Abstract
Background
We aimed to determine the association of neutrophil-to-lymphocyte ratio (NLR) with non-arteritic anterior ischemic optic neuropathy (NAION).
Methods
We conducted a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Scopus and Web of Science were searched from the establishment of the database to May 5, 2022 to find the relevant studies. The quality of the included literature was evaluated with the Newcastle–Ottawa scale (NOS). The results are reflected in the form of standard mean difference (SMD) and 95% confidence interval (CI).
Results
Finally, six articles were included in our study. Compared with healthy controls, patients’ NLR levels were significantly higher (SMD = 0.47; CI 95% = 0.30–0.65, p<0.001). The included studies were not statistically heterogeneous (I2 = 0.0%, p = 0.60); thus, the analysis used the fixed-effect model. The pooled sensitivity of NLR was 0.69 (95% CI 0.60–0.67), and the pooled specificity was 0.59 (95% CI 0.50–0.67). The pooled positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR) of NLR were 1.71(95%CI 1.48–1.98), 0.50 (95%CI 0.41–0.62), and 3.38 (95%CI 2.57–4.44), respectively.
Conclusions
Our findings suggest NLR to be a potential marker of NAION, while also implicating a role for inflammation in underlying pathophysiology.
Background
Non-arteritic anterior ischemic optic neuropathy (NAION) is one of the most common and prevalent diseases leading to vision loss in middle-aged patients and elderly [1]. It is the most common subtype of ischemic optic neuropathy characterized by sudden onset vision loss, optic edema upon presentation, and cup-to-disc ratio of 0.2 or less (“disc at risk”) in the same eye [1, 2]. Exact pathophysiology remains unknown; however, it is generally thought to be preceded by relative hypoperfusion to optic nerve head leading to progressive edema and infarction of the optic nerve fibers [2]. Patients with history of hypertension, hypercholesterolemia, diabetes mellitus, cardio- and cerebrovascular disease, and obstructive sleep apnea are at higher risk of developing NAION [2]. A majority of these predisposing conditions are regulated by inflammatory mechanisms [3–6]. Furthermore, there is evidence that cellular inflammation plays a major role after the initial infarct to clear debris and stimulate tissue remodeling [7]. Together, these suggest that inflammatory markers may be useful in diagnosing and assessing clinical progression of NAION, especially given the absence of objective and quantitative measures for diagnosis.
Peripheral blood neutrophil-to-lymphocyte ratio (NLR) is an emerging prognostic biomarker of inflammation and immune function in cardiovascular disease, respiratory disease, kidney disease, lung disease, infection, and cancer [8]. Independent of disease, NLR is also associated with overall mortality [8]. NLR reflects a balance between inflammatory activity and immune response, offering insights into the extent and phase of these processes [9]. Effective regulation of NLR is essential for both disease progression and recovery. Since inflammation may play a role in the development of NAION, recent studies have examined the potential link between NAION and peripheral blood NLR, suggesting an association between higher NLR levels and NAION. Although prior systematic reviews [10–14] have covered NAION risk factors and treatment options, none have explored the NLR–NAION relationshiExisting studies on this topic are all original research [15–20]. This systematic review and meta-analysis aim to compile data from these studies to evaluate the potential of NLR as a biomarker for NAION in the appropriate clinical context. To the best of our knowledge, this is the first manuscript on this context.
Methods
Search strategy
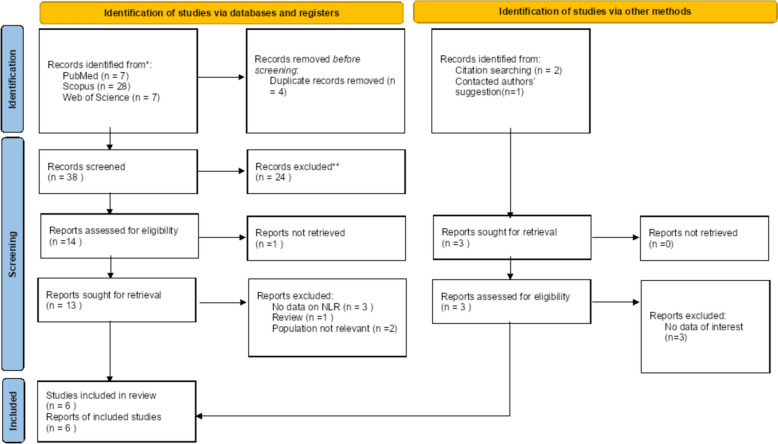
We used the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) standards to perform a systematic review and meta-analysis to collect all published materials, such as grey literature and preprints [21] (Fig. 1). Three main data bases (Scopus, PubMed, and Web of Science) were systematically searched using these key words: “Neutrophil to lymphocyte ratio”, NLR, and “Nonarteritic Anterior Ischemic Optic Neuropathy”. Table 1 illustrates the precise search methodology. The latest update to the search occurred on May 5, 2022. Our search approach was not limited by language or publishing year.
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which includes searches of databases, registers and other sources
Table 1.
Exact search strategy of databases
| Database | Search strategy | Limitation | Date of search | Number of studies |
|---|---|---|---|---|
| PubMed | (“Neutrophil to lymphocyte ratio”[All Fields] OR “NLR”[All Fields]) AND (“Nonarteritic Anterior Ischemic Optic Neuropathy”[All Fields] OR “Non-arteritic Anterior Ischemic Optic Neuropathy”[All Fields]) | None | May 5, 2022 | 7 |
| Scopus | [ALL (“Neutrophil to lymphocyte ratio” OR NLR ) AND ALL (“Nonarteritic Anterior Ischemic Optic Neuropathy” OR “Non-arteritic Anterior Ischemic Optic Neuropathy”)] | None | May 5, 2022 | 28 |
| Web of Science | “Neutrophil to lymphocyte ratio” OR NLR (All Fields) and “Nonarteritic Anterior Ischemic Optic Neuropathy” OR “Non-arteritic Anterior Ischemic Optic Neuropathy” (All Fields) | None | May 5, 2022 | 7 |
Inclusion and exclusion criteria
The PICOS concept is used to determine whether investigations are eligible. The following were the inclusion criteria:
Population: patients with NAION
Intervention: NLR
Control: healthy controls
Outcomes: the prognostic performance of NLR in NAION
Case–control, nested case–control, and cross-sectional studies
Duplicate studies, experimental or animal investigations, editorials, letters, articles with insufficient data, case reports, case series, and studies with overlapping data were excluded.
Data extraction and quality assessment
EndNote software was used for study screening [22]. Duplicate studies were first eliminated. Following that, two writers independently reviewed the titles and abstracts of articles found during the first database search, concentrating on those that were closely related. The same writers received and evaluated the full texts of the studies listed. The meta-analysis eventually contained studies that satisfied the criteria. In addition, we looked through the references of relevant original publications and review papers to see if we could find any more relevant research. A third person was brought in to mediate disagreements among the two writers doing the screening. The following information was separately collected from the included articles by two reviewers: study design and location, first author’s name, publication year, number of controls and cases, mean age, gender, best-corrected visual acuity (BCVA), NAION phase (acute or chronic), mean± standard deviation (SD) of NLR level in cases and controls, or sufficient data for estimating the mean± SD such as median and interquartile range (IQR) or/and range. Two writers independently assessed the quality of the included studies using the Newcastle–Ottawa Scale (NOS) [23].
Statistical analysis
The Standardized Mean Difference (SMD) was provided along with a 95% confidence interval (CI) to indicate the NLR level. Due to variations in laboratory standards for NLR across countries and the impact of factors like ethnicity and geographic location, we opted to use the standardized mean difference (SMD) rather than the mean difference (MD) in our study. SMD enables comparisons across studies with different measurement scales or units, as it standardizes effect size, allowing for a more consistent and widely applicable metric when pooling results. While MD might offer greater statistical power, it is constrained to studies with comparable outcomes and units, which could limit broader relevance. We evaluated the heterogeneity among the outcomes of the studies using both the chi-squared (χ2) test and the I2 statistic. Results with an I2 value exceeding 75% and a P value less than 0.05 for the χ2 test were regarded as indicative of significant result heterogeneity. In such instances, we employed a random effect model for the meta-analysis of the heterogeneous results. Alternatively, if the conditions mentioned earlier were not met, we applied the fixed-effect model. We evaluated the diagnostic efficacy of NLR for NAION through the utilization of a summary receiver operating characteristic (SROC) curve.
To detect potential publication bias, we employed Egger’s linear-regression test along with the funnel plot. For conducting statistical analyses, we employed STATA 12.0 software provided by Stata Corporation in College Station, TX, USA. We considered a P value equal to or less than 0.05 as indicative of statistical significance.
Results
Search and selection of literature
A total of 45 records were retrieved in the database search and manual search of citation list of articles. After the exclusion of duplicates and not relevant records, six studies were included in the systematic review and meta-analysis [15–20], for a total of 251 patients with NAION and 252 healthy controls. The process of inclusion and exclusion is detailed in the PRISMA flow diagram, provided in Fig. 1. The PRISMA checklist for this investigation is included in Supplementary File 1.
Characteristics of the included studies
This meta-analysis included six studies, of whom four were conducted in Turkey, one in Italy and one in Thailand. In terms of document language, all of the documents were written in English language. All of them were retrospective studies. Table 2 shows the overall characteristics of the studies and their quality scores. In total, six research examined NLR levels in patients with NAION and healthy controls[15–20], and five studies reported diagnostic value of NLR for differentiating between patients with NAION and healthy controls, based on ROC curve analysis[15, 17–20]. NOS score of included studies ranged between 6 and 7. In all studies, subjects without any systemic disease were considered as healthy controls. With regards to risk factors affecting the immune system and eyes, all of included studies excluded people with such risk factors including other ocular pathologies, medications that may affect blood parameters, chronic inflammatory disease or autoimmune disease, and any systemic diseases such as renal or liver failure.
Table 2.
General characteristics of included studies
| First author | Years | Country | Design | Mean age | Gender (male percentage) | NLR cutoff value | Sensitivity | Specificity | BCVA | NAION group | Healthy controls | Healthy control definition | Exclusion criteria | NAION phase | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | NLR | N | NLR | ||||||||||||||
| Polat [19] | 2015 | Turkey | R | 60.1 | 51.11% | 1.94 | 60 | 63 | 3.13 | 45 | 2.44 ± 1.00 | 50 | 1.85 ± 0.50 | Subjects without any systemic disease | Other ocular pathologies, using antiplatelet or anticoagulant agents, malignancy, coronary artery disease, chronic liver or heart disease | Acute | 7 |
| Gunes [15] | 2017 | Turkey | R | 54.7 | 42.9% | 1.64 | 85 | 41 | 1.08 | 56 | 3.62 ± 4.37 | 56 | 2.09 ± 0.96 | Subjects without any systemic disease | Other ocular pathologies, neurological disease, chronic autoimmune disease or inflammatory disease, active infection, | Acute | 6 |
| Inanc [16] | 2018 | Turkey | R | 66.66 | 41.66% | 2.25 | 66 | 73 | – | 33 | 2.04 ± 0.42 | 35 | 1.97 ± 0.31 | Healthy people from outpatient clinic of ophthalmology with simple refractive errors | Other ocular pathologies, alcohol abuse, chronic smoking, inflammatory diseases, atrial fibrillation, any systemic disease, hepatic or renal failure | Acute | 6 |
| Kocak [17] | 2020 | Turkey | R | 64.7 | 42.85% | 1.79 | 71 | 59 | – | 50 | 2.57 ± 1.49 | 44 | 1.98 ± 1.05 | Healthy people from outpatient clinic of ophthalmology | Other ocular pathologies, using medications that may affect blood parameters, chronic inflammatory disease or autoimmune disease, any systemic diseases | Acute | 7 |
| Pinna [18] | 2021 | Italy | R | – | – | _ | _ | _ | – | 37 | 2.44 ± 0.99 | 37 | 1.95 ± 0.81 | Subjects without NAION | Not declared | Acute | 7 |
| Sinsawad [20] | 2021 | Thailand | R | 59.20 | 56.7% | 1.89 | 66.7 | 65 | – | 30 | 2.25 ± 0.95 | 30 | 1.96 ± 0.90 | patients with cataract without any systemic disease | Using any kind of treatment for NAION, other neuro-ophthalmic diseases, glaucoma diseases, retinal diseases, alcohol drinking and smoking, underlying disease that can affect the blood test results | Acute | 7 |
N: Number; NLR: Neutrophil-to-lymphocyte ratio; R: Retrospective; BCVA: best-corrected visual acuity; NOS: the Newcastle–Ottawa scale
Difference in NLR level between patients with NAION and healthy controls
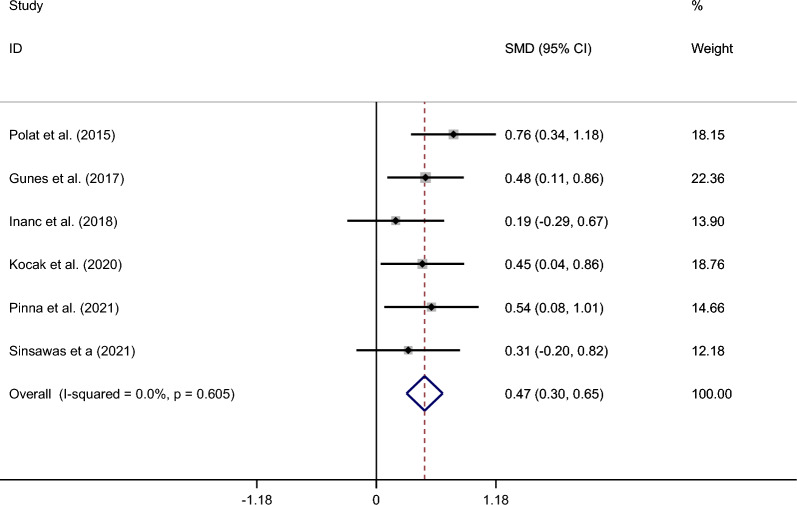
Compared with healthy controls, patients’ NLR levels were significantly higher (SMD = 0.47; CI 95% = 0.30–0.65, p < 0.001). The included studies were not statistically heterogeneous (I2 = 0.0%, p = 0.60); thus, the analysis used the fixed-effect model Fig. 2.
Figure 2.
Meta-analysis of differences in NLR level between patients with NAION and healthy controls
Diagnostic value of NLR for differentiating between patients with NAION and healthy controls
The pooled sensitivity of five studies was 0.69 (95% CI 0.60–0.67), and the pooled specificity was 0.59 (95% CI 0.50–0.67). The pooled positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR) of NLR were 1.71(95%CI 1.48–1.98),0.50 (95%CI 0.41–0.62), and 3.38 (95%CI 2.57–4.44), respectively Fig. 3.
Figure 3.
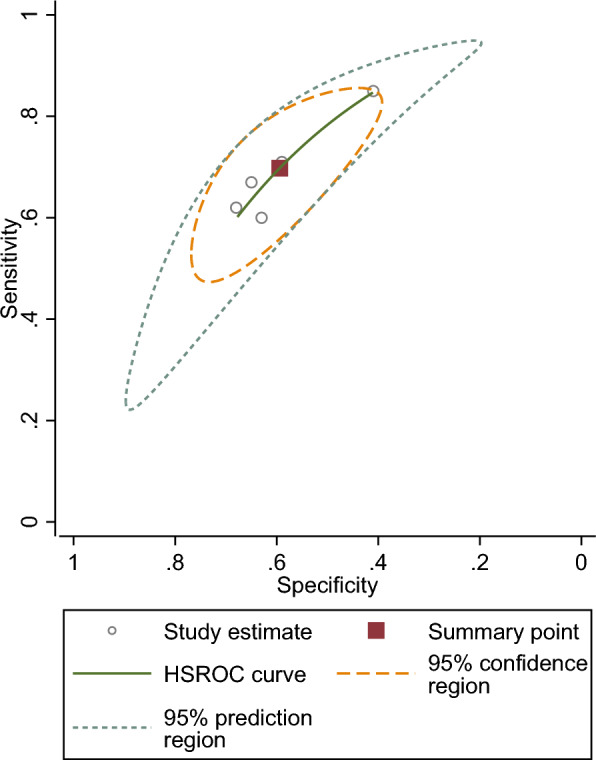
SROC curve of included studies assessing diagnostic value of NLR for NAION
Publication bias
As shown in Fig. 4, there was no publication bias among included studies (Egger’s test p = 0.34).
Figure 4.
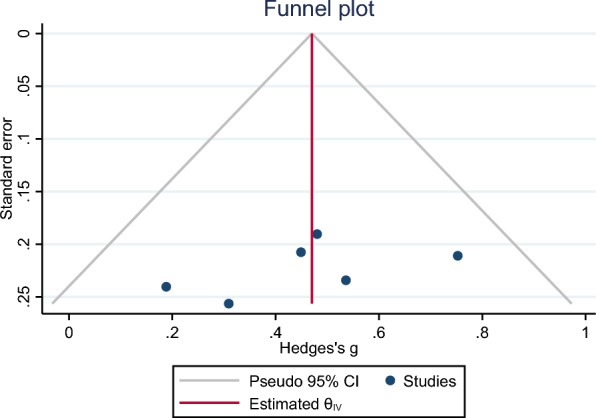
Funnel plot assessing publication bias
Discussion
Our study found that NLR was higher in patients with NAION than in healthy controls in six retrospective studies. No significant publication bias was measured. SROC analysis of the pooled data demonstrates a relationship between NLR and NAION. This could be used to help identify mechanisms of pathology and treatment, both of which remain elusive.
NAION is the most common ischemic optic neuropathy in general and most common optic nerve disorder for patients over 50 years of age [24]. It is distinguished clinically from arteritic anterior ischemic optic neuropathy (AAION), which is from giant cell arteritis. NAION is characterized by acute and painless loss of vision [25]. A swollen, pallid optic disk (the optic nerve head [ONH]), with a small or absent cup is a pathognomonic finding that results from obstructions in the posterior ciliary artery and associated vasculature. The exact mechanism is unknown and likely multifactorial. Loss of vision generally stabilizes following the initial incident, and occurrence in one eye does not guarantee the same contralateraly [26]. Associations between smoking, hypertension, and diabetes and NAION have been consistently reported [2, 27], along with obstructive sleep apnea, renal failure, and other inflammatory processes [28, 29].
There are several possibilities for the inadequate perfusion and subsequent optic disk injury seen in NAION: Thrombotic and hypoperfusion ischemia have both been reported [7, 30]. Arnold et al. found delayed filling of the prelaminar nerve head vasculature to precede disk edema, axonal atrophy and swelling [31]. They also described axoplasmic stasis as contributing to laminar crowding and a compartment syndrome mechanism [31]. Co-occurring inflammatory conditions, like those commonly found in NAION patients, exacerbate preexisting variations in microvascular and orbital anatomy and influence the nerve sheath’s propensity for injury [30]. Cell and molecular pathology that follows injury is difficult to study, however, because NAION is not lethal and so histology is rare. Since the optic nerve is a CNS structure, it is reasonable to believe NAION pathology could mirror that of other, better-understood CNS ischemic conditions [32]. Post-injury CNS tissue, for example, shows neutrophils peaking within the first week, coinciding with disruption of the blood–brain barrier and thrombus forming NETs [33]. Resident microglia and peripheral macrophage levels remain elevated over a longer period (on the order of a month), with myeloid subtypes remaining active for different lengths of time [34]. The composition of leukocytes in the injured tissue, moreover, predicts hemorrhagic complications and outcomes more broadly, even when considered independently of infection [35].
Histology of animal and human NAION lesions are consistent with the inflammatory cellular progression of CNS ischemia overall [7]. Tissue edema in a non-human primate model (pNAION) increases in the first week and reperfusion can be seen between 3 and 7 days [36]. Neutrophils respond to the site of injury and peak before microglia and peripheral macrophages take hold, with the monocytes peaking around 21–35 days. Early breakdown of the blood–retinal barrier (BRB) in pNAION resolves by day 14 [32]. Rodent (rAION) models, as well, have shown acute inflammation of the optic nerve axon 24 h post-infarct, with peripheral macrophages appearing between days 3 and 14 [37]. Evidence, thus, suggests the optic nerve is exposed to complex immunological interplay in a manner consistent with more common systemic diseases [37].
The NLR is an easy value to calculate that shows a relationship to disease progression and activity in a number of conditions [8, 38–41]. The ratio is more stable than the absolute counts of either cell type, making it a better indicator of overall immune activity than each value on its own. Neutrophils respond to sterile injury—such as hypoxic, inadequately perfused optic nerve tissue—in essentially the same pro-inflammatory manner as used to clear infection [42].
The elevated NLR value in our investigation might be explained by two different mechanisms. First, it has been discovered that the early stage of NAION is characterized by neutrophil-mediated cellular inflammation [7, 37]. Elevated neutrophil count has been linked to ischemic injury, according to several publications [43–45]. An indication of neutrophil-mediated microvascular plugging may be the relationship between impaired microvascular perfusion and neutrophilia. The relationship between low-grade inflammation and atherosclerosis is the second potential mechanism. Immune cells and different inflammatory factors have a significant contribution to the development and progression of atherosclerotic lesions [46, 47]. Given that atherosclerosis is a risk factor for NAION, increased NLR levels may suggest low-grade chronic inflammation.
In addition to NLR, other complete blood count (CBC)-based biomarkers have been shown to play a significant role in the diagnosis and prediction of NAION. For instance, Pinna et al. in 2019 revealed that median values of mean platelet volume (MPV; p = 0.01), dNLR [dNLR = neutrophils/(white blood cells–neutrophils)] (p = 0.01), and red cell distribution width (RDW; p = 0.015) were all significantly higher in NAION patients. In the mentioned study, there were no significant differences between two groups in terms of neutrophils, lymphocytes, white blood cells, and PLR [18]. In Kocak et al.’s study monocyte, platelet, and neutrophil counts were greater in the NAION group compared to control group, but the difference was statistically insignificant (p > 0.05). The SII in the NAION group were greater than in the control group (p = 0.011). SII had an area under the curve of 0.66, and SII of > 417 indicated NAION with a specificity of 49% and sensitivity of 71%. Monocyte-to-lymphocyte ratio (MLR) and PLR did not significantly vary between the groups (p = 0.347 and p = 0.105, respectively) [17]. Inanc et al. in 2018 demonstrated that mean platelet volume (MPV), Plateletcrit, and platelet distribution width (PDW) is higher among NAION and patients with arteritic anterior ischemic neuropathy (AAION) groups compared with healthy controls. Just in the AAION group compared to the control and NAION groups, the mean NLR was significantly higher, indicating that platelet function plays a critical role in AIONs and that NLR may be utilized to distinguish AAION from NAION [16]. Recently, other inflammatory biomarkers have been researched in addition to the biomarkers that are based on CBC. Micieli et al. in 2017 illustrated that in Aqueous Humor, the mean level of IL-2 (5.56 ± 1.27 pg/mL) was significantly lower in the NAION group when compared to the cataract control group (16.6 ± 14.0 pg/mL; P = .002) and the mean level of vascular endothelial growth factor (VEGF) (94.1 ± 40.4 pg/mL) was significantly higher in the NAION group than in the cataract control group (52.2 ± 20.8 pg/mL; P = .010). However, there was not a significant difference in term of IL-1β, TNF-α, IL-6, and IL-8 [48]. According to Mesentier-Louro et al.'s research in 2021, the best biomarker candidates for acute NAION were MCP-2, Eotaxin-3, TRAIL, and TPO. CXCL10 and IL-1α were shown to be the most effective treatment targets in chronic NAION [49]. In this research, comprehensive plasma profiling showed considerable differences in cytokine profiles between mouse model and human with NAION compared with controls, which validates increased inflammation. In human NAION, 21 cytokines elevated more than 1.5 times over control levels, whereas none dropped more than 0.5 times. Four cytokines rose > 1.5x in mouse NAION, whereas two dropped to 0.5x. Monocyte–chemoattractant protein MCP3 and C–C motif chemokine CCL11 (the protein which is associated with human aging) were the most increased cytokine in both mouse models and human with NAION. IL1a, CXCL5, and CXCL13 were the cytokines that increased the highest in human chronic NAION, along with CCL11. Bilateral NAION exhibited the most dramatic elevations in these cytokines. Plasma from three human NAION patients increased angiogenesis and disrupted the endothelial barrier in cultured human retinal endothelial cells [50].
Our analysis is limited by the retrospective design of the included studies, their relatively small sample sizes, and the lack of blood samples drawn at multiple points over the course of a clinically relevant period.
Conclusion
Our study showed that NLR could predict NAION with high sensitivity and specificity. NLR level is elevated in patients with NAION than healthy controls. However, prospective studies, preferably randomized and multicenter, are needed to establish whether NLR has predictive value for visual acuity and prognosis in NAION. Furthermore, future studies could address whether use of other biomarkers, such as ESR, systemic immune inflammation index (SII), platelet-to-lymphocyte ratio (PLR), or platelet distribution width, which have also been found to correspond to NAION, help refine NLR’s diagnostic value.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Acknowledgements
Not applicable.
Abbreviations
- NLR
Neutrophil-to-lymphocyte ratio
- NAION
Non-arteritic anterior ischemic optic neuropathy
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- NOS
Newcastle–Ottawa scale
- SMD
Standard mean difference
- CI
Confidence interval
- IQR
Interquartile range
- SROC
Summary receiver operating characteristic
- AAION
Arteritic anterior ischemic optic neuropathy
- BRB
Blood–retinal barrier
- rAION
Rodent
- CBC
Complete blood count
- RDW
Red cell distribution width
- MLR
Monocyte-to-lymphocyte ratio
- MPV
Mean platelet volume
- PDW
Platelet distribution width
- VEGF
Vascular endothelial growth factor
- SII
Systemic immune inflammation index
- PLR
Platelet-to-lymphocyte ratio
- BCVA
Best-corrected visual acuity
Author contributions
E.K: study design, draft the initial manuscript, revision of the manuscript, data collection; Sh.N: supervision of data collection, drawing the tables, searching the databases; J.V: data collection, statistical analysis; I.S: supervision of data collection, initial analysis; H.B: statistical analysis, data collection; A.Gh: design and conceptualization of the study, writing the initial draft; B.L-W, A.M.E.M: revision of the manuscript, data collection; Sh.Kh: statistical analysis, study design, revision of the manuscript. All authors read and approved the final manuscript and are responsible for data review.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
8. References
- 1.Hayreh SS. Ischemic optic neuropathy. Prog Retinal Eye Res. 2009;28(1):34–62. [DOI] [PubMed] [Google Scholar]
- 2.Raizada K, Margolin E. Non-arteritic anterior ischemic optic neuropathy, in StatPearls. St. Petersburg: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 3.Stapleton PA, et al. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm (Lond). 2010;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick DM, Van Beusecum J, Kirabo A. The role of inflammation in hypertension: novel concepts. Curr Opin Physiol. 2021;19:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsalamandris S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfaddagh A, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. 2020;4: 100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salgado C, et al. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch Ophthalmol. 2011;129(12):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song M, et al. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11(1):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–88. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, et al. The association between phosphodiesterase type 5 inhibitor use and risk of non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Sex Med. 2018;6(3):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatziralli IP, et al. Cardiometabolic factors and risk of non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260(5):1445–56. [DOI] [PubMed] [Google Scholar]
- 12.Tong YX, et al. Optical coherence tomography evaluation of retinal nerve fiber layer thickness in non-arteritic anterior ischemic optic neuropathy and primary open angle glaucoma: a systematic review and meta-analysis. Int J Ophthalmol. 2022;15(8):1370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou Y, et al. Microvascular alterations detected by optical coherence tomography angiography in non-arteritic anterior ischaemic optic neuropathy: a meta-analysis. Acta Ophthalmol. 2022;100(2):e386–95. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, et al. Steroids in the treatment of nonarteritic anterior ischemic optic neuropathy: a PRISMA-compliant meta-analysis. Medicine (Baltimore). 2019;98(46): e17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunes A, et al. Neutrophil to lymphocyte ratio in patients with nonarteritic anterior ischemic optic neuropathy. Korean J Ophthalmol. 2017;31(2):159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inanc M, et al. Could platelet indices and neutrophil to lymphocyte ratio be new biomarkers for differentiation of arteritic anterior ischemic neuropathy from non-arteritic type? Neuro Ophthalmol. 2018;42(5):287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocak N, et al. Serum inflammatory biomarkers in patients with nonarteritic anterior ischemic optic neuropathy. Korean J Ophthalmol. 2020;34(6):478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinna A, et al. Complete blood cell count indexes in non-arteritic anterior ischemic optic neuropathy. Acta Ophthalmol. 2019. 10.1111/j.1755-3768.2019.5244.31486592 [Google Scholar]
- 19.Polat O, et al. Neutrophil-to-lymphocyte ratio as a marker in patients with non-arteritic anterior ischemic optic neuropathy. Balk Med J. 2015;32(4):382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinsawad P, Boonhaijaroen T. Neutrophil to lymphocyte ratio as a marker in patients with non-arteritic anterior ischemic optic neuropathy in HRH princess maha chakri sirindhorn medical center. J Med Assoc Thail. 2021;104(9):44–50. [Google Scholar]
- 21.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906. [DOI] [PubMed] [Google Scholar]
- 22.Gotschall T. EndNote 20 desktop version. J Med Libr AssocJMLA. 2021;109(3):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ga W. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. In: 3rd Symposium on Systematic Reviews: Beyond the Basics. Oxford, UK; 2000. p. 3–5.
- 24.Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol. 2004;17(1):27–35. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg-Cohen N, et al. Elevated plasma levels of interleukin 8 in patients with acute anterior ischaemic optic neuropathy. Br J Ophthalmol. 2004;88(12):1538–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss JN, Levy S, Benes SC. Stem cell ophthalmology treatment study: bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig. 2017;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trial IOND. Research GrouCharacteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the ischemic optic neuropathy decompression trial. Arch Ophthalmol. 1996;114:1366–74. [DOI] [PubMed] [Google Scholar]
- 28.Mojon DS, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120(5):601–5. [DOI] [PubMed] [Google Scholar]
- 29.Servilla KS, Groggel GC. Anterior ischemic optic neuropathy as a complication of hemodialysis. Am J Kidney Dis Off J National Kidney Found. 1986;8(1):61–3. [DOI] [PubMed] [Google Scholar]
- 30.Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23(2):157–63. [DOI] [PubMed] [Google Scholar]
- 31.Arnold AC. Anterior ischemic optic neuropathy. In seminars in ophthalmology. Abingdon: Taylor & Francis; 1995. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein SL, et al. Functional and cellular responses in a novel rodent model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2003;44(10):4153–62. [DOI] [PubMed] [Google Scholar]
- 33.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–57. [DOI] [PubMed] [Google Scholar]
- 34.Berchtold D, et al. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol. 2020;30(6):1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semerano A, et al. Leukocyte counts and ratios are predictive of stroke outcome and hemorrhagic complications independently of infections. Front Neurol. 2020;11:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CS, et al. A primate model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2008;49(7):2985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein SL, Johnson MA, Miller NR. Nonarteritic anterior ischemic optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res. 2011;30(3):167–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahzouni P, Shavakhi M. Prostate-specific membrane antigen expression in neovasculature of glioblastoma multiforme. Adv Biomed Res. 2019;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahadiat S-A, et al. Role of vitamin D in pathogenesis, diagnosis, and treatment of inflammatory diseases. Kindle. 2022;2(1):1–119. [Google Scholar]
- 40.Shavakhi M, et al. Prognostic role of neutrophil to lymphocyte ratio in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2022;2022(1):1554079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanzadeh M, et al. Prognostic role of neutrophil to lymphocyte ratio in allergic rhinitis: a systematic review and meta-analysis. Indian J Otolaryngol Head Neck Surg. 2024;76(1):1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolaczkowska E, Kubes. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. [DOI] [PubMed] [Google Scholar]
- 43.Pitsavos C, et al. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev Diabet Stud RDS. 2007;4(2):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106(1):56–61. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi N, et al. White blood cell count as a risk factor for hypertension; a study of Japanese male office workers. J Hypertens. 2002;20(5):851–7. [DOI] [PubMed] [Google Scholar]
- 46.Pant S, et al. Inflammation and atherosclerosis—revisited. J Cardiovasc Pharmacol Ther. 2014;19(2):170–8. [DOI] [PubMed] [Google Scholar]
- 47.Hansen PR. Chronic inflammatory diseases and atherosclerotic cardiovascular disease: innocent bystanders or partners in crime? Curr Pharm Design. 2018;24(3):281–90. [DOI] [PubMed] [Google Scholar]
- 48.Micieli JA, et al. Aqueous humor cytokines in patients with acute nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2017;177:175–81. [DOI] [PubMed] [Google Scholar]
- 49.Mesentier-Louro LA, et al. Immunoprofiling of nonarteritic anterior ischemic optic neuropathy. Transl Vis Sci Technol. 2021;10(8):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesentier-Louro LA, et al. Identification of novel biomarkers of nonarteritic anterior ischemic optic neuropathy using cytokine profiling. Investig Ophthalmol Vis Sci. 2020;61(7):4586–4586. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.