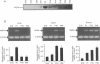Abstract
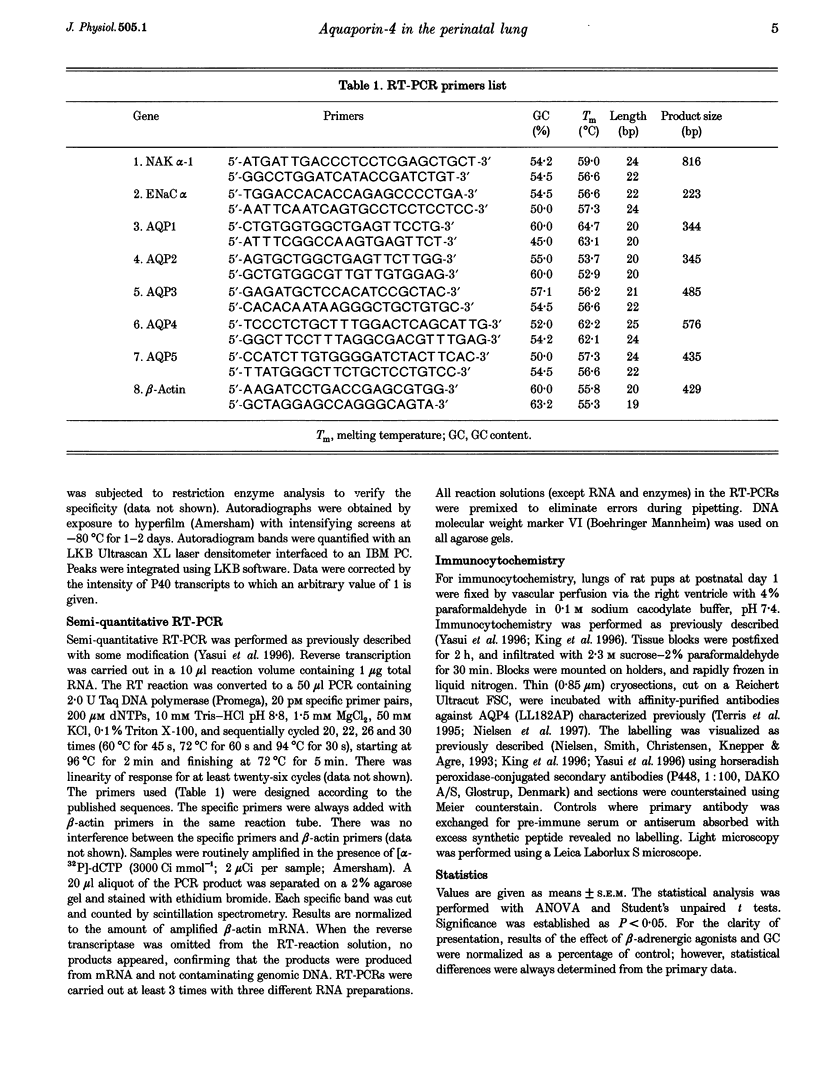
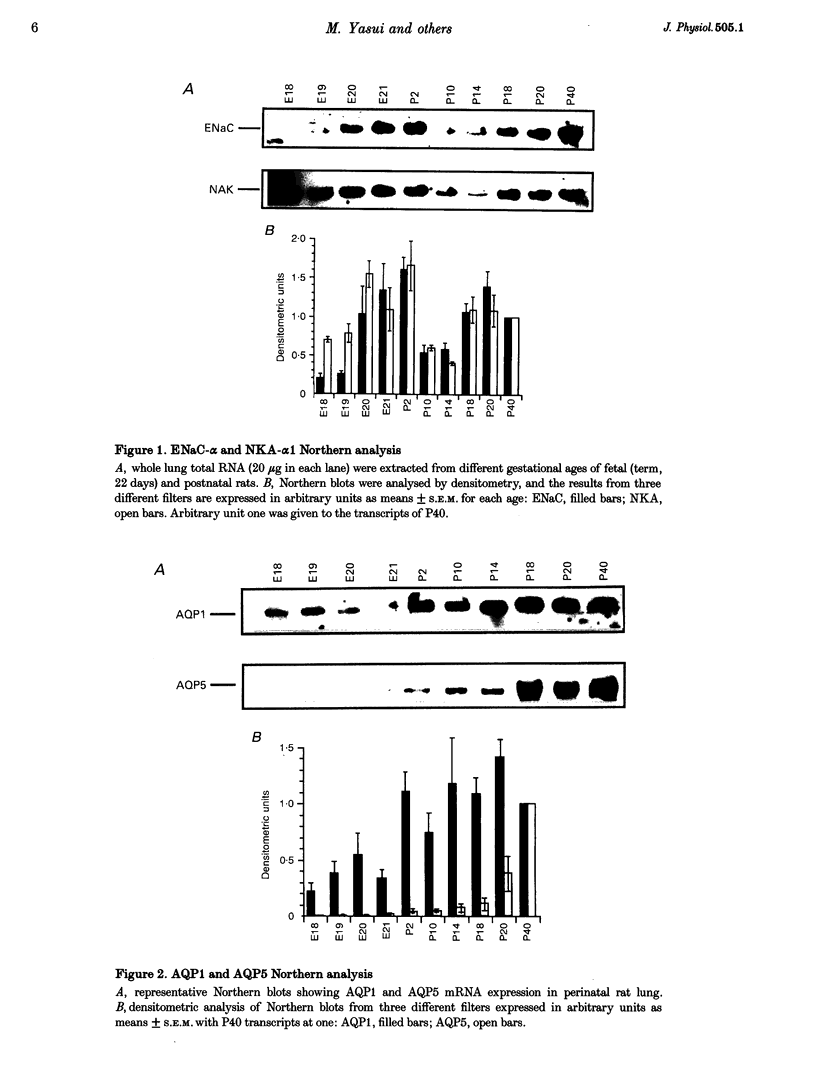
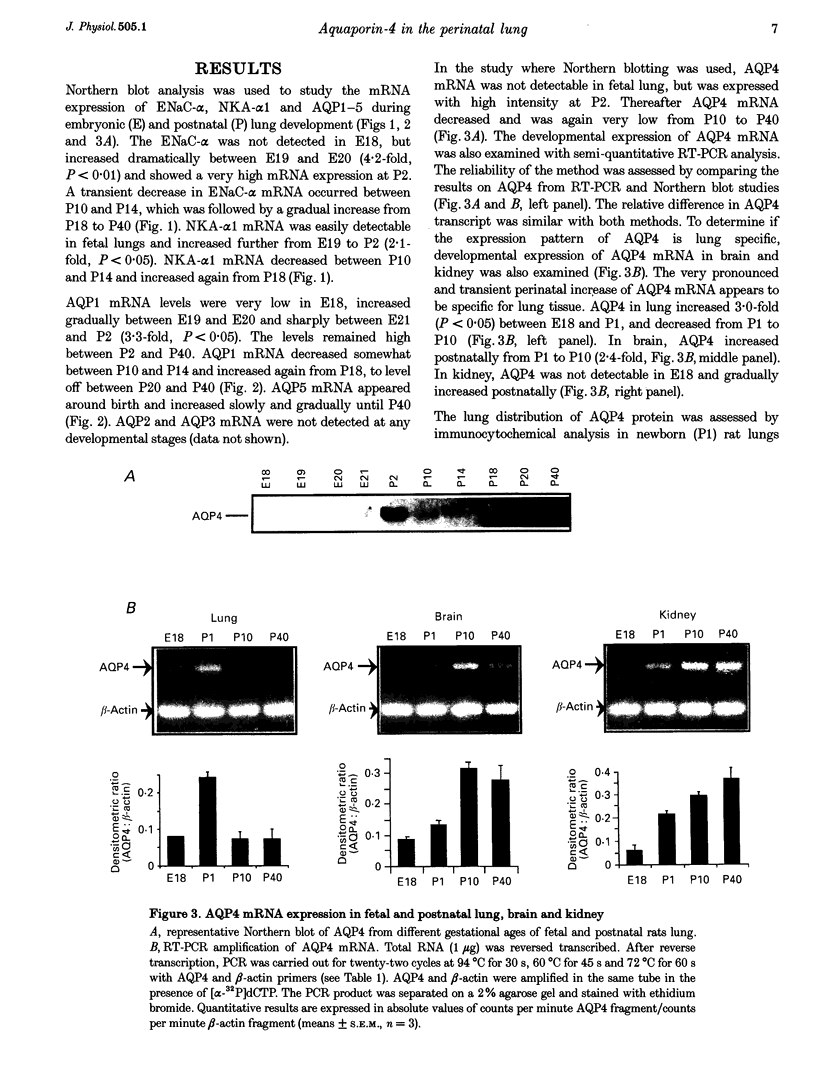
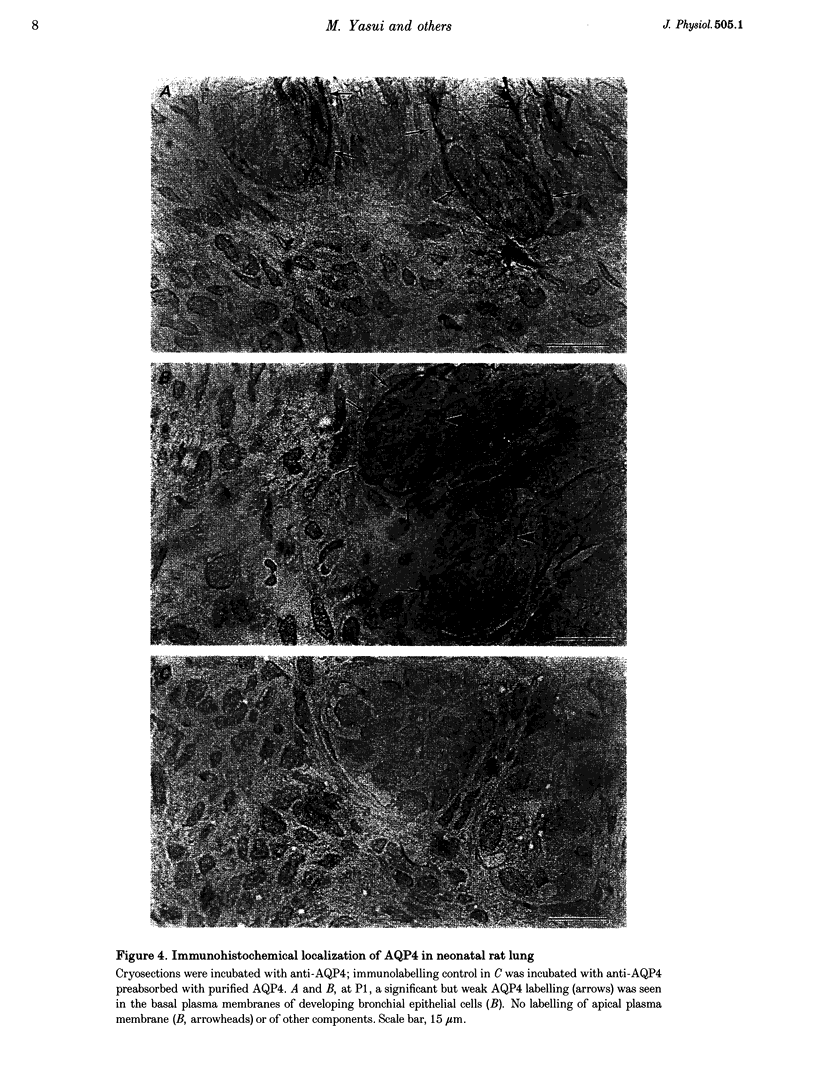
1. At birth, rapid removal of lung liquid from potential airspaces is required to establish pulmonary gas exchange. To investigate the role for water channels, aquaporins (AQP) and ion transporters in this process, the mRNA expression of AQP, Na+,K(+)-ATPase and the amiloride-sensitive Na+ channel (ENaC) were studied in the fetal and postnatal rat lung. 2. The mRNA expression of all transporters studied increased postnatally. 3. The following water channels were expressed in the lung, AQP1, 4 and 5. The most specific perinatal induction pattern was observed for AQP4. A sharp and transient increase of AQP4 mRNA occurred just after birth coinciding with the time course for clearance of lung liquid. This transient induction of AQP4 mRNA at birth was lung-tissue specific. Around birth there was a moderate increase in AQP1 mRNA, which was not transient. AQP5 increased continuously until adulthood. 4. Fetal lung AQP4 mRNA was induced by both beta-adrenergic agonists and glucocorticoid hormone, which are factors that have been suggested to accelerate the clearance of lung liquid. 5. Immunocytochemistry revealed that AQP4 was located in the basolateral membranes of bronchial epithelia in newborn rats, consistent with the view that this is the major site for perinatal lung liquid absorption. 6. The Na+,K(+)-ATPase alpha 1 subunit and ENaC alpha-subunit mRNA also increased around birth, suggesting that they co-operatively facilitate lung liquid clearance at birth. 7. These data indicate that removal of lung liquid at birth is associated with pronounced and well-synchronized changes in the expression of AQP and the ion transporters studied. The transient perinatal induction of AQP4, which could be prenatally induced by beta-adrenergic agonists, and the localization of this water channel strongly suggest that it plays a critical role for removal of lung liquid at the time of birth.
Full text
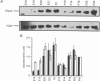
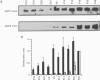
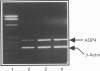
PDF

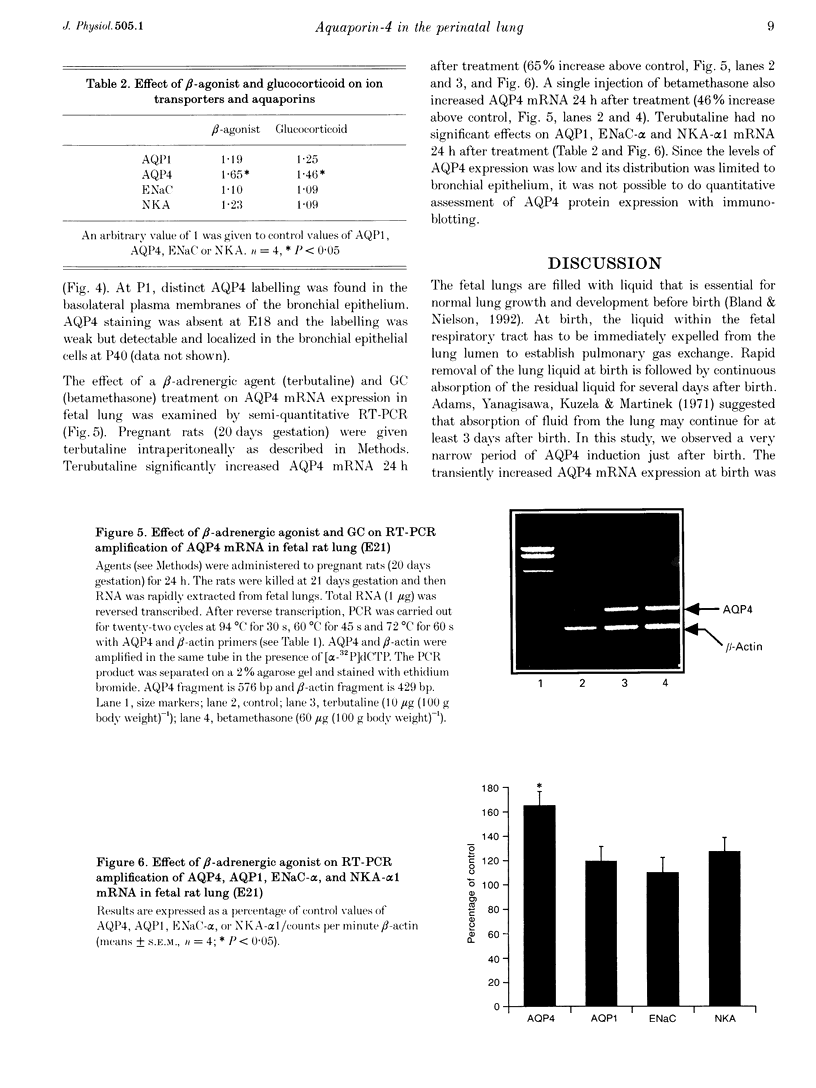


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams F. H., Yanagisawa M., Kuzela D., Martinek H. The disappearance of fetal lung fluid following birth. J Pediatr. 1971 May;78(5):837–843. doi: 10.1016/s0022-3476(71)80356-6. [DOI] [PubMed] [Google Scholar]
- Agre P., Brown D., Nielsen S. Aquaporin water channels: unanswered questions and unresolved controversies. Curr Opin Cell Biol. 1995 Aug;7(4):472–483. doi: 10.1016/0955-0674(95)80003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P. L. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989 May;10(2):165–181. doi: 10.1210/edrv-10-2-165. [DOI] [PubMed] [Google Scholar]
- Barker P. M., Markiewicz M., Parker K. A., Walters D. V., Strang L. B. Synergistic action of triiodothyronine and hydrocortisone on epinephrine-induced reabsorption of fetal lung liquid. Pediatr Res. 1990 Jun;27(6):588–591. doi: 10.1203/00006450-199006000-00010. [DOI] [PubMed] [Google Scholar]
- Bland R. D., Bressack M. A., McMillan D. D. Labor decreases the lung water content of newborn rabbits. Am J Obstet Gynecol. 1979 Oct 1;135(3):364–367. doi: 10.1016/0002-9378(79)90706-3. [DOI] [PubMed] [Google Scholar]
- Bland R. D., McMillan D. D., Bressack M. A., Dong L. Clearance of liquid from lungs of newborn rabbits. J Appl Physiol Respir Environ Exerc Physiol. 1980 Aug;49(2):171–177. doi: 10.1152/jappl.1980.49.2.171. [DOI] [PubMed] [Google Scholar]
- Bland R. D., Nielson D. W. Developmental changes in lung epithelial ion transport and liquid movement. Annu Rev Physiol. 1992;54:373–394. doi: 10.1146/annurev.ph.54.030192.002105. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Olver R. E., Ramsden C. A., Strang L. B., Walters D. V. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol. 1983 Nov;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsi G., Wang Z. M., Akusjärvi G., Aperia A. Sensitive periods for glucocorticoids' regulation of Na+,K(+)-ATPase mRNA in the developing lung and kidney. Pediatr Res. 1993 Jan;33(1):5–9. doi: 10.1203/00006450-199301000-00002. [DOI] [PubMed] [Google Scholar]
- Chapman D. L., Carlton D. P., Cummings J. J., Poulain F. R., Bland R. D. Intrapulmonary terbutaline and aminophylline decrease lung liquid in fetal lambs. Pediatr Res. 1991 Apr;29(4 Pt 1):357–361. doi: 10.1203/00006450-199104000-00006. [DOI] [PubMed] [Google Scholar]
- Cheng J. B., Goldfien A., Ballard P. L., Roberts J. M. Glucocorticoids increase pulmonary beta-adrenergic receptors in fetal rabbit. Endocrinology. 1980 Nov;107(5):1646–1648. doi: 10.1210/endo-107-5-1646. [DOI] [PubMed] [Google Scholar]
- Folkesson H. G., Matthay M. A., Frigeri A., Verkman A. S. Transepithelial water permeability in microperfused distal airways. Evidence for channel-mediated water transport. J Clin Invest. 1996 Feb 1;97(3):664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson H. G., Matthay M. A., Hasegawa H., Kheradmand F., Verkman A. S. Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E., Barker P., Gatzy J., Beermann F., Verdumo C., Schmidt A., Boucher R., Rossier B. C. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996 Mar;12(3):325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- King L. S., Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- King L. S., Nielsen S., Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest. 1996 May 15;97(10):2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Nagelhus E. A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O. P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997 Jan 1;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brodovich H., Canessa C., Ueda J., Rafii B., Rossier B. C., Edelson J. Expression of the epithelial Na+ channel in the developing rat lung. Am J Physiol. 1993 Aug;265(2 Pt 1):C491–C496. doi: 10.1152/ajpcell.1993.265.2.C491. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A. N., Desgranges M. F., Masson M., Colle E., Shatz R., Gelfand M. M. The antenatal use of betamethasone in the prevention of respiratory distress syndrome: a controlled double-blind study. Pediatrics. 1979 Jan;63(1):73–79. [PubMed] [Google Scholar]
- Raina S., Preston G. M., Guggino W. B., Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995 Jan 27;270(4):1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Schellhase D. E., Shannon J. M. Effects of maternal dexamethasone on expression of SP-A, SP-B, and SP-C in the fetal rat lung. Am J Respir Cell Mol Biol. 1991 Apr;4(4):304–312. doi: 10.1165/ajrcmb/4.4.304. [DOI] [PubMed] [Google Scholar]
- Tchepichev S., Ueda J., Canessa C., Rossier B. C., O'Brodovich H. Lung epithelial Na channel subunits are differentially regulated during development and by steroids. Am J Physiol. 1995 Sep;269(3 Pt 1):C805–C812. doi: 10.1152/ajpcell.1995.269.3.C805. [DOI] [PubMed] [Google Scholar]
- Terris J., Ecelbarger C. A., Marples D., Knepper M. A., Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol. 1995 Dec;269(6 Pt 2):F775–F785. doi: 10.1152/ajprenal.1995.269.6.F775. [DOI] [PubMed] [Google Scholar]
- Umenishi F., Carter E. P., Yang B., Oliver B., Matthay M. A., Verkman A. S. Sharp increase in rat lung water channel expression in the perinatal period. Am J Respir Cell Mol Biol. 1996 Nov;15(5):673–679. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Manton M. A., Darovec-Beckerman C., Adams K. G., Moore J. J. beta-Adrenergic receptors in the developing rabbit lung. Am J Physiol. 1981 Apr;240(4):E351–E357. doi: 10.1152/ajpendo.1981.240.4.E351. [DOI] [PubMed] [Google Scholar]
- Yasui M., Marples D., Belusa R., Eklöf A. C., Celsi G., Nielsen S., Aperia A. Development of urinary concentrating capacity: role of aquaporin-2. Am J Physiol. 1996 Aug;271(2 Pt 2):F461–F468. doi: 10.1152/ajprenal.1996.271.2.F461. [DOI] [PubMed] [Google Scholar]