Abstract
Background
It is not clear if conventional liver fat cutoff of 5.56% weight which has been used for identifying fatty liver in western populations is also applicable for Asians. In Asian women of reproductive age, we evaluate the optimum metabolic syndrome (MetS)-linked liver fat cutoff, the specific metabolomic alterations apparent at this cutoff, as well as prospective associations of preconception liver fat levels with gestational dysglycemia.
Methods
Liver fat (measured by magnetic resonance spectroscopy), MetS, and nuclear magnetic resonance (NMR)-based plasma metabolomic profiles were assessed in 382 Asian women, who were planning to conceive. Ninety-eight women went on to become pregnant and received an oral glucose tolerance test at week 26 of gestation.
Results
The optimum liver fat cutoff for diagnosing MetS was 2.07%weight. Preconception liver fat was categorized into Low (liver fat < 2.07%), Moderate (2.07% ≤ liver fat < 5.56%), and High (liver fat ≥ 5.56%) groups. Individual MetS traits showed worsening trends, going from Low to Moderate to High groups. Multiple plasma metabolomic alterations, previously linked to incident type 2 diabetes (T2D), were already evident in the Moderate group (adjusted for ethnicity, age, parity, educational attainment, and BMI). Both a cross-sectional multi-metabolite score for incident T2D and mid-gestational glucose area under the curve showed increasing trends, going from Low to Moderate to High groups (p < 0.001 for both). Gestational diabetes incidence was 2-fold (p = 0.23) and 7-fold (p < 0.001) higher in the Moderate and High groups relative to the Low group.
Conclusions
In Asian women of reproductive age, moderate liver fat accumulation below the conventional fatty liver cutoff was not metabolically benign and was linked to gestational dysglycemia. The newly derived cutoff can aid in screening individuals before adverse metabolic phenotypes have consolidated, which provides a longer window for preventive strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03779-0.
Keywords: Metabolomics, Magnetic resonance spectroscopy, Metabolic dysfunction-associated steatotic liver disease (MASLD), Metabolic syndrome, Gestational diabetes
Background
Elevated hepatic triglyceride accumulation can have lipotoxic effects and increases predisposition to progressive liver diseases (steatohepatitis, fibrosis, and eventually cirrhosis). However, most of the mortality risks linked to elevated hepatic triglyceride accumulation are due to extrahepatic morbidities explained by its strong associations with type 2 diabetes (T2D), cardiovascular disease, and chronic kidney disease [1]. This has led to the recent consensus nomenclature of metabolic dysfunction-associated steatotic liver disease (MASLD) by three international liver associations—defined as abnormal liver fat accumulation, in the presence of at least one cardiometabolic risk factor [2]. Notably, the precise cutoff for identifying abnormal liver fat was not determined in this new nomenclature, likely due to the great diversity of methods currently in use for assessing liver fat accumulation. These include quantitative fat assessments obtained either invasively using biochemical triglyceride extraction from liver biopsies or noninvasively using magnetic resonance imaging/spectroscopy (MRI/MRS), semi-quantitative fat assessments based on histopathological grading of liver biopsies, noninvasive qualitative fat assessment using B-mode ultrasound, and indirect assessment using circulating liver enzymes (gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT)).
The seminal work on identifying a cutoff for “abnormal” liver fat was done by LS Szczepaniak et al. [3]. Based on MRS-based measurement of liver fat, this work identified 55.6 mg/g or 5.56% weight as the upper limit of normal, based on the 95th percentile of liver fat in subjects from the Dallas Heart Study, who had a low risk for hepatic steatosis (defined as BMI < 25 kg/m2, no T2D, and normal fasting glucose and alanine transaminase levels). This population-based cutoff has been widely used, with the implicit assumption that liver fat accumulation below this level is metabolically benign. However, this cutoff has been shown to represent a level at which common clinical metabolic phenotypes have already consolidated [4]. Hence, the threshold at which metabolic alterations emerge is likely to be lower [5] and may vary in different populations. Identifying this threshold is important for prospective risk stratification, early targeted interventions, and understanding the true prevalence of metabolically adverse liver fat accumulation. While there has been prior work which found metabolic risk-based cutoffs for fatty liver to be lower than the conventional population-based cutoffs [6], there are limited studies in Asians, in whom metabolic risks emerge at relatively lower levels of BMI and central adiposity [7, 8].
The current work has three major goals. Firstly, we aimed to identify a metabolic-risk-linked cutoff for diagnosing abnormal liver fat accumulation in a multi-ethnic preconception cohort of reproductive age Asian women. Secondly, we aimed to assess if liver fat accumulation above this metabolic-risk-linked cutoff, but below the conventional cutoff, is already linked to alterations in the plasma metabolome. This can provide a detailed look at specific alterations at the level of lipoprotein metabolism, lipoprotein subclasses concentrations and composition, glycolytic metabolites, ketone bodies, renal function, amino acids, fatty acids, and inflammatory markers, beyond just the common MetS risk factors. Finally, we aimed to assess the prospective association of preconception liver fat levels with gestational dysglycemia.
Methods
Study population and participant characteristics
Study subjects that had both nuclear magnetic resonance (NMR) metabolomics and liver MRS data at preconception were identified from the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO) cohort [9]. S-PRESTO is a multi-ethnic (Indian, Chinese, Malay, or mixed ethnicities) Asian prospective longitudinal mother–offspring cohort which recruited women aged 18 to 45 years between February 2015 and October 2017 based on the following criteria: (1) planning to conceive within the next one year and (2) intention to reside in Singapore for the next 5 years. Detailed exclusion criteria are listed in the supplementary section (Additional File 1: SPRESTO Exclusion Criteria). This research is being reported in line with the STROBE criteria [10].
The cohort had a total of 1032 participants, of which 382 women had MRS-based liver fat imaging data measured at the preconception imaging visit. Of the 382 women, 98 went on to become pregnant. Interviewer-administered questions at enrolment were used to collect information on participant characteristics. The participant characteristics are shown in Table 1.
Table 1.
Participant characteristics
| Characteristics N = 382 | Mean ± SD/% |
|---|---|
| Age (years) | 31.5 ± 3.9 |
| BMI (kg/m2) | 23.6 ± 5.4 |
| - Underweight | 10.0% |
| - Normal weight | 46.8% |
| - Overweight | 15.3% |
| - Obese | 27.9% |
| Ethnicity | |
| - Chinese | 68.8% |
| - Malay | 18.1% |
| - Indian | 10.7% |
| - Mixed | 2.4% |
| Educational status | |
| - Below university | 40.1% |
| - University | 59.9% |
| Parity | |
| - Nulliparous | 66.9% |
| - Parous | 33.1% |
| OGTT glucose levels (%) | |
| - Normoglycemic rangea | 88.1% |
| - Prediabetes rangeb | 10.1% |
| - Diabetes rangec | 1.9% |
aNormoglycemic range: fasting glucose < 6.1 mmol/l and 2-h glucose < 7.8 mmol/l
bPrediabetes range: fasting glucose: 6.1–6.9 mmol/l or 2-h glucose: 7.8–11 mmol/l
cDiabetes range: fasting glucose > 6.9 mmol/l or 2-h glucose > 11 mmol/l
Briefly, the women were relatively young (31.5 ± 3.9 years (mean ± SD)) and lean (56.8% underweight and normal weight). Most were university educated (59.9%), nulliparous (66.9%), normoglycemic (88.1%), and of Chinese ethnicity (68.8%). There were no statistically significant differences in the characteristics of participants in the cohort who had liver imaging data and those who did not (Additional File 1: Table S1).
Liver fat measurement using magnetic resonance spectroscopy
At the preconception imaging visit, liver fat was measured by single voxel proton magnetic resonance spectroscopy (1H-MRS) from 8 cm3 voxels localized in the left and right lobe of the liver using a 3 T clinical MRI scanner. Care was taken to avoid visible blood vessels and the liver boundary. Localized MRS was performed using the point-resolved spectroscopy (PRESS) sequence (TR/TR = 33/2000 ms), with (averages = 4) and without water suppression (averages = 1). Respiratory motion was handled by breath-holding. Area of the water peak (4.7 ppm) in the water-unsuppressed spectrum and lipid peaks (sum of lipid peaks between 0 and 3 ppm) in the water-suppressed spectrum were quantified using LCModel [11]. Visual quality control checks were performed to reject spectra with artifacts. Data from 382 women (97%) were usable after the checks. T2 correction of the water and lipid peaks was performed using the mean of water and fat T2 relaxation values reported in literature [12–14]. The T2 corrected water lipid peak areas were used to estimate liver fat percentage by weight using validated methods [3]. A correction factor of 0.914 was used to take into account lipid peaks that cannot be directly quantified as they were masked by the water peak [13]. Overall liver fat (%weight) was derived by averaging the liver fat measured from the voxels placed in the right and left lobes. Derivations for the conversion of liver fat expressed in %weight to fat fraction units (and vice versa) are described in the supplementary digital content.
Metabolic assessments
Weight, height, BMI, waist circumference, and blood pressure were measured at the pre-conception visit. Fasting plasma glucose (FG) (overnight fast of 8–14 h) and serum triglyceride, total cholesterol, and HDL cholesterol were measured using a Beckman AU5800 analyzer (Beckman Coulter). LDL cholesterol was calculated using the Friedewald equation (LDL-cholesterol (mmol/L) = total cholesterol (mmol/L) − HDL-cholesterol (mmol/L) − triglyceride (mmol/L)/2.2) [15]. Serum liver enzymes levels (gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT)) were measured by colorimetric assay (Beckman AU5800 analyzer). Metabolic syndrome (MetS) was diagnosed using the harmonized MetS criteria (elevated abdominal obesity (≥ 80 cm in Asian females), hypertriglyceridemia (≥ 1.7 mmol/L or drug treatment for elevated TG), reduced HDL (< 1.3 mmol/L in females or drug treatment for low HDL), high blood pressure (≥ 130/85 mmHg or antihypertensive drug treatment), elevated fasting glucose (≥ 100 mg/dL or drug treatment of elevated glucose)) [16]. At week 26 of gestation, an oral glucose tolerance test (OGTT) was performed in the 98 women who became pregnant. The glucose response was assessed at 5 timepoints (0, 30, 60, 90, and 120 min). The area under the curve (AUC) of the glucose response was determined using the trapezoid approximation (complete data for AUC calculation was available in 63 women). Gestational diabetes mellitus (GDM) status using the International Association of Diabetes and Pregnancy Study (IADPSG) criteria [17] (data for IADPSG GDM classification available in 91 women).
Plasma metabolomics
From fasting blood samples collected at the preconception visit, circulating maternal levels of 249 plasma biomarkers were profiled using the Nightingale Health’s proton NMR Metabolomics Platform. The NMR panel was also used to compute the multi-metabolite prospective risk score for incident T2D [18]. Briefly, this multi-metabolite score comprised of three metabolites: phenylalanine, non-esterified cholesterol in large HDL, and the ratio of cholesteryl ester to total lipid in large VLDL. We also derived the ratio of cholesteryl ester (CE) to free cholesterol (FC) for each HDL subfraction as well as for the total CE/total free cholesterol as proxies of hepatic lecithin:cholesterol acyltransferase (LCAT) activity. Individual biomarker readouts were log10 transformed (because of skewness) and then z-transformed before further downstream statistical analysis.
Statistical analysis
Statistical analyses were performed in SPSS Statistics v26 (IBM) and R (v 4.2.2). R packages dplyr v.1.1.0, ggpubr v0.6.0, ggplot2 v3.4.2, ggforestplot v0.1.0, and Epiviz v0.0.1 were used for data analysis and visualization.
Receiver operator curve (ROC) analysis was performed to assess the ability of continuous liver fat (in %weight) to diagnose MetS. The optimum liver fat cutoff for predicting MetS was obtained using the maximum value of the Youden index. Continuous liver fat was converted to a categorical variable (low, moderate, high) based on the following thresholds (< metabolic-risk based cutoff, ≥ metabolic-risk based cutoff and < conventional cutoff of 5.56%, ≥ conventional cutoff of 5.56%).
NMR biomarker values below the limit of quantification were replaced by half of the minimum value [19]. Subsequently, all the metabolomic biomarkers were log10 transformed and z-score standardized. Analysis of covariance (ANCOVA), adjusted for BMI, age, ethnicity, education level and parity, was used to assess the adjusted mean difference (AMD) in individual NMR metabolomic markers (outcome variable) in the Moderate and High liver fat categories (exposure variable) relative to the Low group (in SD units of the log10 transformed NMR-metabolite). The confounders were selected based on known risk factors for both liver fat accumulation and metabolic alterations in literature. We corrected for multiple testing using the Benjamini-Hochberg (BH) method. Biomarkers with adjusted p-value (BH-adj p-value) < 0.05 were considered statistically significant.
We tested for increasing/decreasing trends in clinical metabolic characteristics, liver enzymes, the multi-metabolite risk score for incident T2D, and glucose AUC at mid-gestation, while going from the Low to Moderate to High liver fat groups, using the Jonckheere-Terpstra test. Values of liver enzymes ALT and GGT which were below the limit of detection were replaced by a value determined by dividing the respective limit of detection by the square root of 2. Risk ratios (RR) and confidence intervals for GDM incidence at mid-gestation were derived using modified Poisson regression [20].
Results
MetS associated liver fat cutoff
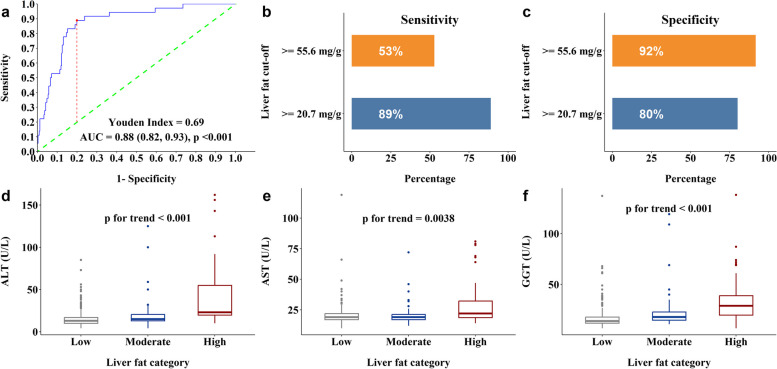
The ROC analysis revealed that continuous liver fat levels had a high ability to discriminate between women, with and without MetS (AUC = 0.88, 95% CI 0.82–0.93, p < 0.001) (Fig. 1a). The optimum liver fat cutoff based on the highest Youden Index was 2.07% weight (20.7 mg/g or fat fraction% of 3.2%). The sensitivity and specificity for classifying MetS using the metabolic-risk-based liver fat cutoff of 2.07% weight and the conventional fatty liver cutoff of 5.56% weight are shown in Fig. 1b and c, respectively.
Fig. 1.
Box plots of metabolic-risk-based liver fat cutoff and liver enzymes across the different liver fat categories. a Receiver operator curve analysis for predicting MetS with continuous liver fat levels as the sole predictor, 95% CI indicated in brackets (n = 372). b Sensitivity of proposed vs conventional liver fat cutoffs. c Specificity of proposed vs conventional liver fat cutoffs. d ALT (U/L) (n = 373), e AST (U/L) (n = 373), f GGT (U/L) (n = 373). p for trend values represents trend of each liver enzyme with increasing liver fat
Since these cutoffs have been variously reported in %weight or fat fraction%, we have reported the cutoffs in both units. The newly derived cutoff is 63% lower than the conventional population-based cutoff of 5.56% weight commonly used in MRS based liver fat studies and 36% lower than the commonly used proton density fat fraction cutoff of 5% in MRI based liver fat studies. While the conventional liver fat cutoff of 5.56% weight had a high specificity (92%) for diagnosing MetS in our cohort, it had very low sensitivity (53%). The newly derived metabolic-risk-linked cutoff provided a good trade-off between sensitivity (89%) and specificity (80%). Based on the newly derived cutoff, the preconception liver fat was categorized into Low (liver fat < 2.07%) (n = 277), Moderate (2.07% ≤ liver fat < 5.56%) (n = 54), and High (liver fat ≥ 5.56%) (n = 51) groups.
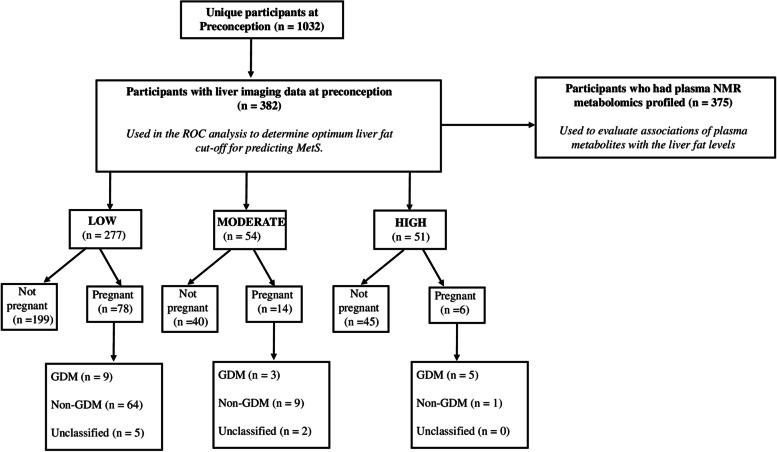
Only 98 women with preconception liver fat data progressed to the pregnancy stage (28.0% in the Low group, 25.9% in the Moderate group, and 11.8% in the High group) as shown in the participant flow chart in Fig. 2.
Fig. 2.
Flowchart of participant numbers (unclassified GDM cases were due to missing values of either 1-h plasma glucose, 2-h plasma glucose, or both)
Association of liver fat with clinical metabolic characteristics
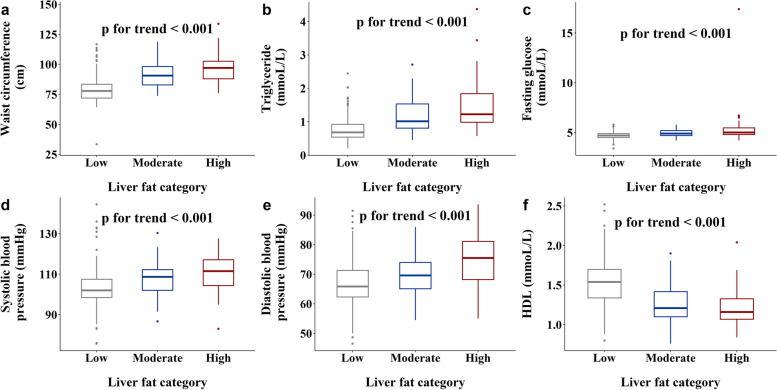
We found increasing trends for liver enzymes (ALT, AST and GGT) (Fig. 1d–f) going from Low to Moderate to High liver fat groups. We also found increasing trends for waist circumference (Fig. 3a), plasma TG (Fig. 3b), fasting plasma glucose (Fig. 3c), systolic (Fig. 3d), and diastolic blood pressure (Fig. 3e) and decreasing trends for plasma HDL (Fig. 3f) going from Low to Moderate to High liver fat groups.
Fig. 3.
Box plots of metabolic traits in MetS across the different liver fat categories: a waist circumference (cm) (n = 380), b triglyceride (mmoL/L) (n = 373), c fasting glucose (mmoL/L) (n = 380), d systolic blood pressure (mmHg) (n = 381), e diastolic blood pressure (mmHg) (n = 381), f HDL (mmoL/L) (n = 373)
Association of liver fat with plasma NMR metabolome
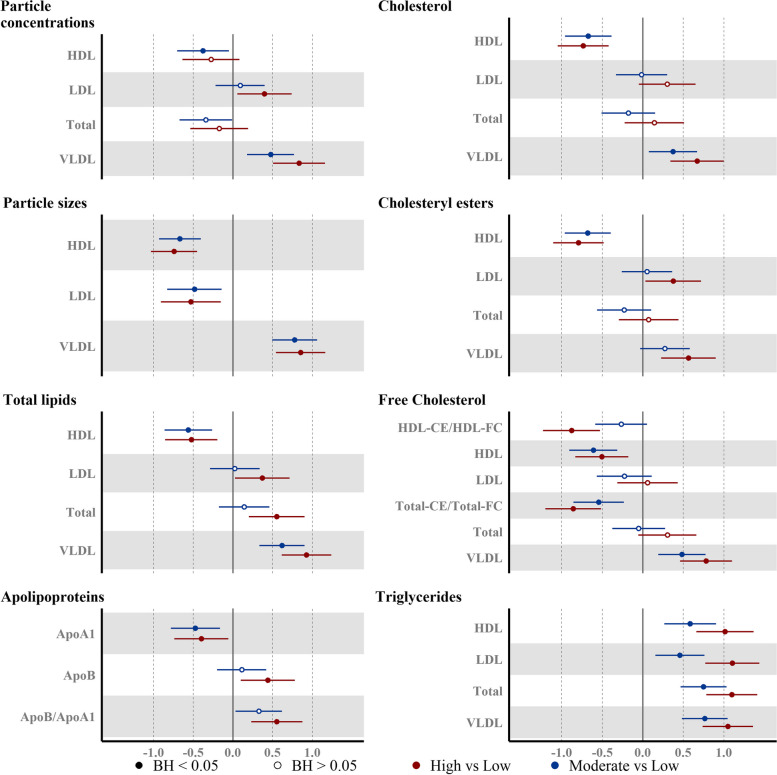
AMD of 141 out of the 255 metabolites/metabolite ratios passed the BH-adj p-value < 0.05 threshold for the Moderate vs Low group comparison, while 187 metabolites were significantly different in the High vs Low group comparison. There was a directional consistency and a graded response between the Moderate vs Low and High vs Low results for most of the metabolites (Additional File 1: Fig. S1, Additional File 1: Table S2 and S3). Overall, the magnitude of AMD of the NMR biomarkers was more marked in High-Low comparisons than with Moderate-Low comparisons (expressed as SD units of log10-transformed metabolomic marker). More detailed descriptions of metabolome-wide associations with liver fat groups (reference: Low liver fat) are provided below, particularly focusing on the alterations already evident in the Moderate group. Details on the associations of liver fat with relative composition of lipoprotein subfractions can be found in Additional File 1: Table S2 and S3 and Additional File 1: Fig. S1. Overall, we observed that the trends in absolute concentrations of lipoprotein subcomponents were more strongly driven by particle concentrations than the relative composition of the lipoprotein subfractions.
Apolipoproteins
Apolipoprotein B (ApoB) levels, reflecting total number of atherogenic particles (VLDL, LDL, and intermediate density lipoproteins (IDL)) was elevated only in the High group (AMD (95% CI): 0.44 (0.10, 0.78)). On the other hand, apolipoprotein A1 (APOA1), the structural protein in HDL particles was already lower in the Moderate group (Fig. 4) (AMD: − 0.40 (− 0.74, − 0.05)).
-
(b)
VLDL
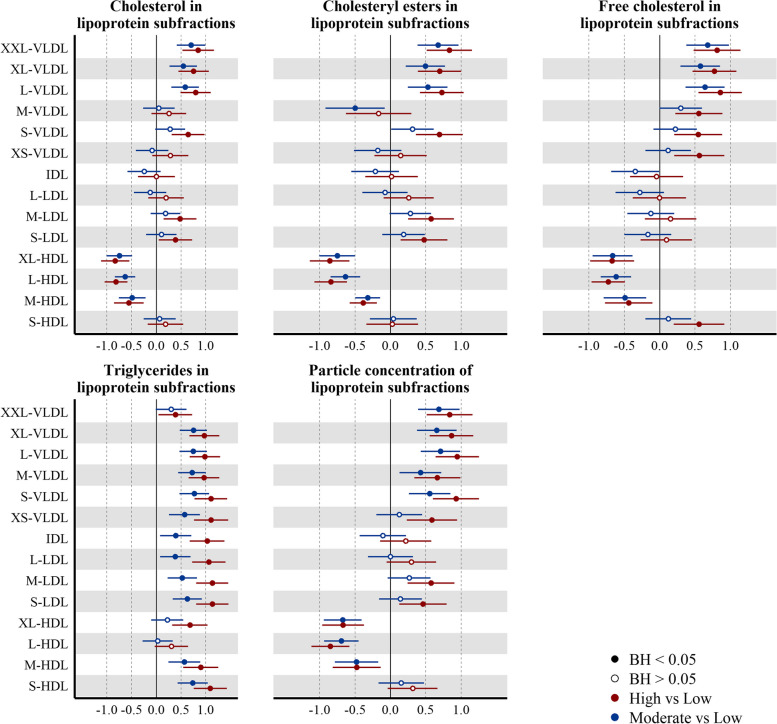
Both VLDL mean particle size and concentration were already elevated in the Moderate group (AMD: 0.78 (0.49, 1.06), AMD: 0.47 (0.18, 0.77)) compared to the Low group (Fig. 4). The concentrations of all VLDL subfractions were elevated in the High group, whereas in the Moderate group, all subfractions other than very small (XS) VLDL particles were elevated (Fig. 5). Total triglyceride content in VLDL (VLDL-TG) (Fig. 4) as well triglyceride in individual VLDL subfractions (Fig. 5) were elevated in both High and Moderate groups (VLDL-TG (AMD: 0.76 (0.48, 1.05)). In addition to the increased triglyceride load, phospholipids (PL), FC, and CE content of the larger (L, XL, and XXL) VLDL subfractions were already elevated in Moderate group.
Fig. 4.
Associations between the plasma lipoprotein and lipid levels and liver fat accumulation. Point estimates represent the beta coefficients for 1-SD change (95% CI) (n = 372) in the log10 transformed NMR-metabolite in the liver fat category, with respect to Low liver fat category (liver fat < 2.07%). Model adjusted for ethnicity, age, parity, education level, and BMI at pre-conception. Hollow/filled circles were/were not statistically significant with BH-adj p-values < 0.05, as determined by the Benjamini-Hochberg (BH) method
Fig. 5.
Associations between the liver fat accumulation and plasma lipoprotein composition and concentration. Point estimates represent the beta coefficients for 1-SD change (95% CI) (n = 372) in the log10 transformed NMR-metabolite in the liver fat category, with respect to Low liver fat category (liver fat < 2.07%). Model adjusted for ethnicity, age, parity, education level, and BMI at pre-conception. Hollow/filled circles were/were not statistically significant with BH-adj p-values < 0.05, as determined by the Benjamini-Hochberg (BH) method
-
(c)
IDL and LDL
IDL concentrations were not elevated in both Moderate (AMD: − 0.11 (− 0.43, 0.22)) and High groups (Fig. 5). While concentrations of small and medium LDL subfractions were elevated in the High group, no LDL subfraction concentrations were elevated in the Moderate group. Triglyceride load in IDL particles (AMD: 0.39 (0.07, 0.71)) and LDL subfractions were higher (Fig. 5) and mean LDL particle size (AMD: − 0.48 (− 0.83, − 0.14)) was lower in the Moderate group (Fig. 4).
-
(d)
HDL-related markers
Marked changes in HDL particles were evident in the Moderate group—these included lower particle concentrations (all subfractions except small HDL) (Fig. 5), mean particle size (AMD: − 0.67 (− 0.93, − 0.40)) (Fig. 4), cholesterol ester and free cholesterol (in medium and large HDL), and higher triglycerides (in small and medium HDL). We also found the ratio of CE/FC to be reduced in all HDL subfractions, even in the Moderate group.
-
(e)
Fatty acids
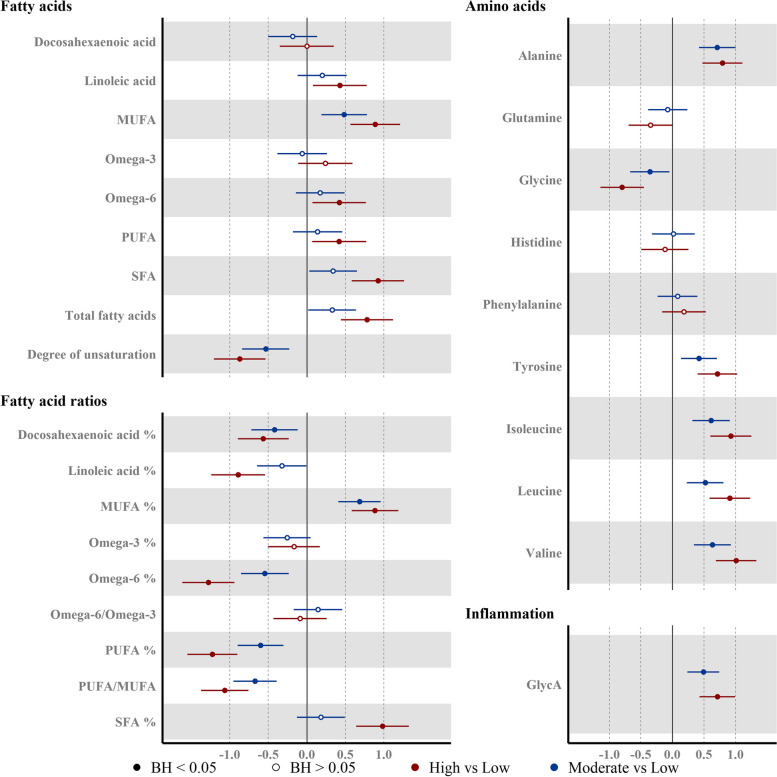
Absolute concentrations of monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA) and saturated fatty acid (SFA) were all higher in the High group, whereas only MUFA was higher in the Moderate group (AMD: 0.48 (0.19, 0.78)) (Fig. 6). Relative levels of total polyunsaturated fatty acid (PUFA%) (AMD: − 0.60 (− 0.90, − 0.30)), total omega-6 fatty acid (Omega-6%) (AMD: − 0.55 (− 0.86, − 0.23)), and the omega-3 fatty acid% (AMD: − 0.26 (− 0.57, 0.05)), docosahexaenoic acid% (AMD: − 0.42 (− 0.72, − 0.12)) were lower in the Moderate group.
Fig. 6.
Associations between the liver fat accumulation and plasma fatty acids and polar metabolites. Point estimates represent the beta coefficients for 1-SD change (95% CI) (fatty acids: n = 371, polar metabolites (except valine): n = 372, valine: n = 369) in the log10 transformed NMR-metabolite in the liver fat category, with respect to Low liver fat category (liver fat < 2.07%). Model adjusted for ethnicity, age, parity, education level, and BMI at pre-conception. Hollow/filled circles were/were not statistically significant with BH-adj p-values < 0.05, as determined by the Benjamini-Hochberg (BH) method
-
(f)
Inflammatory marker and amino acids
The inflammatory marker glycoprotein acetyl (GlycA) was higher (AMD: 0.49 (0.23, 0.74)) in the Moderate group. We also observed marked alterations in the amino acid profiles even in the Moderate group; these included higher levels of alanine (AMD: 0.71 (0.42, 1.00)), tyrosine (AMD: 0.42 (0.14, 0.71)), and branched chain amino acids (isoleucine, leucine, and valine) and lower levels of glycine (AMD: − 0.36 (− 0.67, − 0.04)) (Fig. 6).
Association of liver fat with incident T2D risk score and glucose AUC and GDM incidence at mid-gestation
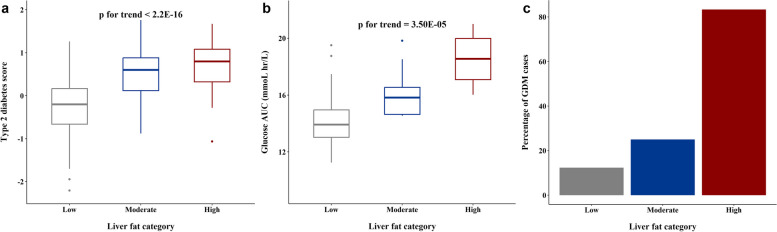
The prospective multi-metabolite risk score (derived from phenylalanine, non-esterified cholesterol in large HDL and the ratio of cholesteryl ester to total lipid in large VLDL) for incident T2D showed an increasing trend from Low to Moderate to High liver fat groups (Fig. 7a). Since phenylalanine was not associated with the liver fat groups, the above trend was likely driven by lipid composition of large HDL and VLDL particles. The mid-gestational glucose AUC showed an increasing trend from Low to Moderate to High liver fat groups (Fig. 7b). GDM incidence was 2.03-fold (RR = 2.03 (95% CI 0.64 to 6.44, p = 0.23)) and 6.76-fold (RR = 6.76 (3.33 to 13.73), p < 0.001) higher in Moderate and High groups respectively as compared to Low group (Fig. 7c).
Fig. 7.
Associations between the liver fat accumulation and metabolic outcomes. a Box plot of multi-metabolite score for incident T2D across the different liver fat categories (n = 375). b Box plot of glucose AUC at mid-gestation across the different liver fat categories. c Percentage of GDM cases at mid-gestation across the different liver fat categories (n = 91). p for trend values represents trend of T2D score and Glucose AUC in the study with increasing liver fat
Discussion
We found 2.07% weight to be the optimum metabolic-risk-linked cutoff for diagnosing abnormal liver fat accumulation in reproductive age Asian women. Notably, this cutoff is nearly 60% lower than the conventional population-based cutoff of 5.56% weight commonly used in MRS based liver fat studies and about a third lower than the commonly used proton density fat fraction cutoff of 5% in MRI-based liver fat studies. The newly derived metabolic-risk-linked cutoff also provided much better trade-offs between sensitivity and specificity for diagnosing MetS. A comparison of the newly derived metabolic-risk-based liver fat cutoff with MRI/MRS based cutoffs for abnormal liver fat accumulation identified in prior literature is shown in (Table 2).
Table 2.
Comparison of newly derived metabolic-risk-linked fatty liver cutoff with MRS/MRI-based abnormal liver fat cutoffs identified in prior literature
| No | Abnormal liver fat cutoff | Basis for cutoff | Reference | |
|---|---|---|---|---|
| % weight | Fat fraction % | |||
| 1 | 2.07% | 3.2a% | Optimum threshold for predicting MetS | Current work |
| 2 | 5.56% | 8.42a% | 95th percentile of liver fat in subjects at low risk of hepatic steatosis | LS Szczepaniak et al. [3] |
| 3 | 1.94b% | 3.00% | Sensitivity of hepatic steatosis detection by histology | P Nasr et al. [21] |
| 4 | 3.26b% | 5% | Threshold for mild steatosis—expert opinion | J Starekova et al.[22] |
| 5 | 2.41b% | 3.71% | Optimum threshold for steatosis detection by histology | CC Park et al. [23] |
| 6 | 4.19b% | 6.4% | Optimum threshold for steatosis detection by histology | A Tang et al. [24] |
| 7 | 2.27b% | 3.50% | Optimum threshold for predicting MetS | JL Rehm et al. [6] |
| 8 | 2.27b% | 3.50% | Sensitivity of hepatic steatosis detection by histology | S Park et al. [25] |
| 9 | 0.96b% | 1.50% | Emergence of hepatic insulin resistance | F Bril et al. [4] |
aFat -fraction% estimated from liver fat % weight
bLiver fat % weight estimated from fat -fraction %
Nasr et al. [21] and Park et al. [23] found that optimum MRI based liver fat fraction cutoffs based on the ability to identify histological steatosis were 3% (in Swedish adults with mean age of 53.3) and 3.5% (in White adults with mean age of 50.8 years), respectively (corresponding to a liver fat %weight of 1.94% and 2.27%, respectively). Similar to the current work, Rehm et al. [6] also derived a metabolic-risk based cutoff based on the ability to diagnose MetS in a cohort of young healthy females (11–22 years of age, predominantly African-American and White) and found the optimum fat fraction cutoff to be 3.5% (~ 2.27%weight). Thus, our finding of a lower cutoff for abnormal liver fat accumulation in Asian women of reproductive age, relative to the conventional population-based cutoff, is consistent with earlier studies which derived risk-based cutoffs in other cohorts with different ethnic/racial profiles. Based on the liver fat cutoff of 2.07%, the prevalence of “abnormal” liver fat in our cohort (27.5%) was more than double the prevalence (13.4%) identified using the conventional 5.56% weight, indicating a much larger at-risk population.
We studied the specific metabolic perturbations that emerged even at moderate levels of liver fat below conventional liver fat cutoffs. We found statistically significant trends for worsening MetS-linked traits (dysglycemia, dyslipidemia, central obesity, and increased blood pressure), going from Low to Moderate to High levels of liver fat. We also found statistically significant increasing trends for liver enzymes AST, ALT, and GGT, which are suggestive of subclinical disease processes of liver injury and oxidative stress, even at moderate levels of liver fat [26].
We found an unfavorable VLDL profile in both groups, characterized by larger VLDL particles, higher VLDL concentration, and a higher VLDL triglyceride load. There was also a concomitant reduction in the mean diameter of both LDL and HDL particles. We found ApoB, the main structural lipoprotein in VLDL, IDL, and LDL to be elevated in the High liver fat group, but not in the Moderate group. The VLDL-TG secretion rate has been previously reported to be elevated in nonalcoholic fatty liver disease (NAFLD) [27]. Thus, moderate liver fat accumulation below conventional cutoffs may represent an early stage of steatosis, when the net increase in intrahepatic fatty acids has not yet stimulated increased production of ApoB, which requires more TG to be packaged within the VLDL particles and more TG to be stored within the liver.
In addition to increased TG in VLDL, we also found enriched levels of LDL-TG and HDL-TG in the respective lipoprotein subfractions even in the Moderate liver fat group. CE levels were increased in the larger-sized VLDL subfractions (XXL, XL, L) with concomitant decrease in CE load in all HDL subfractions (except S-HDL) in the Moderate group. These findings are suggestive of higher plasma cholesteryl ester transfer protein (CETP) activity even at moderate liver fat levels, which results in a high net transfer of TGs from VLDL to HDL and LDL and of CEs from HDL to VLDL[28]. These trends have been observed earlier in NAFLD [28]. Elevated levels of plasma CETP are one of the drivers of the atherogenic lipid profile in NAFLD. Hence, the observed changes may reflect the onset of an atherogenic profile.
This specific pattern of larger VLDL particles and smaller HDL and LDL particles has also been linked to incident T2D in women [29]. The Moderate liver fat group also had lower concentrations of larger-sized HDL subfractions and of HDL cholesterol. These patterns are suggestive of reduced capacity for reverse cholesterol transport. We found further supporting evidence for this in our analysis of the total CE/FC ratio and within individual HDL subfractions. LCAT activity, assessed by plasma total-CE/Total-FC as well as the CE/FC in the largest HDL particles (XL-HDL), was lower in both Moderate and High groups. LCAT is an important player in the metabolism of lipoprotein in plasma and triglycerides in the liver [30]. There have been mixed reports regarding the association of LCAT with NAFLD, with reports of either positive [31, 32] or no significant associations [33, 34]. These differing results could be due to the different ways in which NAFLD was assessed in these studies (fatty liver index-based [31, 32], liver biopsy-based [35], ultrasonography-based [36]).
The Nightingale NMR panel provides a readout of the inflammatory marker, GlycA. This is a composite biomarker that simultaneously captures the glycosylation states of several acute-phase proteins [37] and is more stable than C-reactive protein (CRP) [38]. The inflammatory marker, GlycA, was elevated in both Moderate and High groups. Prior studies have established the link between systemic inflammation and NAFLD [39] as well as hepatic steatosis [40]. Positive associations of GlycA levels with higher levels of liver fat have also been reported [41].
Higher plasma levels of BCAAs—leucine, isoleucine, and valine—were linked to moderate levels of liver fat, as were higher levels of alanine and tyrosine. These amino acid dysregulations have been previously reported with higher levels of liver fat [41–43]. Cross-sectionally, elevated BCAAs are positively associated with NAFLD [44]. Furthermore, the association of NAFLD with T2D development has been reported to be partially mediated by BCAAs [44]. We found lower glycine levels even in the Moderate group. Impaired glycine metabolism has been previously hypothesized to be a causative factor for NAFLD [45].
In terms of circulating fatty acids, higher absolute levels of MUFA were seen in the Moderate liver fat group, while higher absolute levels of MUFA, PUFA, and SFA were seen in the High group. Relative to total fatty acids, lower levels of omega-3 and omega-6 fatty acids (primarily reflecting dietary PUFA intake) were already evident in the Moderate group. These patterns are concordant with earlier reports [41].
Overall, the NMR metabolomics analysis showed directionally similar patterns in both Moderate and High groups for most of the metabolomics markers. The metabolite patterns observed even at moderate levels of liver fat had multiple indicators of a predisposition towards an atherogenic state and early-stage steatosis.
Interestingly, the alterations observed in the Moderate group in lipoprotein profiles, amino acids, fatty acids, glucose, and inflammatory markers are consistent with metabolic profiles previously linked to incident T2D [18, 46]. Furthermore, we found an increasing trend for the prospective multi-metabolite score for incident T2D, while going from Low to Moderate to High groups in our cohort. Thus, moderate liver fat accumulation below conventional cutoffs does not seem to be metabolically benign. Rather, it may be a sensitive barometer of early pathophysiological metabolic perturbations.
Since the SPRESTO participants were planning to conceive, subclinical metabolic alterations at preconception could lead to a more detrimental health status due to the physiological challenge of pregnancy. Although not the primary focus of the current study, it was noteworthy that far fewer women in the High group progressed to the pregnancy stage (11.8% in the High group vs 28.0% in Low group and 25.9% in Moderate group) within 1 year after recruitment, suggesting that higher preconception liver fat levels might a risk factor for lower fecundability. These trends are concordant with earlier work in the same cohort linking preconception obesity and unhealthy lifestyle factors to lower fecundability [47]. We also observed a 2-fold higher incidence of GDM in the Moderate group and 6.76-fold higher incidence in the High group as compared to the Low group. The RR associated with this 2-fold increase in GDM incidence was not significant, likely due to the low number of GDM cases in this group (3 GDM cases out of the 14 successful pregnancies in the Moderate group). Interestingly, the continuous gestational glucose AUC was found to increase going from the Low to Moderate to High groups. Gestational glucose AUC has been linked to adverse pregnancy outcomes irrespective of GDM status [48]. Our findings suggest that even moderate accumulation of liver fat below conventional fatty liver cutoffs at the preconception stage can have adverse metabolic effects during pregnancy. Higher GDM incidence can lead to maternal health complications during pregnancy and postpartum [49, 50] as well as long-term adverse health consequences in the offspring [51].
A key implication of our finding is that the use of conventional MRS-based liver fat cutoffs or fatty liver assessments insensitive to low levels of liver fat may result in underestimation of the true prevalence of pathological liver fat accumulation and its associated disease burden. The main limitation of this study is that the metabolic risk-linked liver fat cutoffs were derived in a cohort that included only reproductive age women of Chinese, Indian, and Malay ethnicities. Hence, replication in larger and more diverse cohorts that include both sexes and wider age ranges is warranted, to evaluate the generalizability of the findings. However, concordant reports of lower metabolic risk-based liver cutoffs in other populations suggest that our finding may not just be an Asian-specific phenomenon. Another limitation of our study is that due to the prospective design starting from preconception, only a quarter of the women went on to become pregnant, with a small number of incident GDM cases. This limited the sample size for evaluating gestational outcomes. However, we have also shown a significant trend of increasing mid-gestational continuous glucose AUC values from Low to Moderate to High liver fat groups. Increase in glucose AUC values has also been linked to maternal health adversities during pregnancy independent of GDM status.
The strengths of our study include the deployment of the MRS technique, which is sensitive to very low levels liver fat (unlike more common imaging modalities like B-mode ultrasound or proxy assessments like liver enzymes) in a cohort of relatively healthy (predominantly normoglycemic with low MetS prevalence) reproductive age women. This allowed us to identify the optimum liver fat cutoffs for diagnosing MetS. The use of NMR metabolomic profiles allowed us to characterize in detail the specific metabolic perturbations that are already apparent at the derived cutoff. The prospective preconception design of the study allowed us to probe the links between moderate preconception liver fat levels and gestational dysglycemia.
Conclusions
The metabolic risk-linked cutoff in our cohort of reproductive age Asian women was much lower than conventional fatty liver cutoffs. Our findings suggest that the at-risk population with abnormal liver fat accumulation may be underestimated with the conventional cutoff. Using the newly derived cutoff may allow for early screening of individuals in whom metabolic dysregulation is still emerging, enabling early preventive approaches. Our findings also highlight preconception liver fat as an intervention target to mitigate the adverse pregnancy outcomes linked to gestational dysglycemia. Given the prohibitive cost of MRS-based liver fat assessments, development of more scalable approaches that are sensitive to low levels of liver fat will be important for translating the findings for the wider population. The use of NMR biomarkers seems promising for developing such approaches, given the range of metabolomic alterations that were already apparent even at moderate liver fat levels.
Supplementary Information
Acknowledgements
"We thank the S-PRESTO study group that includes Airu Chia, Anna Magdalena Fogel, Anne Eng Neo Goh, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, Bee Wah Lee, Bobby Kyungbeom Cheon, Candida Vaz, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Dawn Xin Ping Koh, Desiree Y Phua, Doris Ngiuk Lan Loh, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Faidon Magkos, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Izzuddin Bin Mohd Aris, Jeannie Tay, Jerry Kok Yen Chan, Jia Xu, Joanne Su-Yin Yoong, Johan Gunnar Eriksson, Jonathan Tze Liang Choo, Jonathan Y Bernard, Jonathan Yinhao Huang, Jun Shi Lai, Karen Mei Ling Tan, Keith M Godfrey, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kothandaraman Narasimhan, Kok Wee Chong, Kuan Jin Lee, Li Chen, Lieng Hsi Ling, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Khee-Shing Leow, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Ngee Lek, Oon Hoe Teoh, Priti Mishra, Queenie Ling Jun Li, Sambasivam Sendhil Velan, Seng Bin Ang, Shirong Cai, Si Hui Goh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Teng Hong Tan, Tong Wei Yew, Varsha Gupta, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yin Bun Cheung, Yiong Huak Chan and Zai Ru Cheng."
Abbreviations
- 1H-MRS
Proton magnetic resonance spectroscopy
- ALT
Alanine aminotransferase
- AMD
Adjusted mean difference
- ApoA1
Apolipoprotein A1
- ApoB
Apolipoprotein B
- AST
Aspartate aminotransferase
- BCAA
Branched-chain amino acid
- C
Cholesterol
- CE
Cholesteryl ester
- CETP
Cholesteryl ester transfer protein
- CRP
C-reactive protein
- CSE-MRI
Chemical shift-encoded MRI
- FC
Free cholesterol
- FG
Fasting plasma glucose
- GDM
Gestational diabetes mellitus
- GGT
Gamma-glutamyl transferase
- GlycA
Glycoprotein acetyls
- HDL-C
HDL cholesterol
- HDL-P
HDL particle concentration
- HDL-TG
Triglyceride in HDL
- IDL
Intermediate-density lipoprotein
- IDL-TG
Triglyceride in IDL
- LCAT
Lecithin::cholesterol acyltransferase
- LDL-TG
Triglyceride in LDL
- MAFLD
Metabolic-associated fatty liver disease
- MetS
Metabolic syndrome
- MRS
Magnetic resonance spectroscopy
- MUFA
Monounsaturated fatty acid
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- PL
Phospholipid
- PLTP
Phospholipid transfer protein
- PRESS
Point-resolved spectroscopy
- PUFA
Polyunsaturated fatty acid
- RR
Risk ratio
- ROC
Receiver operator curve
- SFA
Saturated fatty acid
- T2D
Type 2 diabetes
- VLDL-TG
Triglyceride in VLDL
- XL
Very large
- XXL
Extremely large
Authors' contributions
P.D.G., Y.S.C., K.H.T., J.G.E., K.M.G., S.Y.C., J.K.Y.C., P.M. and N.M. contributed to the study conceptualization and design. P.D.G., Y.S.C., K.H.T., S.Y.C. and J.K.Y.C. provided funding and operational support for the S-PRESTO cohort. S.S.V. and M.V.F. provided planning and operational support for the MRI visit. N.M., S.A.S. and J.Y. conducted the post-processing of MRI data. D.W. provided expert advice on statistical analysis, data visualization and carried out manuscript revision. P.M. and N.M. conducted the statistical analysis of the data, and P.M. carried out the data visualization. P.M. and N.M. interpreted the results and wrote the initial version of the manuscript. All authors read and approved the final manuscript.
Funding
The study is supported by the National Research Foundation (NRF) under the Open Fund-Large Collaborative Grant (OF-LCG; MOH-000504) administered by the Singapore Ministry of Health's National Medical Research Council (NMRC) and the Agency for Science, Technology and Research (A*STAR). In RIE2025, the study is supported by funding from the NRF's Human Health and Potential (HHP) Domain, under the Human Potential Programme.
Data availability
"Supporting data is provided in the supplementary material. Other data is not publicly available due to ethical considerations and can be provided upon reasonable request, subject to approval from the S-PRESTO cohort’s Executive Committee."
Declarations
Ethics approval and consent to participate
Ethical approval was obtained (No. 2014/692/D) from the SingHealth Centralized Institutional Review Board, and written informed consent was obtained from all women. The S-PRESTO study is registered at ClinicalTrials.gov (NCT 03531658).
Consent for publication
Not applicable.
Competing interests
'DW is supported by the Wellcome Trust (17068/Z/19/Z). DW is additionally supported by the Academy of Medical Sciences Professorship (APR7_1002) and the Engineering and Physical Sciences Research Council (EP/V029045/1). KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health and Care Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (NIHR203319)) and Alzheimer’s Research UK (ARUK-PG2022A-008). K.M.G. received reimbursement for speaking at conferences sponsored by companies selling nutritional products. S.C. has received reimbursement from the Expert Group on Inositol in Basic and Clinical Research (EGOI; a not-for-profit academic organisation) and Nestlé Nutrition Institute for speaking at conferences. K.M.G., S.Y.C. and Y.S.C. are part of an academic consortium that has received research funding from Société Des Produits Nestlé S.A. and BenevolentAI Bio Ltd, and are co-inventors on patents filed on nutritional factors and metabolic risk outside the submitted work. All other authors declare that they have nothing to disclose.'
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yi M, Peng W, Feng X, Teng F, Tang Y, Kong Q, et al. Extrahepatic morbidities and mortality of NAFLD: an umbrella review of meta-analyses. Aliment Pharmacol Ther. 2022;56(7):1119–30. [DOI] [PubMed] [Google Scholar]
- 2.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. [DOI] [PubMed] [Google Scholar]
- 4.Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–44. [DOI] [PubMed] [Google Scholar]
- 5.Rotman Y, Neuschwander-Tetri BA. Liver fat accumulation as a barometer of insulin responsiveness again points to adipose tissue as the culprit. Hepatology (Baltimore, MD). 2017;65(4):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25(10):2921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64(1):42–61. [DOI] [PubMed] [Google Scholar]
- 8.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35(3):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loo EXL, Soh SE, Loy SL, Ng S, Tint MT, Chan SY, et al. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur J Epidemiol. 2021;36(1):129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. [DOI] [PubMed] [Google Scholar]
- 12.Chabanova E, Bille DS, Thisted E, Holm J-C, Thomsen HS. MR spectroscopy of liver in overweight children and adolescents: investigation of 1H T2 relaxation times at 3T. Eur J Radiol. 2012;81(5):811–4. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24(7):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250(1):95–102. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes IAo, Panel PSGC. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahola-Olli AV, Mustelin L, Kalimeri M, Kettunen J, Jokelainen J, Auvinen J, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62(12):2298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RA, Moore SC, Playdon M, Meirelles O, Newman AB, Milijkovic I, et al. metabolites associated with lean mass and adiposity in older Black men. J Gerontol A Biol Sci Med Sci. 2017;72(10):1352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 21.Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, et al. Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology. 2017;153(1):53-5.e7. [DOI] [PubMed] [Google Scholar]
- 22.Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: state of the art. Radiology. 2021;301(2):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598-607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, et al. Accuracy of MR imaging–estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274(2):416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Kwon JH, Kim SY, Kang JH, Chung JI, Jang JK, et al. Cutoff values for diagnosing hepatic steatosis using contemporary MRI-proton density fat fraction measuring methods. Korean J Radiol. 2022;23(12):1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Beek JH, de Moor MH, de Geus EJ, Lubke GH, Vink JM, Willemsen G, et al. The genetic architecture of liver enzyme levels: GGT ALT and AST. Behav Genet. 2013;43(4):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59(5):1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta Mol Cell Biol Lipids. 2000;1529(1):245–56. [DOI] [PubMed] [Google Scholar]
- 31.Nass KJ, van den Berg EH, Gruppen EG, Dullaart RPF. Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease. Eur J Clin Invest. 2018;48(9):e12988. [DOI] [PubMed] [Google Scholar]
- 32.Janac J, Zeljkovic A, Jelic-Ivanovic Z, Dimitrijevic-Sreckovic V, Miljkovic M, Stefanovic A, et al. The association between lecithin–cholesterol acyltransferase activity and fatty liver index. Ann Clin Biochem. 2019;56(5):583–92. [DOI] [PubMed] [Google Scholar]
- 33.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36(2):211–28. [PubMed] [Google Scholar]
- 34.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9(2):155–67. [PubMed] [Google Scholar]
- 35.McCullough A, Previs SF, Dasarathy J, Lee K, Osme A, Kim C, et al. HDL flux is higher in patients with nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2019;317(5):E852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fadaei R, Poustchi H, Meshkani R, Moradi N, Golmohammadi T, Merat S. Impaired HDL cholesterol efflux capacity in patients with non-alcoholic fatty liver disease is associated with subclinical atherosclerosis. Sci Rep. 2018;8(1):11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunoni AR, Salum GA, Hoffmann MS, Goulart AC, Barreto SM, Canhada S, et al. Prospective associations between hsCRP and GlycA inflammatory biomarkers and depression: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Affect Disord. 2020;271:39–48. [DOI] [PubMed] [Google Scholar]
- 38.Duprez DA, Jacobs DR Jr. GlycA, a composite low-grade inflammatory marker, predicts mortality: prime time for utilization? J Intern Med. 2019;286(5):610–2. [DOI] [PubMed] [Google Scholar]
- 39.Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27(5):412–21. [DOI] [PubMed] [Google Scholar]
- 40.Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham Heart Study. Clin Gastroenterol Hepatol. 2019;17(6):1157-64.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnatiuc Friedrichs L, Trichia E, Aguilar-Ramirez D, Preiss D. Metabolic profiling of MRI-measured liver fat in the UK Biobank. Obesity (Silver Spring). 2023;31(4):1121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaggini M, Carli F, Rosso C, Buzzigoli E, Marietti M, Della Latta V, et al. Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology. 2018;67(1):145–58. [DOI] [PubMed] [Google Scholar]
- 43.Yamakado M, Tanaka T, Nagao K, Imaizumi A, Komatsu M, Daimon T, et al. Plasma amino acid profile associated with fatty liver disease and co-occurrence of metabolic risk factors. Sci Rep. 2017;7(1):14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Berg EH, Flores-Guerrero JL, Gruppen EG, de Borst MH, Wolak-Dinsmore J, Connelly MA, et al. Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: role of circulating branched-chain amino acids. Nutrients. 2019;11(3):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rom O, Liu Y, Liu Z, Zhao Y, Wu J, Ghrayeb A, et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci Transl Med. 2020;12(572):eaaz2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bragg F, Kartsonaki C, Guo Y, Holmes M, Du H, Yu C, et al. The role of NMR-based circulating metabolic biomarkers in development and risk prediction of new onset type 2 diabetes. Sci Rep. 2022;12(1):15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loy SL, Ku CW, Tiong MMY, Ng CST, Cheung YB, Godfrey KM, et al. Modifiable risk factor score and fecundability in a preconception cohort in Singapore. JAMA Netw Open. 2023;6(2):e2255001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Wei Y, Sun WJ, Yang H. The area under the Curve (AUC) of oral glucose tolerance test (OGTT) could be a measure method of hyperglycemia in all pregnant women. Open J Obstet Gynecol. 2019;9(2):186–95.
- 49.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma AK, Singh S, Singh H, Mahajan D, Kolli P, Mandadapu G, et al. Deep insight of the pathophysiology of gestational diabetes mellitus. Cells. 2022;11(17):2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
"Supporting data is provided in the supplementary material. Other data is not publicly available due to ethical considerations and can be provided upon reasonable request, subject to approval from the S-PRESTO cohort’s Executive Committee."