Additionally, calibration curves were generated to evaluate the calibration performance of the nomogram.
Statistical analysis
Statistical analyses were conducted using SPSS version 26 (IBM SPSS, Inc., Armonk, NY), and R software (R Foundation for Statistical Computing, Vienna, Austria version 4.1.0). Categorical variables were compared using the chi-square test or Fisher’s exact test, while continuous variables were assessed using the independent t-test or Mann-Whitney U test. The ICC was calculated with R package “psych”. The mRMR analysis was carried out by the “mRMRe” package. The LASSO Cox regression was conducted with R package “glmnet”; Univariable and multivariable Cox regression analyses, as well as Kaplan-Meier analysis, were conducted using the “survival” and “survminer” packages.; Calculating the AUC (95% confidence interval (CI)) and comparing different ROC curves were performed by R package “timeROC”. Nomograms and calibration curves were created using the “rms” and “survival” packages. A two-sided P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
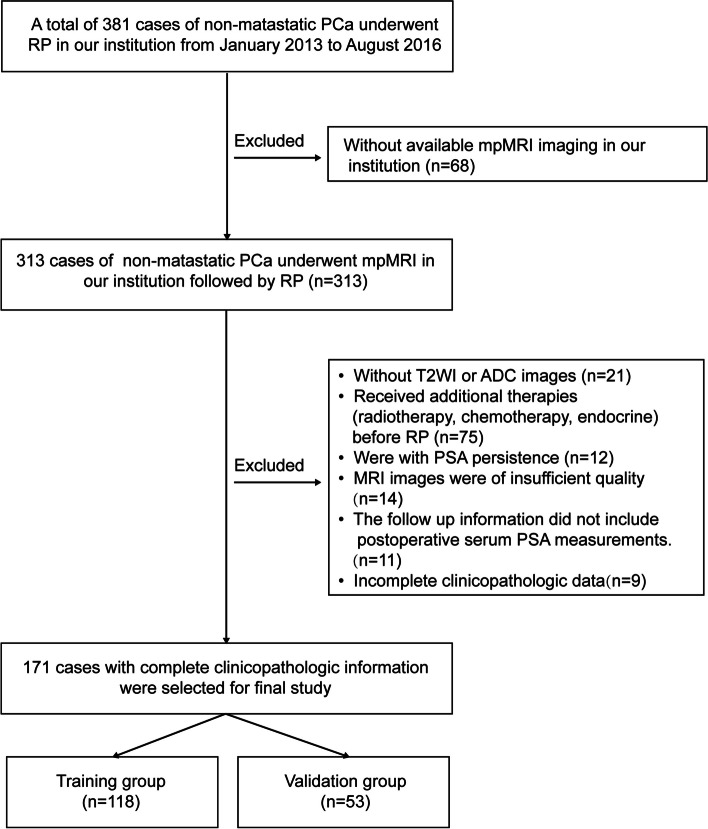
The flow diagram of this study is presented in Fig. 1. The baseline clinical characteristics information of 356 non-metastatic PCa patients were listed in Table 1. The mean (standard deviation (SD)) age of all patients was 69.19 (6.46) years. The Initial PSA at diagnosis of all patients was 21.57 (6.20–97.04) ng/ml. The number of patients with ISUP group ≤ 3 was 194 (54.49%), and the number of patients with ISUP group > 3 was 162 (45.51%). There were no significant differences observed in Age, BMI, initial PSA level, ISUP grading group, Lymph nodes metastasis, Positive surgical margin, Peri-prostatic fat involvement, Perineal invasion, Lymphovascular invasion as well as BCR rates between the training group, validation group, and testing group.
Fig. 1.
Flow diagram of this study
Table 1.
Baseline characteristics of 356 PCa patients
| Patient Characteristics | Training group (n = 118) | Validation group (n = 53) | Testing group (n = 185) | P value |
|---|---|---|---|---|
| Age at diagnosis (years) | 68.53 ± 5.93 | 68.36 ± 6.44 | 69.87 ± 6.76 | 0.868a/0.078b/0.148c |
| BMI (kg/m2) | 23.86 ± 2.69 | 23.06 ± 2.62 | 23.83 ± 4.01 | 0.071a/0.962b/0.185c |
| Initial PSA at diagnosis (ng/ml) | 21.24 (8.09–97.04) | 20.86 (6.20–48.87) | 21.43 (6.46–54.67) | 0.412a/0.074b/0.616c |
| Pathological T stage | 0.001a/0.001b/0.291c | |||
| ≤ pT2c | 81 (68.64%) | 22 (41.51%) | 92 (49.72%) | |
| ≥ pT3 | 37 (31.36%) | 31 (58.49%) | 93 (50.27%) | |
| ISUP grading group of surgical specimens | 0.872a/0.766b/0.957c | |||
| ≤ 3 | 63 (53.39%) | 29 (54.72%) | 102 (55.13%) | |
| >3 | 55 (46.61%) | 24 (45.28%) | 83 (44.97%) | |
| Lymph nodes metastasis | 0.281a/0.603b/0.109c | |||
| Yes | 13 (11.00%) | 9 (17.00%) | 17 (9.21%) | |
| No | 105 (89.00%) | 44 (83.00%) | 168 (90.89%) | |
| Positive surgical margin | 0.775a/0.486b/0.410c | |||
| Yes | 45 (38.14%) | 19 (35.85%) | 78 (42.17%) | |
| No | 73 (61.87%) | 34 (64.15%) | 107 (53.83%) | |
| Peri-prostatic fat involvement | 0.646a/0.726b/0.850c | |||
| Yes | 3 (2.54%) | 2 (3.77%) | 6 (3.25%) | |
| No | 115 (97.46%) | 51 (96.23%) | 179 (96.75%) | |
| Perineal invasion | 0.981a/0.677b/0.773c | |||
| Yes | 11 (9.32%) | 5 (9.43%) | 20 (11.85%) | |
| No | 107 (90.68%) | 48 (90.57%) | 165 (89.15%) | |
| Lymphovascular invasion | 0.686a/0.704b/0.484c | |||
| Yes | 16 (13.56%) | 6 (11.32%) | 27 (15.13%) | |
| No | 102 (86.44%) | 47 (88.68%) | 158 (84.86%) | |
| Biochemical recurrence | 0.160a/0.521b/0.318c | |||
| Yes | 36 (30.51%) | 22 (41.51%) | 63 (34.05%) | |
| No | 82 (69.49%) | 31 (58.49%) | 122 (65.95%) | |
| Median Follow-up (months) | 52.01 (44.61–59.41) | 53 (47.07–59.22) | 49.44 (44.16–54.84) |
PCa Prostate cancer, BMI Body mass index, PSA Prostate-specific antigen, ISUP International Society of Urological Pathology
aTraining group vs. Validation group
bTraining group vs. Testing group
cValidation group vs. Testing group
The median follow-up duration in the study was 51.07 months, ranging from 46.48 to 55.67 months. The number of patients who experienced BCR was 121 (33.98%). During the follow-up of the 121 patients who experienced BCR, eleven (9.09%) patients developed local recurrences. Of these, eight patients received salvage radiotherapy, and 3 patient received salvage radiotherapy combined with androgen deprivation therapy (ADT) (leuprorelin, 3.75 mg every four weeks). Additionally, fifteen (12.40%) patients developed distant metastases. Of these, ten patients were treated with ADT combined with abiraterone acetate (1000 mg daily) plus prednisone (5 mg daily) or enzalutamide (160 mg daily), and 5 patients were treated with ADT combined with chemotherapy (docetaxel, dose of 75 mg/sqm every three weeks, six cycles).
Identification of periprostatic fat and intratumoral radiomics feature
We extracted 2632 radiomics features form the periprostatic fat ROI (1316 radiomics features from ADC and 1316 radiomics features from T2WI) and intratumoral ROI (1316 radiomics features from ADC and 1316 radiomics features from T2WI), respectively. After ICC analysis, the count of periprostatic fat features decreased to 1937 (899 features from ADC and 1038 features from T2WI), while the count of intratumoral features decreased to 1831 (932 features from ADC and 899 features from T2WI). The remaining features were ranked by the mRMR algorithm to retain the top 40 periprostatic fat and intratumoral radiomics features for the construction of radiomic scores (Supplementary Table 3).
Construction and validation of radiomics
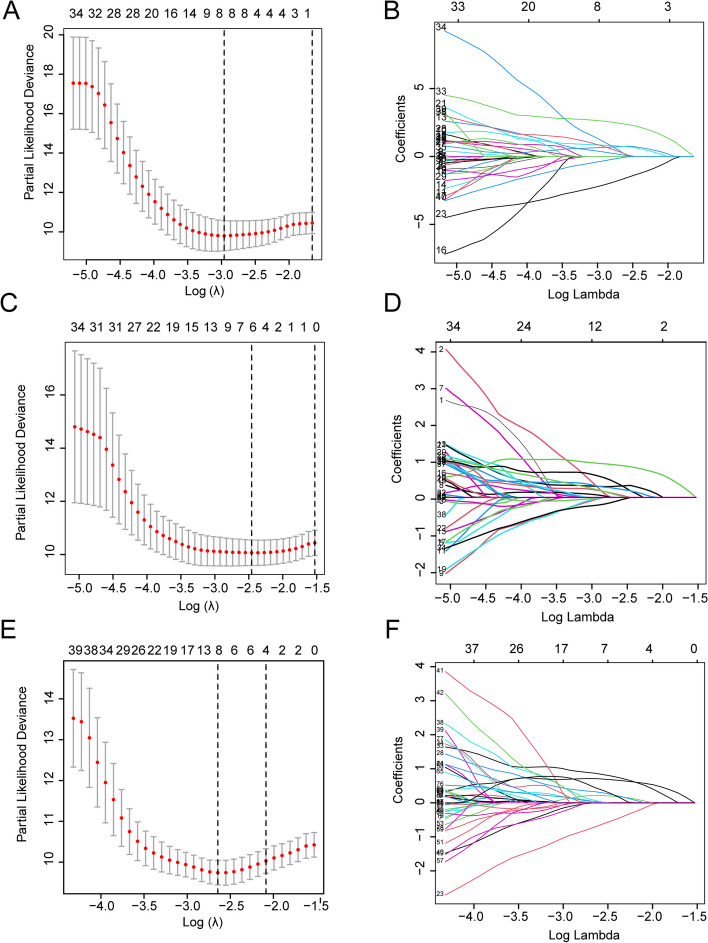
By employing LASSO Cox regression analysis, we identified eight radiomics features for constructing the periprostatic fat radiomics score, six features for constructing the intratumoral radiomics score, and another eight features for constructing the periprostatic fat-intratumoral radiomics score (Table 2; Fig. 2). The formula for the radiomics scores was as following.
Table 2.
LASSO COX regression analyses constructing three radiomics scores for predicting bRFS in patient with non-metastatic PCa receiving RP treatment
| ROIs | MRI sequences | Group and filters | Feature class | Feature | Coef |
|---|---|---|---|---|---|
| Periprostatic fat radiomics features | |||||
| Periprostatic fat | ADC | Wavelet (HLL) | GLSZM | Large Area High Gray Level Emphasis | 0.349 |
| T2WI | Wavelet (HLL) | NGTDM | Contrast | −2.132 | |
| ADC | Wavelet (HHH) | GLSZM | Small Area Emphasis | 0.661 | |
| T2WI | Original | Shape | Major Axis Length | 0.839 | |
| ADC | LoG (sigma = 3.0.mm 3D) | GLCM | Sum Squares | 2.613 | |
| ADC | Wavelet (HHH) | GLCM | Idn | 1.160 | |
| T2WI | Wavelet (LHH) | GLSZM | Zone Variance | 0.777 | |
| T2WI | Wavelet (HLH) | GLSZM | Large Area Emphasis | −0.343 | |
| Intratumoral radiomics features | |||||
| Intratumoral region | ADC | wavelet (LHH) | First order | Interquartile Range | 0.837 |
| ADC | Wavelet (HLL) | First order | Skewness | 0.000 | |
| ADC | Wavelet (HHL) | GLCM | Joint Energy | 0.328 | |
| ADC | LoG (sigma = 5.0.mm 3D) | GLRLM | Run Length Non Uniformity Normalized | 0.183 | |
| ADC | LoG (sigma = 2.0.mm 3D) | GLCM | Sum Entropy | 0.180 | |
| T2WI | Wavelet (LLL) | GLCM | Idn | −0.002 | |
| Periprostatic fat-intratumoral radiomics features | |||||
| Periprostatic fat | T2WI | Wavelet (HLL) | NGTDM | Contrast | −0.708 |
| Periprostatic fat | ADC | wavelet (HHH) | GLSZM | Small Area Emphasis | 0.215 |
| Periprostatic fat | T2WI | Original | Shape | Major Axis Length | 0.327 |
| Periprostatic fat | ADC | LoG (sigma = 3.0.mm 3D) | GLCM | Sum Squares | 0.869 |
| Periprostatic fat | T2WI | Wavelet (LHH) | GLSZM | Zone Variance | 0.072 |
| Intratumoral region | ADC | Wavelet (LHH) | First order | Interquartile Range | 0.713 |
| Intratumoral region | ADC | LoG (sigma = 5.0.mm 3D) | GLRLM | Run Length Non Uniformity Normalized | 0.448 |
| Intratumoral region | ADC | LoG (sigma = 3.0.mm 3D) | GLCM | Imc2 | 0.035 |
LASSO The Least absolute shrinkage and selection operator, bRFS biochemical recurrence-free survival, PCa Prostate cancer, RP radical prostatectomy, ROIs Regions of interests, MRI Magnetic resonance imaging, Coef Coefficient, ADC Apparent-diffusion coefficient, T2WI T2-weighted imaging, GLSZM Gray-level size zone matrix, NGTDM neighboring gray tone difference matrix, GLCM Gray-level co-occurrence matrix, GLRLM Gray-level run length matrix, LoG Laplacian of Gaussian
Fig. 2.
LASSO Cox regression analyses of three radiomics models. A, C, E The partial likelihood deviance was plotted versus log (λ). The y-axis indicates the partial likelihood deviance, while the lower x-axis indicates the log (λ) and the upper x-axis represents the average number of predictors. Dotted vertical lines were drawn at the optimal values using the minimum criteria and 1 standard error of the minimum criteria. The tuning parameter (λ) was selected in the LASSO model via 10-fold cross-validation based on minimum criteria. A The lambda.min used in the LASSO algorithm for the periprostatic fat radiomics score is 0.05181153; B The lambda.min used in the LASSO algorithm for the intratumoral radiomics score is 0.08555169; C The lambda.min used in the LASSO algorithm for the periprostatic fat-intratumoral radiomics score is 0.07075123. The coefficients (y-axis) were plotted against log (lambda) and (B) 8 features with nonzero coefficients were selected to build periprostatic fat radiomics model, D 6 features with nonzero coefficients were selected to build intratumoral radiomics model, F and 8 features with nonzero coefficients were selected to build periprostatic fat-intratumoral radiomics model. LASSO, the Least absolute shrinkage and selection operator
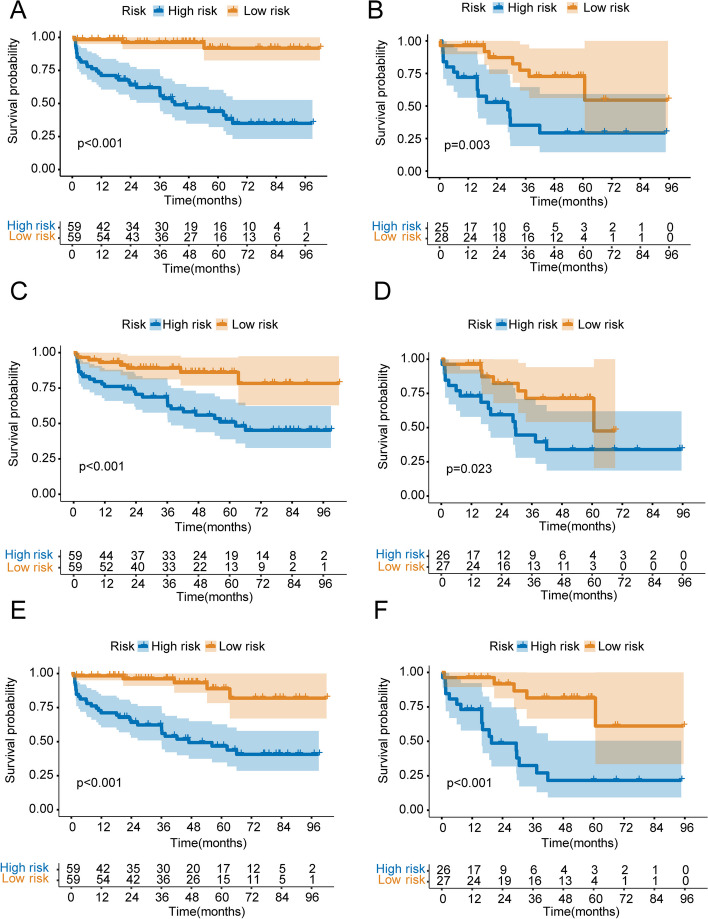
The risk score of each patient was calculated based on radiomics scores. All patients were classified into high-risk and low-risk groups according to the median risk score. Kaplan-Meier analysis conducted in training and validation groups were illustrated in Fig. 3. Survival analysis demonstrated that the bRFS of patients with high-risk was significantly decreased compared with that of patients with low-risk.
Fig. 3.
Kaplan-Meier curve analyses of three radiomics models. A periprostatic fat radiomics model in the training group. B periprostatic fat radiomics model in the validation group. C intratumoral radiomics model in the training group. D intratumoral radiomics model in the validation group. I periprostatic fat-intratumoral radiomics model in the training group. F periprostatic fat-intratumoral radiomics model in the validation group
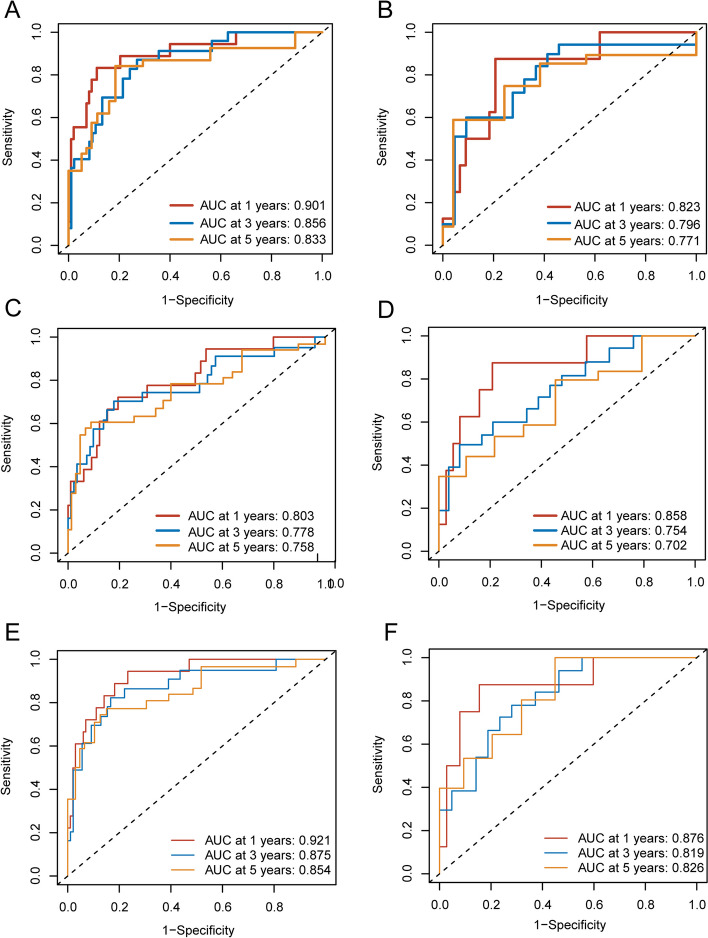
The ROC curves of the three models in the training and validation groups were shown in Fig. 4. The AUCs of the periprostatic fat radiomics model in the training group were 0.901 (95%CI, 0.817–0.982), 0.856 (95%CI, 0.741–0.923), 0.833 (95%CI, 0.687–0.921) at 1-year, 3-years and 5-years bRFS in training cohort (Fig. 4A), and the AUCs in the validation group were 0.823 (95%CI, 0.663–0.965), 0.796 (95%CI, 0.656–0.943), 0.771 (95%CI, 0.642–0.905) at 1-year, 3-years and 5-years bRFS (Fig. 4B). The AUCs of the intratumoral radiomics model in the training group were 0.803 (95%CI, 0.640–0.889), 0.778 (95%CI, 0.606–0.865), 0.758 (95%CI, 0.600–0.827) at 1-year, 3-years and 5-years (Fig. 4C), and the AUCs in the validation group were 0.858 (0.731–0.961), 0.754 (95%CI, 0.624–0.895), 0.702 (95%CI, 0.610–0.863) at 1-year, 3-years and 5-years (Fig. 4D). The AUCs of the periprostatic fat-intratumoral radiomics model in the training group were 0.921 (95%CI, 0.857–0.981), 0.875 (95%CI, 0.763–0.950), 0.854 (95%CI, 0.706–0.923) at 1-year, 3-years and 5-years (Fig. 4E), and the AUCs in the validation group were 0.858 (95%CI, 0.731–0.961), 0.754 (95%CI, 0.624–0.895), 0.702 (95%CI, 0.610–0.863) for 1-year, 3-years and 5-years bRFS (Fig. 4F).
Fig. 4.
ROC curve analyses of one-year, three-years, five-years for bRFS of three radiomics models. A periprostatic fat radiomics model in the training group. B periprostatic fat radiomics model in the validation group. C intratumoral radiomics model in the training group. D intratumoral radiomics model in the validation group. I periprostatic fat-intratumoral radiomics model in the training group. F periprostatic fat-intratumoral radiomics model in the validation group. ROC, the receiver operating characteristic. bRFS, biochemical recurrence-free survival
The performance of each radiomics score was compared and summarized in Table 3. The periprostatic fat-intratumoral radiomics score achieved the highest AUC values for 1-year (AUC: 0.921, 95% CI: 0.857–0.981), 3-year (AUC: 0.875, 95% CI: 0.763–0.950), and 5-year (AUC: 0.854, 95% CI: 0.706–0.923) bRFS. The periprostatic fat-intratumoral radiomics score showed a significant improvement in predicting 1-year (AUC: 0.803; 95%CI: 0.640–0.889; P = 0.010) and 3-year bRFS (AUC: 0.778; 95%CI: 0.606–0.865; P = 0.017) compared to the intratumoral radiomics score. Whereas periprostatic fat radiomics model and intratumoral radiomics model demonstrated similar performance for 1-year (AUC: 0.901, 95% CI: 0.817–0.982; P = 0.051), 3-year (AUC: 0.856, 95% CI: 0.741–0.923; P = 0.136), and 5-year (AUC: 0.833, 95% CI: 0.687–0.921; P = 0.241) bRFS. Therefore, the periprostatic fat-intratumoral radiomics model was identified for further analysis.
Table 3.
Comparing ROC curves of three radiomics scores
| Radiomics scores | Training group (n = 118) | Validation group (n = 53) | ||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | P value | AUC (95% CI) | P value | |||
| 1-Years bRFS | ||||||
| Periprostatic fat-intratumoral vs. Intratumoral | 0.921 (0.857–0.981) | 0.803 (0.640–0.889) | 0.010 | 0.876 (0.764–0.980) | 0.858 (0.731–0.961) | 0.467 |
| Periprostatic fat-intratumoral vs. Periprostatic fat | 0.921 (0.857–0.981) | 0.901 (0.817–0.982) | 0.413 | 0.876 (0.764–0.980) | 0.823 (0.663–0.965 | 0.478 |
| Periprostatic fat vs. Intratumoral | 0.901 (0.817–0.982) | 0.803 (0.640–0.889) | 0.051 | 0.823 (0.663–0.965) | 0.858 (0.731–0.961) | 0.595 |
| 3-Years bRFS | ||||||
| Periprostatic fat-intratumoral vs. Intratumoral | 0.875 (0.763–0.950) | 0.778 (0.606–0.865) | 0.017 | 0.819 (0.696–0.931) | 0.754 (0.624–0.895) | 0.073 |
| Periprostatic fat-intratumoral vs. Periprostatic fat | 0.875 (0.763–0.950) | 0.856 (0.741–0.923) | 0.359 | 0.819 (0.696–0.931) | 0.796 (0.656–0.943) | 0.768 |
| Periprostatic fat vs. Intratumoral | 0.856 (0.741–0.923) | 0.778 (0.606–0.865) | 0.136 | 0.796 (0.656–0.943) | 0.754 (0.624–0.895) | 0.533 |
| 5-Years bRFS | ||||||
| Periprostatic fat-intratumoral vs. Intratumoral | 0.854 (0.706–0.923) | 0.758 (0.600–0.827) | 0.062 | 0.826 (0.710–0.935) | 0.702 (0.610–0.863) | 0.021 |
| Periprostatic fat-intratumoral vs. Periprostatic fat | 0.854 (0.706–0.923) | 0.833 (0.687–0.921) | 0.787 | 0.826 (0.710–0.935) | 0.771 (0.642–0.905) | 0.273 |
| Periprostatic fat vs. Intratumoral | 0.833 (0.687–0.921) | 0.758 (0.600–0.827) | 0.214 | 0.771 (0.642–0.905) | 0.702 (0.610–0.863) | 0.867 |
ROC Receiver operating characteristic, bRFS biochemical recurrence-free survival, AUC Area Under Curve, CI Confidence interval
Construction and validation of clinical model
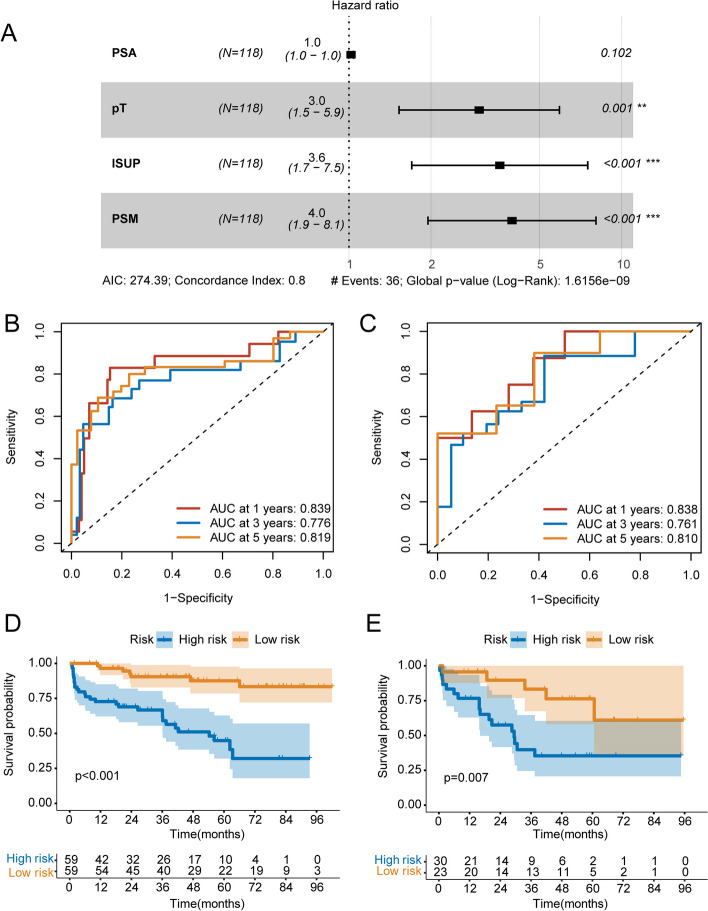
Univariable Cox regression analysis revealed that initial PSA at diagnosis (P = 0.05; HR = 1.024; 95%CI, 1.007–1.042), pathological T stage (P = 0.002; HR = 2.780; 95%CI, 1.438–5.374), ISUP grading group of surgical specimens (P < 0.001; HR = 3.466; 95%CI, 1.699–7.069), positive surgical margin (P < 0.001; HR = 4.401; 95%CI, 2.215–8.744) were associated with bRFS of PCa patients receiving RP treatment (Table 4). A clinical model was constructed using multivariate Cox regression analysis based on the results obtained from univariable Cox regression analysis (Fig. 5A).
Table 4.
Univariate and multivariate Cox regression analyses exploring prognostic factors for predicting bRFS in patients with non-metastatic PCa receiving RP treatment
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis | 1.017 (0.961–1.076) | 0.549 | - | - |
| BMI | 0.931 (0.825–1.050) | 0.244 | - | - |
| Initial PSA at diagnosis | 1.024 (1.007–1.042) | 0.005 | 1.017 (0.997–1.039) | 0.102 |
| Pathological T stage | 2.780 (1.438–5.374) | 0.002 | 3.006 (1.527–5.918) | 0.001 |
| ISUP grading group of surgical specimens | 3.466 (1.699–7.069) | < 0.001 | 3.575 (1.700–7.519) | < 0.001 |
| Positive surgical margin | 4.401 (2.215–8.744) | < 0.001 | 3.96 (1.950–8.062) | < 0.001 |
| Lymph nodes metastasis | 1.005 (0.355–2.847) | 0.992 | - | - |
bRFS biochemical recurrence-free survival, PCa Prostate cancer, RP radical prostatectomy, HR Hazard ratio, CI Confidence interval, BMI Body mass index, PSA Prostate-specific antigen, ISUP International Society of Urological Pathology
Fig. 5.
Construction and validation of clinical model. A multivariate Cox regression analysis of clinical characteristics based on univariable Cox regression analysis. B ROC curve analyses of one-year, three-years, five-years for bRFS of clinical model in the training group. C ROC curve analyses of one-year, three-years, five-years for bRFS of clinical model in the validation group. D Kaplan-Meier curve analyses of clinical model in the training group. I Kaplan-Meier curve analyses of clinical model in the validation group. ROC, the receiver operating characteristic. bRFS, biochemical recurrence-free survival
The risk score of each patient was calculated based on the clinical risk scores. All patients were categorized into high-risk and low-risk groups according to the median risk score. As illustrated in Fig. 5B and E. Kaplan-Meier analysis revealed that patients with a high-risk profile experienced significantly shorter bRFS in comparison to those in the low-risk group (P < 0.001), with an AUC of 0.839, 0.776, and 0.819 for 1-year, 3-year, and 5-year bRFS, respectively, in the training group. And in the validation group, high-risk patients had significantly shorter bRFS than low-risk patients (P = 0.007), with an AUC of 0.838, 0.761, and 0.810 for 1-year, 3-year, and 5-year bRFS, respectively.
Development and validation of combined radiomics-clinical nomogram
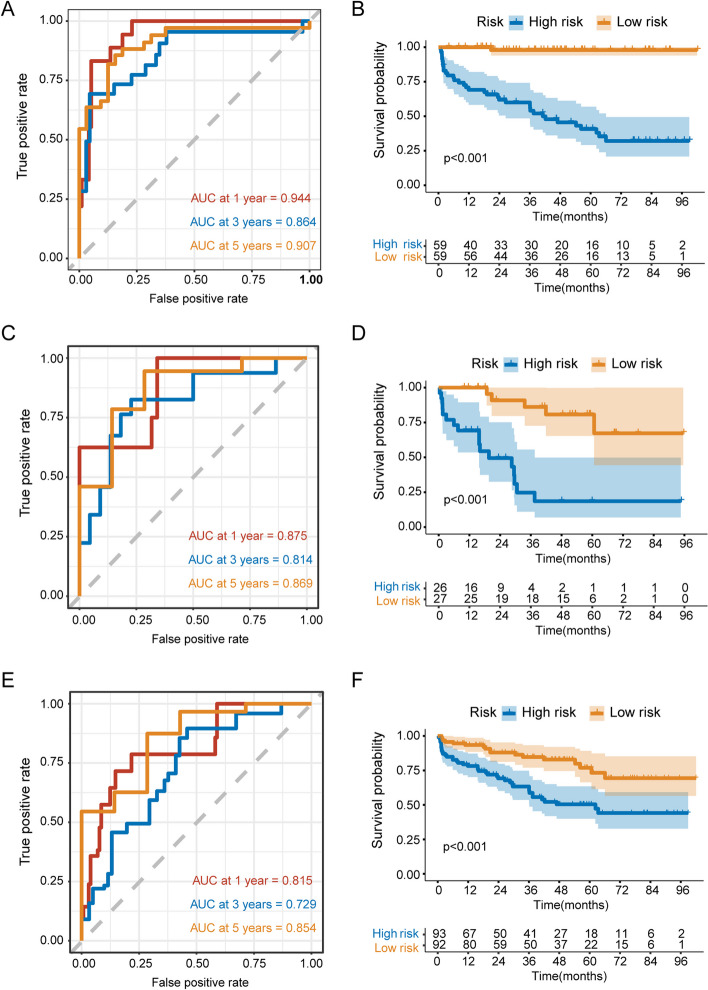
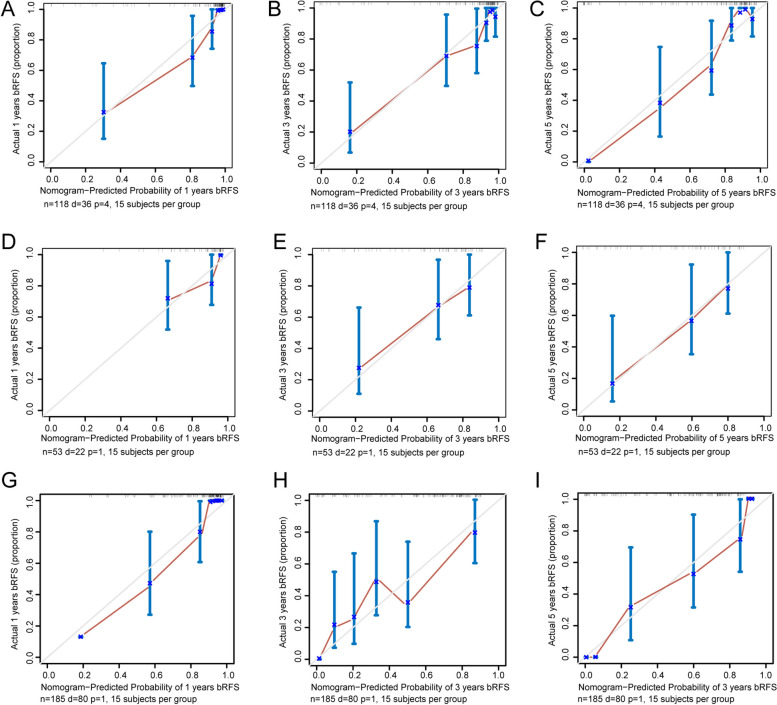
Based on the periprostatic fat-intratumoral radiomics score and significant clinical characteristics, a combined radiomics-clinical nomogram for predicting bRFS of PCa patients was developed by using multivariate Cox regression analysis (Fig. 6). The AUCs of radiomics-clinical nomogram in the training group were 0.944 (95%CI, 0.912–0.990), 0.864 (95%CI, 0.772–0.969), 0.907 (95%CI, 0.836–0.982) at 1-year, 3-years and 5-years bRFS in training cohort (Fig. 7A), the AUCs in the validation group were 0.875 (95%CI, 0.750–0.998), 0.814 (95%CI, 0.676–0.953), 0.869 (95%CI, 0.712–0.997) at 1-year, 3-years and 5-years bRFS (Fig. 7C), and AUCs in the testing group were 0.815 (95%CI, 0.664–0.921), 0.729 (95%CI, 0.641–0.883), 0.854 (95%CI, 0.702–0.913) (Fig. 7E). Kaplan-Meier analysis revealed that patients with high-risk experienced significantly shorter bRFS in comparison to those in the low-risk group (P < 0.001) in the training group, validation group, and testing group (Fig. 7B, D and F). In addition, the calibration curves of the radiomics-clinical nomogram revealed that the predicted and observed survival probability of 1-years, 3-year and 5-year bRFS had high degree of consistency in the training group, validation group, and testing group (Fig. 8).
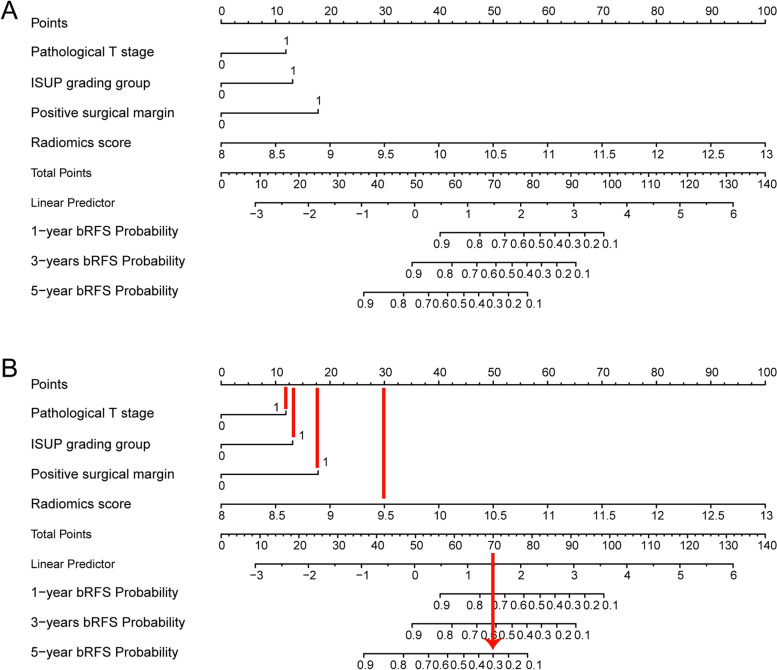
Fig. 6.
The radiomics-clinical nomogram. A Nomogram for predicting one-year, three-years, five-years bRFS of PCa patients receiving RP treatment. B How to use: (1) locate the patient’s radiomic score, Pathological T stage, ISUP grading group, Positivity surgical margin and then draw a straight line on the top dot axis to obtain the corresponding score; (2) sum the scores obtained; (3) find the final sum on the total point axis and draw a straight line down to assess the risk of bRFS in patients with PCa. bRFS, biochemical recurrence-free survival. PCa, prostate cancer
Fig. 7.
ROC curve and Kaplan-Meier curve analyses of the radiomics-clinical nomogram. ROC curve analyses of one-year, three-years, five-years for bRFS of combined radiomics-clinical nomogram in the training group (A), validation group (C), and testing group I. Kaplan-Meier curve analyses of radiomics-clinical nomogram in the training group (B), validation group (D), and testing group (F). ROC, the receiver operating characteristic. bRFS, biochemical recurrence-free survival. PCa, prostate cancer. RP, radical prostatectomy
Fig. 8.
Calibration curves of the combined radiomics-clinical nomogram. A-C Calibration curves of the combined radiomics-clinical nomogram for one-year, three-years, five-years bRFS in the training group. D-F Calibration curves of the combined radiomics-clinical nomogram for one-year, three-years, five-years bRFS in the validation group. G-I Calibration curves of the combined radiomics-clinical nomogram for one-year, three-years, five-years bRFS in the validation group. bRFS, biochemical recurrence-free survival
The comparison of ROC curves for the optimal radiomics score, the clinical model, and the radiomics-clinical nomogram was presented in Table 5. The radiomics-clinical nomogram demonstrated the highest AUC values for 1-year bRFS (AUC: 0.944, 95%CI: 0.912–0.990) and 5-year bRFS (AUC: 0.907, 95%CI: 0.836–0.982) in the training group. Furthermore, the radiomics-clinical nomogram exhibited a substantial improvement compared to clinical model in predicting 1-year (AUC: 0.944; 95%CI, 0.912–0.990 vs. AUC: 0.839; 95%CI, 0.661–0.928; P = 0.009), 3-year (AUC: 0.864; 95%CI, 0.772–0.969 vs. AUC: 0.776; 95%CI, 0.602–0.872; P = 0.008), and 5-year bRFS (AUC: 0.907; 95%CI, 0.836–0.982 vs. AUC: 0.819; 95%CI, 0.687–0.915; P = 0.027) respectively. Additionally, no statistically significant disparity was observed between the performance of the periprostatic fat-intratumoral radiomics score and the clinical model.
Table 5.
Comparing ROC curves of optimal radiomics model, clinical model and radiomics-clinical nomogram
| Predictive models | Training group (n = 118) | Validation group (n = 53) | Testing group (n = 185) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P value | AUC (95% CI) | P value | AUC (95% CI) | P value | ||||
| 1-Years bRFS | |||||||||
| Nomogram vs. Radiomics model | 0.944 (0.912–0.990) | 0.921 (0.857–0.981) | 0.329 | 0.875 (0.750–0.998) | 0.876 (0.744–0.978) | 0.915 | 0.815 (0.664–0.921) | 0.807 (0.623–0.911) | 0.201 |
| Nomogram vs. Clinical model | 0.944 (0.912–0.990) | 0.839 (0.661–0.928) | 0.009 | 0.875 (0.750–0.998) | 0.838 (0.685–0.980) | 0.402 | 0.815 (0.664–0.921) | 0.779 (0.615–0.893) | 0.553 |
| Radiomics model vs. Clinical model | 0.921(0.857–0.981) | 0.839 (0.661–0.928) | 0.123 | 0.876 (0.744–0.978) | 0.838 (0.685–0.980) | 0.616 | 0.807 (0.623–0.911) | 0.779 (0.615–0.893) | 0.679 |
| 3-Years bRFS | |||||||||
| Nomogram vs. Radiomics model | 0.864 (0.772–0.969) | 0.875 (0.762–0.949) | 0.705 | 0.814 (0.676–0.953) | 0.819 (0.696–0.945) | 0.897 | 0.729 (0.641–0.883) | 0.714 (0.626–0.842) | 0.424 |
| Nomogram vs. Clinical model | 0.864 (0.772–0.969) | 0.776 (0.602–0.872) | 0.008 | 0.814 (0.676–0.953) | 0.761 (0.616–0.914) | 0.241 | 0.729 (0.641–0.883) | 0.703 (0.605–0.847) | 0.386 |
| Radiomics model vs. Clinical model | 0.875 (0.762–0.949) | 0.776 (0.602–0.872) | 0.115 | 0.819 (0.696–0.945) | 0.761 (0.616–0.914) | 0.477 | 0.714 (0.626–0.842) | 0.703 (0.605–0.847) | 0.553 |
| 5-Years bRFS | |||||||||
| Nomogram vs. Radiomics model | 0.907 (0.836–0.982) | 0.854 (0.706–0.923) | 0.050 | 0.869 (0.712–0.997) | 0.826 (0.630–0.997) | 0.142 | 0.854 (0.702–0.913) | 0.817 (0.712–0.955) | 0.658 |
| Nomogram vs. Clinical model | 0.907 (0.836–0.982) | 0.819 (0.687–0.915) | 0.027 | 0.869 (0.712–0.997) | 0.810 (0.689–0.998) | 0.721 | 0.854 (0.702–0.913) | 0.805 (0.704–0.942) | 0.349 |
| Radiomics model vs. Clinical model | 0.854 (0.706–0.923) | 0.819 (0.687–0.915) | 0.876 | 0.826 (0.630–0.997) | 0.810 (0.689–0.998) | 0.448 | 0.817 (0.712–0.955) | 0.805 (0.704–0.942) | 0.695 |
ROC Receiver operating characteristic, bRFS biochemical recurrence-free survival, AUC Area Under Curve, CI Confidence interval
Discussion
To our knowledge, the present study is the first to investigate the value of a nomogram constructed based on periprostatic fat radiomics in predicting the BFS in localized PCa patients after RP. Our findings revealed that the periprostatic fat radiomics score and intratumoral radiomics score exhibited comparable predictive ability for bRFS in PCa patients. However, upon combining the periprostatic fat radiomics features with intratumoral radiomics features, the periprostatic fat-intratumoral radiomics score exhibited superior performance in predicting bRFS compared to the intratumoral radiomics score alone. Furthermore, the combined radiomics-clinical nomogram exhibited a high predictive performance to estimate bRFS of PCa patients after RP treatment.
Establishing an assessment model for bRFS in PCa patients is crucial for guiding treatment plans, influencing follow-up schedules, and enabling adjuvant therapy decisions. Firstly, by accurately stratifying patients based on their risk of BCR, the nomogram could guide clinicians in tailoring post-surgical treatment strategies. For instance, high-risk patients identified through the nomogram may benefit from closer monitoring or early initiation of adjuvant therapies such as ADT or radiotherapy, potentially improving long-term outcomes by catching recurrences earlier. This targeted approach can lead to more personalized and effective treatment pathways, maximizing the benefit of additional therapies in patients most likely to experience recurrence. Secondly, the nomogram could also significantly influence follow-up schedules. Patients with a higher predicted risk of recurrence might require more frequent PSA monitoring, imaging, and clinical assessments, enabling timely interventions if necessary. Conversely, patients with a lower predicted risk could follow standard or less intensive follow-up protocols, thereby reducing unnecessary hospital visits, medical costs, and patient anxiety. This stratified follow-up strategy could enhance resource allocation, allowing healthcare systems to focus efforts on high-risk patients while minimizing the burden on low-risk individuals. Thirdly, the predictive power of the nomogram may help in making informed decisions regarding the need for adjuvant therapies. For example, patients identified as high-risk may be recommended for early initiation of ADT or radiotherapy post-prostatectomy, which could play a pivotal role in preventing disease progression and improving long-term survival rates. On the other hand, low-risk patients might be spared from unnecessary adjuvant treatments, reducing their exposure to the associated side effects and preserving their quality of life. This individualized approach underscores the value of a radiomics-clinical model in supporting shared decision-making between patients and clinicians.
Previous studies have provided evidence of the potential of radiomics features derived from MRI to estimate BCR in PCa patients following initial treatment. For instance, Shiradkar et al. demonstrated the utility of radiomics features derived from ADC and T2WI MRI in predicting BCR of PCa patients receiving RP [19]. Shahait et al. proposed a prediction model that integrates radiomic features extracted from MRI and other clinicopathological parameters for post-surgical bRFS; which performed better than other traditional models such as Decipher and CAPRA [20–22]. Bourbonne et al. developed an MRI-derived radiomics classifier that effectively predicted BCR among patients with PCa underwent RP, which was successfully validated and achieved an accuracy of 0.76 [23, 24]. However, these studies only extracted and modeled radiomics features within the intratumoral region. In our study, we simultaneously evaluated the predictive value of radiomics features derived from intratumoral and periprostatic fat region for predicting bRFS. ROC curve analysis revealed a strong relationship between intratumoral region radiomic features and bRFS, which was consistent with previous research. Furthermore, we observed a noteworthy finding that the AUCs of both periprostatic fat and intratumoral radiomics features were significantly increased, indicating a substantial improvement in the predictive efficiency by adding periprostatic fat radiomic features. Additionally, the construction of a nomogram combining radiomic features of the periprostatic fat and intratumoral region, along with clinicopathological features, achieved the highest AUC value. These findings underscore the significance of periprostatic fat radiomics in assessing tumor recurrence in clinical practice.
In recent years, researchers have observed features in the adipose tissue surrounding tumors that are linked to tumor progression, and these features can be captured and analyzed by radiomics methods. For example, Jayaprakasam et al. investigated the association between MRI radiomics features of mesorectal fat and clinical outcomes in patients with locally advanced rectal cancer. Their findings revealed that radiomics features derived from mesorectal fat exhibited predictive capabilities for various clinical parameters, including local and distant recurrence, pathological complete response, as well as post-treatment T and N categories in patients with locally advanced rectal cancer [25]. Ahn et al. discovered that radiomics features extracted from perigastric adipose tissue on 2-deoxy-2-[18 F] fluoro-D-glucose PET/CT ([18 F] FDG PET/CT) were substantial prognostic indicators for predicting recurrence-free survival among patients with gastric cancer [26]. Shaish et al. conducted a study investigating the potential value of pretreatment MRI-based radiomics extracted from locally advanced rectal cancer and the surrounding mesorectal compartment in predicting significant clinical outcomes. The optimal predictive performance was observed when utilizing the mesorectal compartment for predicting pathological complete response and when employing a combined approach that leveraged both the mesorectal compartment and tumor region to predict tumor regression grade and neoadjuvant rectal score [27]. In our study, periprostatic fat radiomics model and intratumoral radiomics model demonstrated similar performance, and combined periprostatic fat-intratumoral radiomics features were more prognostic of bRFS compared to intratumoral radiomics features. These research findings suggested that the radiomics features of the peritumoral adipose tissue contain important prognostic information.
Periprostatic fat radiomics features could reflect the different biological characteristics, metabolic activity, and pathological features of the periprostatic fat tissue [28, 29]. The predictive ability of these radiomics features in relation to bRFS of PCa may be attributed to various factors. First, one reason for this predictive ability may be the detection of microscopic characteristic changes in periprostatic fat that are not visible to the naked eye but that play a vital role in tumor development. Abd Elmageed et al. demonstrated that PCa triggers pro-tumorigenesis in periprostatic adipose tissue. Their findings unveiled that the medium utilized for culturing PCa cells triggered tumor-like alterations in preadipocytes, encompassing epithelial-mesenchymal transition, genetic variability, and the formation of tumor-like lesion in vivo [30]. These results propose that a persistent positive feedback loop between PCa and the periprostatic environment hastens the progressive deterioration of the disease. Second, the structure and metabolic level of periprostatic adipose tissue have been found to be associated with the growth and spread of PCa [31, 32]. Studies have demonstrated that PCa cells can derive nutrients and energy from periprostatic adipose tissue, promoting tumor growth and metastasis [16, 33, 34]. Coy et al. reported that lipid metabolism disturbances in periprostatic adipose tissue can influence tumor cell processes by inducing metabolic changes [17]. Laurent et al. [35]. shown that the uptake of exogenous lipids promotes tumor cell invasion. These transferred lipids stimulate the expression of one isoform of the pro-oxidant enzyme NADPH oxidase, NOX5. NOX5 increases intracellular reactive oxygen species (ROS), which in turn activate the HIF1/MMP14 pathway, leading to enhanced tumor cell invasion. Gang Liu et al. [36]. reported that prostate-specific expression of the IL-6 transgene has been shown to autonomously induce prostate neoplasm by amplifying inflammation within both the prostate and periprostatic adipose tissue. The identification of radiomics features reflecting the structure and metabolic changes in periprostatic adipose tissue has been instrumental in predicting tumor recurrence. Third, PCa cells may persist in the periprostatic fat tissue following RP or radiotherapy [37]. A thorough analysis of periprostatic tissue radiomics features allows for a more accurate identification of residual cancer foci or lymph node metastasis [38, 39], ultimately improving BCR prediction performance.
This study exhibited several limitations. Firstly, it was a retrospective investigation conducted at a single center, featuring a restricted sample size. Additionally, the nomogram constructed in this study was only internally validated and lacked external validation. It is essential to conduct prospective validation studies in multicenter and large-scale cohorts to confirm these findings. Secondly, manual delineation of the ROI inevitably introduces some degree of variability. To address this, we implemented several measures to minimize bias and enhance the accuracy of ROI delineation. The ROIs were carefully marked by two experienced radiologists with extensive training in prostate MRI. Inter-observer and intra-observer repeatability were quantified by employing the infraclass correlation coefficient (ICC). Features with ICC greater than 0.8 were chosen for further analysis. Thirdly, there is a significant difference in the distribution of pathological T stage between the training and validation cohorts. This difference in T stage distribution may potentially likely impacted the model’s performance. This imbalance in pathological T stage distribution between the training and validation cohorts might potentially likely impacted the model’s performance. The model, having been trained on a cohort with a more balanced pathological T stage distribution, may have been less effective in a population where advanced T stage predominated, as the clinical parameters associated with advanced pathological T stage could potentially overshadow the contributions of the other variables included in the nomogram.
Conclusions
The study found that the combined periprostatic fat-intratumoral radiomics features were more prognostic of bRFS compared to intratumoral radiomics features. The combined radiomics-clinical nomogram exhibited a high predictive performance to estimate bRFS of PCa patients after RP treatment. This study underlined that radiomic features of periprostatic adipose tissue could provide more comprehensive and accurate information on PCa lesions.
Supplementary Information
Supplementary Material 1: Supplementary Figure 1. Schematic diagram of the periprostatic fat ROI outline in the MRI images of PCa patient. (A) is the T2WI sequence with PCa in the periprostatic fat region. (B) is the ADC sequence with PCa in the periprostatic fat region. (C) is the periprostatic fat ROI outline in the T2WI sequence. (D) is the periprostatic fat ROI outline in the ADC sequence. (E) is the generated periprostatic fat ROI in the T2WI sequence. (F) is the generated periprostatic fat ROI in the ADC sequence. ROI, regions of interest; MRI, magnetic resonance imaging; PCa, prostate cancer; T2WI, T2-weighted imaging; ADC, apparent-diffusion coefficient.
Supplementary Material 2: Supplementary Figure 2. Schematic diagram of the intratumoral ROI outline in the MRI images of PCa patient. (A) is the T2WI sequence in the intratumoral region. (B) is the ADC sequence in the intratumoral region. (C) is the intratumoral ROI outline in the T2WI sequence. (D) is the intratumoral ROI outline in the ADC sequence. (E) is the generated intratumoral ROI in the T2WI sequence. (F) is the generated intratumoral ROI in the ADC sequence. ROI, regions of interest; MRI, magnetic resonance imaging; PCa, prostate cancer; T2WI, T2-weighted imaging; ADC, apparent-diffusion coefficient.
Acknowledgements
Not applicable.
Abbreviations
- mpMRI
Multiparametric magnetic resonance imaging
- bRFS
Biochemical recurrence-free survival
- PCa
Prostate cancer
- RP
Receiving radical prostatectomy
- LASSO
Least absolute shrinkage and selection operator
- ROC
Receiver operating characteristic
- AUC
Area Under the Curve
- CI
Confidence interval
- BCR
Biochemical recurrence
- TNF
Tumor necrosis factor
- CCL7
C-C motif chemokine ligand 7
- AJCC/UICC
American Joint Committee on Cancer/ Union for International Cancer Control
- TNM
Tumor, Node, Metastasis
- T2WI
T2 weighted images
- ADC
Apparent Diffusion Coefficient
- PSA
Prostate specific antigen
- EAU
European Association of Urology
- ROI
Regions of interest
- LoG
Laplacian of Gaussian
- GLRLM
gray-level run length matrix
- GLCM
gray-level co-occurrence matrix
- GLDM
gray-level dependence matrix
- GLSZM
gray-level size zone matrix
- NGTDM
neighboring gray tone difference matrix
- ICC
Infraclass correlation coefficient
- [18F] FDG PET/CT
2-deoxy-2-[18 F] fluoro-D-glucose positron emission tomography/computed tomography
Authors’ contributions
Writing – original draft, Xiao-Hui Wu, Zhi-Bin Ke, Ze-Jia Chen, Wen-Qi Liu. Writing – review & editing, Yong Wei, Ning Xu. Methodology, Shao-Hao Chen, Dong-Ning Chen. Formal analysis, Qing-Shui Zheng, Shao-Hao Chen, Yu-Ting Xue. Data curation, Shao-Hao Chen, Xue-Yi Xue. Conceptualization, Yong Wei, Xue-Yi Xue. Visualization, Yong Wei. Project administration, Yong Wei, Ning Xu.
Funding
This work was supported by Science and Technology Innovation Joint Fund project of Fujian province (No. 2021Y9126); Science and Technology Innovation Joint Fund project of Fujian province (No. 2023Y9078); Natural Science Foundation of Fujian Province (No. 2024J01556).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Approved No. of ethic committee: MRCTA, ECFAH of FMU [2024]638), and all patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao-Hui Wu, Zhi-Bin Ke, Ze-Jia Chen and Wen-Qi Liu contributed equally to this work.
Contributor Information
Yong Wei, Email: weiyong2017@fjmu.edu.cn.
Ning Xu, Email: drxun@fjmu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Tan H-J. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–42. [DOI] [PubMed] [Google Scholar]
- 3.Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–87. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. [DOI] [PubMed] [Google Scholar]
- 5.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62. [DOI] [PubMed] [Google Scholar]
- 6.Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19(2):132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spohn SKB, Bettermann AS, Bamberg F, Benndorf M, Mix M, Nicolay NH, et al. Radiomics in prostate cancer imaging for a personalized treatment approach - current aspects of methodology and a systematic review on validated studies. Theranostics. 2021;11(16):8027–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghezzo S, Bezzi C, Presotto L, Mapelli P, Bettinardi V, Savi A, et al. State of the art of radiomic analysis in the clinical management of prostate cancer: a systematic review. Crit Rev Oncol Hematol. 2022;169:103544. [DOI] [PubMed] [Google Scholar]
- 9.Woźnicki P, Westhoff N, Huber T, Riffel P, Froelich MF, Gresser E, et al. Multiparametric MRI for prostate cancer characterization: combined use of radiomics model with PI-RADS and clinical parameters. Cancers (Basel). 2020;12(7):1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Yu B, Zhong F, Guo Q, Li K, Hou Y, et al. MRI-based texture analysis of the primary tumor for pre-treatment prediction of bone metastases in prostate cancer. Magn Reson Imaging. 2019;60:76–84. [DOI] [PubMed] [Google Scholar]
- 11.Abdollahi H, Mahdavi SR, Mofid B, Bakhshandeh M, Razzaghdoust A, Saadipoor A, et al. Rectal wall MRI radiomics in prostate cancer patients: prediction of and correlation with early rectal toxicity. Int J Radiat Biol. 2018;94(9):829–37. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi H, Mofid B, Shiri I, Razzaghdoust A, Saadipoor A, Mahdavi A, et al. Machine learning-based radiomic models to predict intensity-modulated radiation therapy response, Gleason score and stage in prostate cancer. Radiol Med. 2019;124(6):555–67. [DOI] [PubMed] [Google Scholar]
- 13.Gregg JR, Surasi DS, Childs A, Moll N, Ward JF, Kim J, et al. The association of periprostatic fat and grade group progression in men with localized prostate cancer on active surveillance. J Urol. 2021;205(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salji M, Hendry J, Patel A, Ahmad I, Nixon C, Leung HY. Peri-prostatic fat volume measurement as a predictive tool for castration resistance in advanced prostate cancer. Eur Urol Focus. 2018;4(6):858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Wu Y, Huang M, Zhang Y, Bai Z. Automatic prostate and peri-prostatic fat segmentation based on pyramid mechanism fusion network for T2-weighted MRI. Comput Methods Programs Biomed. 2022;223:106918. [DOI] [PubMed] [Google Scholar]
- 16.Fontaine A, Bellanger D, Guibon R, Bruyère F, Brisson L, Fromont G. Lipophagy and prostate cancer: association with disease aggressiveness and proximity to periprostatic adipose tissue. J Pathol. 2021;255(2):166–76. [DOI] [PubMed] [Google Scholar]
- 17.Altuna-Coy A, Ruiz-Plazas X, Sánchez-Martin S, Ascaso-Til H, Prados-Saavedra M, Alves-Santiago M, et al. The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell’s metabolic crosstalk. BMC Med. 2022;20(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura S, Urabe F, Sasaki H, Kimura T, Miki K, Egawa S. Prognostic significance of prostate-specific antigen persistence after radical prostatectomy: a systematic review and meta-analysis. Cancers (Basel). 2021;13(5):948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiradkar R, Ghose S, Jambor I, Taimen P, Ettala O, Purysko AS, et al. Radiomic features from pretreatment biparametric MRI predict prostate cancer biochemical recurrence: preliminary findings. J Magn Reson Imaging. 2018;48(6):1626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Shiradkar R, Leo P, Algohary A, Fu P, Tirumani SH, et al. A novel imaging based Nomogram for predicting post-surgical biochemical recurrence and adverse pathology of prostate cancer from pre-operative bi-parametric MRI. EBioMedicine. 2021;63:103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brajtbord JS, Leapman MS, Cooperberg MR. The CAPRA score at 10 years: contemporary perspectives and analysis of supporting studies. Eur Urol. 2017;71(5):705–9. [DOI] [PubMed] [Google Scholar]
- 22.Ross AE, Johnson MH, Yousefi K, Davicioni E, Netto GJ, Marchionni L, et al. Tissue-based Genomics augments post-prostatectomy risk stratification in a natural history cohort of Intermediate- and high-risk men. Eur Urol. 2016;69(1):157–65. [DOI] [PubMed] [Google Scholar]
- 23.Bourbonne V, Fournier G, Vallières M, Lucia F, Doucet L, Tissot V et al. External validation of an MRI-Derived Radiomics Model to predict biochemical recurrence after surgery for high-risk prostate Cancer. Cancers (Basel). 2020;12(4):814. [DOI] [PMC free article] [PubMed]
- 24.Bourbonne V, Vallières M, Lucia F, Doucet L, Visvikis D, Tissot V, et al. MRI-derived radiomics to guide post-operative management for high-risk prostate cancer. Front Oncol. 2019;9:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaprakasam VS, Paroder V, Gibbs P, Bajwa R, Gangai N, Sosa RE, et al. MRI radiomics features of mesorectal fat can predict response to neoadjuvant chemoradiation therapy and tumor recurrence in patients with locally advanced rectal cancer. Eur Radiol. 2022;32(2):971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn H, Song GJ, Jang S-H, Son MW, Lee HJ, Lee M-S, et al. Predicting the recurrence of gastric cancer using the textural features of perigastric adipose tissue on [18F]FDG PET/CT. Int J Mol Sci. 2022;23(19):11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaish H, Aukerman A, Vanguri R, Spinelli A, Armenta P, Jambawalikar S, et al. Radiomics of MRI for pretreatment prediction of pathologic complete response, tumor regression grade, and neoadjuvant rectal score in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation: an international multicenter study. Eur Radiol. 2020;30(11):6263–73. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Chen J, Ge H, Yan Z, Luo B, Hu X, et al. Radiogenomics to characterize the immune-related prognostic signature associated with biological functions in glioblastoma. Eur Radiol. 2023;33(1):209–20. [DOI] [PubMed] [Google Scholar]
- 29.Zheng H, Miao Q, Liu Y, Mirak SA, Hosseiny M, Scalzo F, et al. Multiparametric MRI-based radiomics model to predict pelvic lymph node invasion for patients with prostate cancer. Eur Radiol. 2022;32(8):5688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toren P, Venkateswaran V. Periprostatic adipose tissue and prostate cancer progression: new insights into the tumor microenvironment. Clin Genitourin Cancer. 2014;12(1):21–6. [DOI] [PubMed] [Google Scholar]
- 32.Cancel M, Pouillot W, Mahéo K, Fontaine A, Crottès D, Fromont G. Interplay between prostate cancer and adipose microenvironment: a complex and flexible scenario. Int J Mol Sci. 2022;23(18):10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figiel S, Pinault M, Domingo I, Guimaraes C, Guibon R, Besson P, et al. Fatty acid profile in peri-prostatic adipose tissue and prostate cancer aggressiveness in african-caribbean and caucasian patients. Eur J Cancer. 2018;91:107–15. [DOI] [PubMed] [Google Scholar]
- 35.Laurent V, Toulet A, Attané C, Milhas D, Dauvillier S, Zaidi F, et al. Periprostatic adipose tissue favors prostate cancer cell invasion in an obesity-dependent manner: role of oxidative stress. Mol Cancer Res. 2019;17(3):821–35. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Zhang J, Frey L, Gang X, Wu K, Liu Q, et al. Prostate-specific IL-6 transgene autonomously induce prostate neoplasm through amplifying inflammation in the prostate and peri-prostatic adipose tissue. J Hematol Oncol. 2017;10(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van den Broeck T, van den Bergh RCN, Briers E, Cornford P, Cumberbatch M, Tilki D, et al. Biochemical recurrence in prostate cancer: the uropean association of urology prostate cancer guidelines panel recommendations. Eur Urol Focus. 2020;6(2):231–4. [DOI] [PubMed] [Google Scholar]
- 38.Kwon YS, Ha Y-S, Modi PK, Salmasi A, Parihar JS, Patel N, et al. Oncologic outcomes in men with metastasis to the prostatic anterior fat pad lymph nodes: a multi-institution international study. BMC Urol. 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korten M, Pose RM, Graefen M, Tilki D, Michl U, Knipper S, et al. Preprostatic lymph nodes in prostate cancer hot or not? Impact on oncological outcome after radical prostatectomy. World J Urol. 2022;40(9):2231–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Figure 1. Schematic diagram of the periprostatic fat ROI outline in the MRI images of PCa patient. (A) is the T2WI sequence with PCa in the periprostatic fat region. (B) is the ADC sequence with PCa in the periprostatic fat region. (C) is the periprostatic fat ROI outline in the T2WI sequence. (D) is the periprostatic fat ROI outline in the ADC sequence. (E) is the generated periprostatic fat ROI in the T2WI sequence. (F) is the generated periprostatic fat ROI in the ADC sequence. ROI, regions of interest; MRI, magnetic resonance imaging; PCa, prostate cancer; T2WI, T2-weighted imaging; ADC, apparent-diffusion coefficient.
Supplementary Material 2: Supplementary Figure 2. Schematic diagram of the intratumoral ROI outline in the MRI images of PCa patient. (A) is the T2WI sequence in the intratumoral region. (B) is the ADC sequence in the intratumoral region. (C) is the intratumoral ROI outline in the T2WI sequence. (D) is the intratumoral ROI outline in the ADC sequence. (E) is the generated intratumoral ROI in the T2WI sequence. (F) is the generated intratumoral ROI in the ADC sequence. ROI, regions of interest; MRI, magnetic resonance imaging; PCa, prostate cancer; T2WI, T2-weighted imaging; ADC, apparent-diffusion coefficient.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.