Abstract
Background
Patients with chronic kidney disease (CKD) are susceptible to vascular calcification and vitamin K deficiency. Matrix gla protein (MGP) is a potent inhibitor of calcification requiring vitamin K for activation. Inactive MGP, i.e. dephosphorylated uncarboxylated MGP (dp-ucMGP), is frequently elevated in CKD along with protein induced by vitamin K absence (PIVKA-II). We investigated whether dp-ucMGP and PIVKA-II are useful markers of aortic calcification in CKD.
Methods
Patients with normal or reduced kidney function underwent a non-contrast computed tomography scan of the entire aorta with subsequent blinded standard calcification scoring of the aortic wall ad modum Agatston. Blood samples were analyzed for plasma concentrations of dp-ucMGP and PIVKA-II.
Results
141 patients (104 with CKD stage 3–5) were included. In patients with/without CKD median (interquartile range) were dp-ucMGP 543 (503–744)/1078 (835–1682) pmol/l (P < 0.01); PIVKA-II 19.3 (16.3–23.5)/21.8 (17.2–36.8) ng/ml (P = 0.33) and aortic Agatston scores 1644 (729–4138)/7172 (2834–15360) (P < 0.01). Agatston score was positively associated with PIVKA-II (β = 0.71, P = 0.014, r2 = 0.04) and tended to be so with dp-ucMGP (β = 0.44, P = 0.08, r2 = 0.02). Age, estimated glomerular filtration rate (eGFR) and smoking status were also associated with Agatston score and remained so, along with PIVKA-II, when adjusted for potential confounders. However, the association between age and aortic Agatston score was stronger than for PIVKA-II, eGFR and smoking-status.
Conclusion
Vitamin K deficiency, as estimated through PIVKA-II, but not dp-ucMGP, is weakly associated with aortic Agatston score. Yet, as markers of aortic calcification, both were outperformed substantially by age, and neither surpassed smoking nor eGFR.
ClinicalTrials.gov identifier
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03876-5.
Keywords: Atherosclerosis, Chronic kidney disease, Vitamin K dependent proteins, Aorta, Calcification, Ageing
Introduction
Patients with chronic kidney disease (CKD) have an excessively high risk of cardiovascular disease [1], and accelerated large artery calcification of the media-intima is an important contributor to this [2]. Furthermore, patients with CKD are also prone to vitamin K deficiency which may be implicated in the processes leading to vascular calcification [3, 4]. A group of proteins termed vitamin K dependent proteins (VKDP) all contain a γ-carboxyglutamate-group (Gla) and a vitamin K-dependent carboxylation and subsequent phosphorylation is necessary for these proteins to achieve their active form. High levels of uncarboxylated forms of VKDP therefore reflect poor functional vitamin K status [5]. Matrix Gla protein (MGP) is a VKDP which in its active form, the carboxylated and phosphorylated MGP (cMGP), has the potential to inhibit vascular calcification. Thus, whereas cMGP is found in healthy vessels, the inactive form, dephosphorylated uncarboxylated MGP (dp-ucMGP) accumulates in calcified vessels [6]. It is well established that dp-ucMGP is highly dependent on kidney function [7, 8]. Patients with CKD generally have high levels of dp-ucMGP which is most likely caused by a combination of increased cMGP usage due to accelerated arterial calcification and a high prevalence of vitamin K deficiency [9]. This can be even further augmented by vitamin K antagonist (VKA) treatment [10, 11]. Another VKDP termed protein induced by vitamin K absence II (PIVKA-II), also known as abnormal prothrombin or uncarboxylated factor II has the advantage of being much less dependent on renal function than dp-ucMGP [12].
Vitamin K has a short half-life in serum, and direct estimation of the vitamin concentration is technically complicated. Therefore, vitamin K levels are most often indirectly estimated through levels of uncarboxylated VKDPs [13]. Of these, dp-ucMGP is believed to be particularly reflective of vascular vitamin K status [14] and there has been substantial interest in dp-ucMGP as a potential biomarker for arterial calcification in CKD. Positive associations between calcification scoring and dp-ucMGP have been described for the abdominal aorta, whereas the relation seem inconsistent for other vascular beds [10, 11, 15–18]. Although much less studied in the setting of arterial calcification in CKD, PIVKA-II seems to be without value in predicting calcification of arteries in other vascular beds than the aorta [17, 18]. Vitamin K is preferentially stored in the liver during vitamin K deficiency [19]. Extra-hepatic vitamin K is depleted before hepatic vitamin K and as a clotting factor, carboxylation of PIVKA-II occurs in the liver. This process can therefore continue after being impaired in non-hepatic tissues. PIVKA-II is therefore believed to be more representative of hepatic vitamin K status and has been labelled an insensitive marker of extrahepatic vitamin K status [13].
The association between dp-ucMGP and calcification of the entire aorta has not been examined and no studies have included patients from all CKD stages as well as patients without CKD [20]. Furthermore, the association between PIVKA-II and aortic calcification remains unknown at all levels of kidney function. This knowledge-gap may be of importance due to the recent interest in the association between aortic calcification and VKDPs in CKD patients in particular.
The present study aimed to answer these questions by investigating the association between calcification score of the entire aorta and the VKDPs dp-ucMGP and PIVKA-II in patients representing the entire spectrum of kidney function from normal kidney function to end stage kidney disease.
Methods
This is a cross-sectional study performed between August 2019 and December 2021. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Central Denmark Region Committee on Health Research Ethics (Journal number 1-10-72-21-19). All study participants gave written informed consent.
Patients
Patients included in this study were part of the Aortic Calcification and Central Blood Pressure in Patients with CKD (ACCEPT) study. The ACCEPT study was a cross-sectional study involving 168 patients scheduled for elective coronary angiography (CAG) [21]. The study participants were evenly distributed in five subgroups with (1) renal function considered normal for age (estimated glomerular filtration rate (eGFR) ≥ 60 ml/min/1.73 m2 with urine albumin/creatinine ratio < 30 mg/g), (2) CKD stage 3a (eGFR 45–59 ml/min/1.73 m2), (3) CKD stage 3b (30–44 ml/min/1.73 m2), (4) CKD stage 4 (15–29 ml/min/1.73 m2), and (5) CKD-stage 5 (< 15 ml/min/1.73 m2). Patients could not be included if there had been changes in antihypertensive treatment 2 weeks prior to CAG, if severe aortic valve stenosis (< 1 cm2) or known significant stenosis of the subclavian or brachial arteries, known chronic arrythmia was present, or if a bilateral arterio-venous dialysis fistula had been created even if one of these had been surgically removed.
Aortic calcification assessment
Aortic calcification scores were generated based on a non-enhanced computed tomography (CT) scan of the entire length of the aorta using 120 kV electrocardiogram-triggered high-pitch spiral acquisition (Siemens SOMATOM Force (Siemens, Forchheim, Germany)) [22]. Calcification of the aortic wall was assessed as Agatston score using VitreaAdvanced (VitreaCore Version 6.4.4., Canon Medical Informatics Inc. Minnetonka, MN, USA) by two independent observers blinded to patient information The analysis began on the CT frame immediately cranial to the coronary arteries. Following the full aortic length, the analysis continued until the aortic bifurcation.
The Agatston score was calculated using a weighted value assigned to the highest density of calcification multiplied by the area of calcium [23]. A standard threshold of 130 Hounsfield unit calcium detection was used. To account for the impact of body size on aorta size, aortic Agatston scores of the entire aorta were adjusted by body surface area (BSA) using the Du Bois formula [24]. Furthermore, the maximal thickness of calcification in each patient was also measured in all segments. Only the maximal thickness at any point in the entire aorta was used in the data-analysis (Fig. 1). For both calcification scores and calcification thickness, the mean of the two scores from the blinded independent observers was used in the analysis. CT scans were re-scored by both observers if inter-scorer variability was > 15%.
Fig. 1.
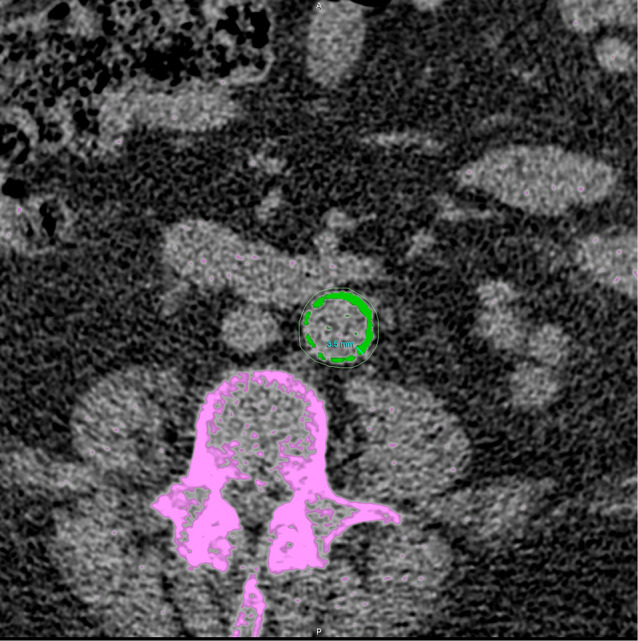
Abdominal transversal CT-image. Aortic calcification counted in the aortic Agatston score is marked in green. Calcifications of the bone (not counted in the aortic Agatston score) is marked in purple. The measurement of maximal aortic calcification thickness of the aortic segment is also shown
Blood samples
On a separate day from the CAG procedure, blood samples were drawn and collected with minimal stasis in evacuated EDTA blood collection tubes. The blood samples were centrifuged for 10 min at 3000 g within 30–60 min and plasma stored at − 80 °C until analysis. Biochemical analyses for PIVKA-II and dp-ucMGP were performed at the Department of Biochemistry and Immunology, Lillebælt Hospital, Kolding; a routine laboratory accredited by Danish Accreditation Fund (DANAK) according to the ISO 15,189 standard that specifies requirements for quality and competence in medical laboratories.
Plasma concentrations of PIVKA-II were measured using a commercially available electrochemiluminescence immunoassay (ECLIA) on the Cobas e 801 analytical unit (Roche, Basel, Switzerland) according to manufacturer’s instructions. The limit of quantification for this assay is 4.5 ng/ml, with an intermediary precision of < 7%. The supplier of the kit used for PIVKA-II analysis reports a median value of 18.7 ng/ml (range 8.4 to 131) in a population of healthy adults with a mean age of 47 years.
dp-ucMGP was measured in plasma using a commercially available ECLIA on the IDS-iSYS Specialty Immunoassay System provided by Immunodiagnostics Systems according to the manufacturer’s instructions (ImmunoDiagnostic Systems Holdings PLC, East Boldon, UK). The limit of quantification for the assay is 300 pmol/l with an intermediary precision of < 8% [25]. The supplier of the kit used for dp-ucMGP analysis reports a value below 750 pmol/L to be normal. The supplier also report 0% cross-reactivity with desphosphorylated-carboxylated MGP and phosphorylated carboxylated MGP.
The most recent plasma creatinine prior to inclusion was obtained from the patient record and used for CKD staging using the CKD-EPI equation (without correction for race) for eGFR [26]. In relation to the study, new blood samples were analyzed for creatinine, HbA1C and lipids while albumin/creatinine ratio was calculated based on a morning spot urine sample. Diabetes was defined as HbA1C above 48 mmol/mol or receiving antidiabetic treatment. Smoking status was based on the patient’s own reporting.
Blood pressure measurements
Blood pressure (BP) was measured on the same day as blood samples collection, following at least 5 min of rest. Initial BP measurements were taken simultaneously on both arms using the WatchBP (Microlife Corporation, Widnau, Switzerland). Immediately thereafter, the arm with the highest BP reading was selected for further measurements using an oscillometric BpTRU device (VSM MedTech Ltd, Vancouver, Canada). The BpTRU device performed six consecutive measurements at 1-5-minute intervals. For analysis, the average of the last five measurements was calculated and used.
Statistical methods
Normal distribution was assessed with QQ-plots and histograms. Patient data are presented as mean ± standard deviation (SD) for normally distributed data and median (interquartile range (IQR)) if not normally distributed. Differences between two groups were tested using students t-test if normally distributed. For skewed data, Wilcoxon rank-sum (Mann-Whitney) test was used. Categorical variables are presented as n (%) and differences were tested using chi2-test.
For continuous data, linear regression models were created. Prior to analysis, some continuous variables were logarithmically transformed using natural logarithm as appropriate. Such transformed variables are denoted in brackets ([]). Associations were tested first in a univariate model. Afterwards, associations were tested in a multiple regression model. Separate models were created for dp-ucMGP and PIVKA-II, respectively. The parameters included in the multivariable regression models with dp-ucMGP and PIVKA-II as dependent variables were: eGFR, age, gender, diabetes status, smoking status and the [calcium-ion x phosphate] product [27]. In multivariable models with [aortic Agatston score] as the dependent variable, we additionally included dp-ucMGP and PIVKA-II. Post-analysis tests for variance inflation factor (VIF) was conducted to assess the degree of collinearity of factors in the multivariable regression models. Furthermore, due to less available data low-density lipoprotein (LDL) cholesterol along with office systolic BP was added to the same multivariable model in a separate analysis. Importantly, due to the known extreme increases in the levels of VKDPs caused by VKA, patients treated with VKA were excluded from the analysis. Finally, as some studies have used dp-ucMGP as a dichotomous variable split at the median [11], we also tested the predictive value in a dichotomized manner above/below median dp-ucMGP and PIVKA-II for aortic calcification in a separate multivariable regression model.
Regression models were tested for normal distribution of residuals with histograms and QQ-plots. Data from regression models are presented as coefficient (β), 95% confidence interval (95% CI) and P-value. A P-value < 0.05 was considered statistically significant. Post-analysis tests for variance inflation factor (VIF) were conducted to assess the degree of collinearity of factors in a model. A VIF of > 5.0 in one or more variables was considered significant evidence of impactful and thereby problematic collinearity [28]. Data was analyzed using STATA (version 17, StataCorp, College Station, TX, USA).
Results
A total of 160 patients from the ACCEPT population had both blood samples and aortic CT scans available for analysis. Seventeen of these were treated with a VKA and therefore excluded. The PIVKA-II and dp-ucMGP-levels of VKA-treated patients are presented in the Supplement. Furthermore, two patients were excluded due to hepatocellular disease known to independently affect PIVKA-II levels [29]. The final cohort included in the present analysis therefore consisted of 141 patients.
The basic characteristics by CKD category are shown in Table 1. Patients with CKD were older and more often had diabetes than patients with normal kidney function. Total cholesterol and LDL cholesterol were similar between the two groups. However, the groups differed on a range of biochemical parameters including 25-OH-vitamin D, parathyroid hormone, calcium-ion and phosphate.
Table 1.
Clinical and biochemical characteristics of the included participants
| Non-CKD (n = 37) |
CKD 3a (n = 28) |
CKD 3b (n = 32) |
CKD 4 (n = 21) |
CKD 5 (n = 23) |
CKD total (n = 104) |
P-value, Non-CKD vs. CKD | |
|---|---|---|---|---|---|---|---|
| Age (years) | 63.5 ± 8.2 | 70.8 ± 7.5 | 71.3 ± 10.0 | 71.2 ± 9.6 | 58.1 ± 11.9 | 68.2 ± 11.1 | 0.02 |
| Female, n (%) | 7 (19) | 5 (18) | 10 (31) | 6 (29) | 5 (22) | 26 (25) | 0.45 |
| BMI (kg/m2) | 27.9 ± 5.0 | 29.6 ± 5.9 | 28.6 ± 4.7 | 27.3 ± 5.0 | 27.0 ± 4.1 | 28.3 ± 5.0 | 0.69 |
| Smoking status, n (current/previously/never) | 3 / 24 / 10 | 3 / 16 / 9 | 3 / 18 / 11 | 3 / 12 / 6 | 5 / 11 / 7 | 14 / 57 / 33 | |
| Systolic BP (mmHg) | 120.6 ± 14.8 | 114.2 ± 16.7 | 116.2 ± 19.6 | 114.8 ± 16.4 | 122.2 ± 20.7 | 116.6 ± 18.5 | 0.25 |
| Diastolic BP (mmHg) | 75.5 ± 9.8 | 70.0 ± 12.2 | 71.4 ± 11.1 | 69.5 ± 10.5 | 73.7 ± 10.5 | 71.1 ± 11.1 | 0.04 |
| Diabetes, n (%) | 5 (14) | 12 (43) | 7 (22) | 11 (52) | 10 (43) | 40 (38) | 0.005 |
| eGFR (ml/min/1.73 m2) | 93.7 (81.9-102.6) | 48.2 (45.6–52.4) | 37.3 (33.1–39.5) | 24.0 (20.7–28.1) | 10.3 (7.0-13.1) | 33.9 (18.0-44.7) | < 0.0001 |
| Dialysis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (52) | 15 (12) | |
| UAC (mg/g) | 3 (0–12) | 13 (1–50) | 30 (7-165) | 208 (31–769) | 1720 (755–2703) | 47 (11–530) | < 0.0001 |
| Ca-ion (mmol/l) | 1.24 ± 0.04 | 1.25 ± 0.05 | 1.23 ± 0.05 | 1.23 ± 0.06 | 1.21 ± 0.05 | 1.23 ± 0.05 | 0.14 |
| Phosphate (mmol/l) | 0.98 (0.85–1.19) | 1.01 (0.94–1.17) | 1.17 (1.02–1.31) | 1.16 (1.00-1.26) | 1.71 (1.40–1.88) | 1.18 (1.00-1.38) | < 0.0001 |
| 25-OH-Vitamin D2 + D3 (nmol/l) | 69.4 ± 32.7 | 86.6 ± 31.3 | 86.7 ± 42.1 | 92.0 ± 45.3 | 82.2 ± 36.3 | 86.7 ± 38.4 | 0.01 |
| PTH (pmol/l) | 5.9 (5.1–8.1) | 9.0 (4.9–13.1) | 12.4 (9.6–17.2) | 18.2 (12.1–23.3) | 32.0 (20.7–54.4) | 15.0 (9.7–23.2) | < 0.0001 |
| Alkaline phosphatase (U/l) | 68 (62–96) | 73 (56–95) | 88 (69–131) | 94 (80–107) | 89 (73–105) | 84 (69–109) | 0.03 |
| LDL cholesterol (mmol/l) | 2.0 (1.7–2.4) | 1.6 (1.4-2.0) | 2.1 (1.7–2.6) | 2.0 (1.7–2.5) | 1.7 (1.5–2.7) | 1.9 (1.6–2.5) | 0.66 |
| Total cholesterol (mmol/l) | 3.9 ± 0.9 | 3.9 ± 1.0 | 4.4 ± 1.2 | 4.3 ± 1.1 | 4.3 ± 1.4 | 4.2 ± 1.2 | 0.42 |
Data are given as mean ± SD or median (interquartile range) unless otherwise stated
BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UAC, urine albumin-creatinine ratio; LDL, low density lipoprotein; PTH, parathyroid hormone
Missing data by category: UAC: 0, 0, 1, 0, 4, Vitamin D: 0, 0, 1, 0, 0, Ca-ion: 0, 1, 0, 0, 0, LDL cholesterol: 1,1,3,2,2, Systolic and diastolic BP: 1, 0, 0, 1, 2
Aortic Agatston score was higher in CKD patients compared with non-CKD patients (Table 2). However, within the CKD group, patients with CKD stage 5 tended to have lower scores than those with less advanced CKD. Notably, CKD stage 5 patients were younger, and the indication for CAG was more frequently part of the evaluation for renal transplantation rather than symptoms of coronary disease. Concentrations of dp-ucMGP were higher in CKD as compared to non-CKD patients, PIVKA-II levels did not show a significant difference between the two groups.
Table 2.
Median (interquartile range) Agatston scores normalized by body surface area, dp-ucMGP and PIVKA-II, by CKD group
| Non-CKD (n = 37) |
CKD 3a (n = 28) |
CKD 3b (n = 32) |
CKD 4 (n = 21) |
CKD 5 (n = 23) |
CKD total (n = 104) |
P-value Non-CKD vs. CKD | |
|---|---|---|---|---|---|---|---|
| Aorta Agatston score |
1644 (729–4138) |
9268 (3665–14991) |
6771 (4354–12873) |
7480 (2686–18036) |
3612 (1697–16008) |
7142 (2638–15692) |
< 0.0001 |
| dp-ucMGP (pmol/l) |
543 (503–744) |
812 (612–968) |
995 (836–1274) |
1027 (877–1329) |
1754 (1111–2715) |
1003 (790–1328) |
< 0.0001 |
| PIVKA-II (ng/ml) |
19.3 (16.3–23.5) |
20.2 (17.5–29.5) |
22.2 (17.8–36.1) |
17.4 (15.5–24.4) |
19.2 (15.7–26.9) |
20.3 (17.0-28.9) |
0.33 |
dp-ucMGP, dephosphorylated uncarboxylated matrix gla protein; PIVKA, protein induced by vitamin K absence
Factors associated with increased dp-ucMGP and PIVKA-II
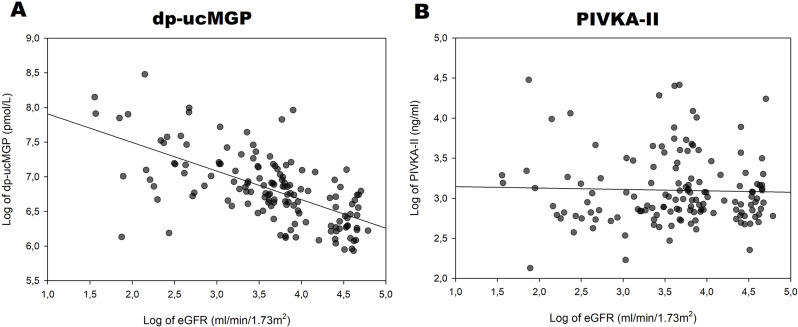
In univariate analysis [dp-ucMGP] increased with decreasing [eGFR] and with increasing [PIVKA-II]-levels (Table 3; Fig. 3). Additionally, [dp-ucMGP] was higher in patients with diabetes (Table 3). Of these factors, the association with [eGFR] was the strongest, with an r2 of 0.40 (Fig. 2A). The associations of [dp-ucMGP] with [PIVKA-II], diabetes and [calcium-ion x phosphate] were all relatively weak with low coefficients of determination (r2) (Table 3; Fig. 3). In a multivariable regression analysis including [eGFR], diabetes status, smoking status, age, [calcium-ion x phosphate] and gender; only [eGFR] and diabetes status remained significantly associated with [dp-ucMGP] levels (Table 3).
Table 3.
Associations in univariate and multivariable models with [dp-ucMGP] as the dependent variable
| Univariate model | Multivariable model (total r2 = 0.44) | |||||
|---|---|---|---|---|---|---|
| β (95% CI) | r 2 | P-value | β (95% CI) | P-value | VIF | |
| Age (years) | -0.001 (-0.009 to 0.007) | 0.00 | 0.727 | 0.002 (-0.004 to 0.008) | 0.566 | 1.07 |
| Gender (female) | 0.023 (-0.176 to 0.223) | 0.00 | 0.817 | 0.003 (-0.153 to 0.158) | 0.972 | 1.06 |
| Diabetes (yes) | 0.269 (0.094 to 0.445) | 0.06 | 0.003 | 0.172 (0.031 to 0.314) | 0.017 | 1.05 |
| Smoking (never vs. former or current) | -0.031 (-0.214 to 0.152) | 0.00 | 0.742 | -0.006 (-0.148 to 0.136) | 0.936 | 1.02 |
| [eGFR] (ml/min/1.73 m2) | -0.412 (-0.497 to -0.328) | 0.40 | < 0.001 | -0.446 (-0.550 to -0.342) | < 0.001 | 1.57 |
| [Calcium-ion x phosphate] (mmol2/l2) | 0.564 (0.238 to 0.890) | 0.08 | < 0.001 | -0.267 (-0.600 to 0.067) | 0.116 | 1.67 |
CI, confidence interval; [], Logarithmically transformed (natural logarithm); eGFR, estimated glomerular filtration rate; dp-ucMGP, dephosphorylated uncarboxylated matrix gla protein; VIF: Variance inflation factor
Fig. 2.
A: Logarithmically transformed eGFR plotted against logarithmically transformed dp-ucMGP. Line represents linear regression. B: Logarithmically transformed eGFR plotted against logarithmically transformed PIVKA-II. Line represents linear regression
Fig. 3.
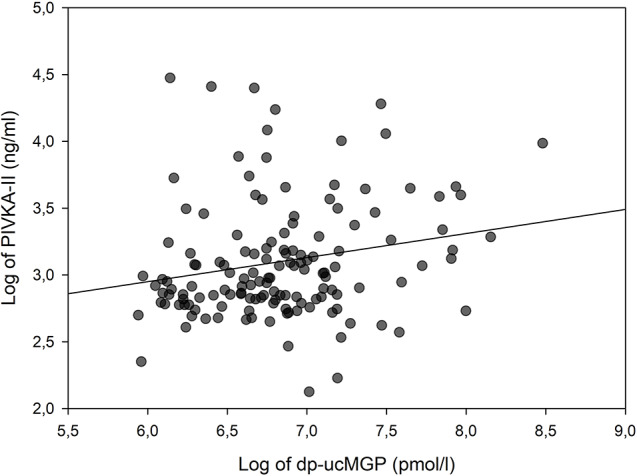
Logarithmically transformed dp-ucMGP plotted against logarithmically transformed PIVKA-II. Line represents linear regression
In contrast, PIVKA-II levels were not higher in patients with CKD and [PIVKA-II] showed no association with [eGFR] (Fig. 2B; Table 4). Moreover, in a multivariable regression model including the same factors ([eGFR], diabetes status, smoking status, age, [calcium-ion x phosphate] and gender), none were found to be significantly associated with [PIVKA-II].
Table 4.
Associations in univariate and multivariable models with [PIVKA-II] as the dependent variable
| Univariate model | Multivariable model (total r2 = 0.01) | |||||
|---|---|---|---|---|---|---|
| β (95% CI) | r 2 | P-value | β (95% CI) | P-value | VIF | |
| Age (years) | 0.001 (-0.006 to 0.008) | 0.00 | 0.728 | 0.001 (-0.006 to 0.008) | 0.824 | 1.07 |
| Gender (female) | 0.013 (-0.158 to 0.186) | 0.00 | 0.878 | 0.032 (-0.148 to 0.212) | 0.724 | 1.06 |
| Diabetes (yes) | -0.056 (-0.212 to 0.100) | 0.00 | 0.478 | -0.059 (-0.222 to 0.105) | 0.481 | 1.05 |
| Smoking (never vs. former or current) | 0.083 (-0.074 to 0.241) | 0.01 | 0.300 | 0.085 (-0.079 to 0.249) | 0.307 | 1.02 |
| [eGFR] (ml/min/1.73 m2) | -0.018 (-0.112 to 0.077) | 0.00 | 0.713 | -0.048 (-0.168 to 0.071) | 0.425 | 1.57 |
| [Calcium-ion x phosphate] (mmol2/l)2 | -0.036 (-0.331 to 0.259) | 0.00 | 0.810 | -0.120 (-0.505 to 0.265) | 0.539 | 1.67 |
CI, confidence interval; [], Logarithmically transformed (natural logarithm); eGFR, estimated glomerular filtration rate; PIVKA-II, protein induced by vitamin K absence II; VIF: variance inflation factor
[Suggested position for Table 4]
VKDPs as markers of aortic calcification
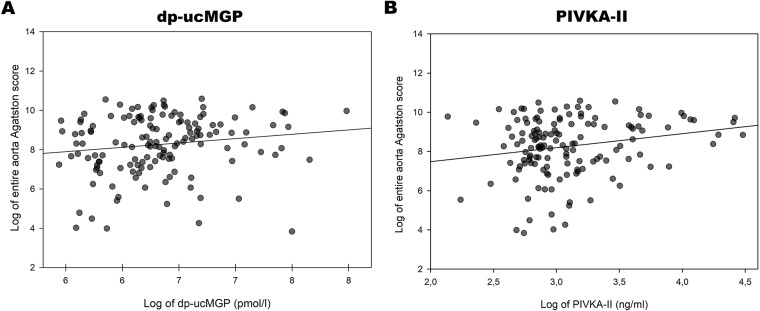
In univariate regression analysis [aortic Agatston score] tended to increase with increasing [dp-ucMGP] (Table 5; Fig. 4A). However, the association between [PIVKA-II] and [aortic Agatston score] was stronger and statistically significant (Table 5; Fig. 4B). Both smoking status and [eGFR] were associated with [aortic Agatston score] (Table 5), but while smoking was associated with higher [aortic Agatston score], the association was inverse for [eGFR]. Notably, r2 for the positive univariate association between age and [aortic Agatston score] was markedly higher than for any other of the tested variables (Table 5).
Table 5.
Associations in univariate and multivariable models with [aortic Agatston score] as the dependent variable
| Univariate model | Multivariable model (total r2 = 0.38) | |||||
|---|---|---|---|---|---|---|
| β (95% CI) | r 2 | P-value | β (95% CI) | P-value | VIF | |
| Age (years) | 0.07 (0.05 to 0.09) | 0.23 | < 0.001 | 0.07 (0.05 to 0.09) | < 0.001 | 1.07 |
| Gender (female) | 0.32 (-0.27 to 0.91) | 0.01 | 0.28 | 0.23 (-0.27 to 0.73) | 0.36 | 1.06 |
| Diabetes (yes) | 0.26 (-0.27 to 0.80) | 0.01 | 0.33 | 0.28 (-0.21 to 0.72) | 0.28 | 1.11 |
| Smoking (never vs. former or current) | 0.56 (0.02 to 1.10) | 0.03 | 0.04 | 0.53 (0.08 to 0.99) | 0.02 | 1.03 |
| [eGFR] (ml/min/1.73 m2) | -0.36 (-0.68 to -0.05) | 0.03 | 0.03 | -0.47 (-0.88 to -0.05) | 0.03 | 2.45 |
| [PIVKA-II] (ng/ml) | 0.71 (0.15 to 1.28) | 0.04 | 0.01 | 0.64 (0.14 to 1.13) | 0.01 | 1.10 |
| [dp-ucMGP] (pmol/l) | 0.44 (-0.05 to 0.93) | 0.02 | 0.08 | -0.16 (-0.73 to 0.41) | 0.59 | 1.94 |
| [Calcium-ion x Phosphate] (mmol2/l2) | 0.21 (-0.45 to 0.88) | 0.00 | 0.53 | 0.33 (-0.75 to 1.40) | 0.55 | 1.71 |
CI, confidence interval; [], Logarithmically transformed (natural logarithm); VIF, Variance inflation factor; eGFR, estimated glomerular filtration rate; PIVKA-II, protein induced by vitamin K absence II; dp-ucMGP, dephosphorylated uncarboxylated matrix gla protein; VIF: Variance inflation factor
Fig. 4.
Logarithmically transformed aortic Agatston score plotted against logarithmically transformed dp-ucMGP (A) and PIVKA-II (B)
In a multivariable model including [dp-ucMGP], [PIVKA-II], age, gender, [eGFR], diabetes status, smoking status and the [calcium-ion x phosphate], only age, [PIVKA-II], [eGFR] and smoking status were significant markers of [aortic Agatston score] (Table 5). The variance inflation factor was relatively low for all included factors in the multivariable model. Furthermore, the multivariable model had an r2-value of 0.38 indicating that a substantial part of the variation in [aortic Agatston score] was unaccounted for by the included variables.
LDL cholesterol was available in 132 patients. In a separate multivariate regression analysis including [LDL cholesterol] as well as office systolic BP and all the above covariates, only [PIVKA-II] and age remained significantly associated with [aortic Agatston score]. In this multivariable model, neither [LDL cholesterol] nor systolic BP were significantly associated with [aortic Agatston score].
No associations were found between [dp-ucMGP] or [PIVKA-II] and maximal calcification thickness in univariate analysis. Only age was significantly associated with calcification thickness (β = 0.06, 95% CI 0.01 to 0.11, P = 0.01, r2 = 0.04) and the association remained significant in multivariate analysis including gender, [eGFR], [dp-ucMGP], [PIVKA-II], diabetes-status, [calcium-ion x phosphate] and smoking.
Replacing [dp-ucMGP] and [PIVKA-II] as continuous variables with dp-ucMGP and PIVKA-II as dichotomous variables (above/below the median) in a multivariable analysis including age, gender, [eGFR], diabetes status, smoking status and [calcium x phosphate] did not alter our findings. Of the two, VKDPs only PIVKA-II remained significantly associated with [aortic Agatston score].
Discussion
The present study is the first to investigate the association between dp-ucMGP and the degree of calcification in the entire aorta in a cohort of patients representing the entire range of kidney function from normal eGFR to CKD stage 5. Similarly, the value of PIVKA-II as a marker of aortic calcification in CKD has not been previously explored. In our adjusted model, eGFR, age, smoking status and, notably, PIVKA-II emerged as significant markers of aortic calcification. We also observed that aortic calcification tended to increase with increasing dp-ucMGP levels. However, the association was not statistically significant, and this signal disappeared in an adjusted multivariable regression model. The association between PIVKA-II and Agatson score supports a role for vitamin K deficiency in arterial calcification in CKD. Our inclusion of patients across a broad spectrum of kidney function allowed us to establish with more certainty, that the association between PIVKA-II and aortic calcification is independent of eGFR.
MGP has been described as one of the most potent inhibitors of vascular calcification [30]. Mice lacking MGP experience rapid arterial calcification leading to death by arterial rupture [31]. Due to the vitamin K dependent nature of MGP, one might expect increased levels of inactive dp-ucMGP to be associated with an increased degree of arterial calcification. However, studies examining this association have shown mixed results. Some reported a rather strong association [10, 11, 15], while in others the association disappeared after adjustment for a range of biochemical markers and patient characteristics [16] and others still found no association [17, 18]. Uncontrolled confounding as well as differences in patient composition and study designs may likely explain these differences [20].
Importantly, while both PIVKA-II and dp-ucMGP are broadly reflective of vitamin K status, dp-ucMGP is believed to mirror vascular vitamin K status [14, 32]. PIVKA-II, on the other hand, may be more reflective of hepatic levels of vitamin K [13]. Our finding that PIVKA-II rather than dp-ucMGP is a better biomarker for calcification in the aorta, is therefore somewhat surprising. Previous studies on the association between PIVKA-II and vascular calcification have been in non-aortic vascular beds and found, with a single exception [33] no significant association [11, 18]. To the best of our knowledge, ours is the first study to investigate the association between PIVKA-II and calcification of the aorta.
In accordance with our findings, previous data showed a weak association between PIVKA-II and dp-ucMGP [5, 34]. Moreover, while dp-ucMGP is independently associated with renal function [7, 8], our data, and the findings of others, suggest that PIVKA-II is not [12]. Although the focus of the present study was on vascular vitamin K status, dp-ucMGP might be too tightly coupled to eGFR for the association to be extricated from the vascular calcification induced by CKD through other mechanisms than those studied. Therefore, in our cohort representing patients at all levels of kidney function, PIVKA-II appears to be more useful than dp-ucMGP for evaluating vitamin K status.
The importance of age as a risk factor for aortic calcification is well established in non-CKD cohorts [35–37]. Our findings suggest that even in patients suffering from CKD, age is of more importance for aortic calcification score than renal function. Furthermore, of the selected covariates, maximal calcification thickness was only dependent on age. Notably, Agatston score does not solely quantify the typical CKD-associated intima media calcification and maximal calcification thickness is also affected by intraluminal calcification, which may have a different pathogenesis [38]. This may explain why only age was a significant predictor of calcification thickness in our model.
The incidence of CKD increases with age, and CKD is in turn associated with vitamin K deficiency. However, our findings suggest that aortic calcification in CKD patients is linked to at least 3 different processes: one dependent on CKD, one dependent on aging and one dependent on vitamin K deficiency. However, despite the inclusion of age, gender, eGFR, diabetes status, smoking status, calcium-ion × phosphate, and both VKDPs in our model, the r² value was only 0.38, indicating that much of the variance in calcification is explained by factors not included in the analysis. Overall, our results suggest that markers of vitamin K deficiency (elevated dp-ucMGP and PIVKA-II) are associated with aortic calcification. Yet, from a clinical perspective, little is gained in the prediction of aortic calcification by analyzing dp-ucMGP and PIVKA-II as age outperforms all of the included markers and as neither of the two VKDPs are better markers of aortic calcification than smoking status or eGFR which are usually more readily available. Future studies could explore the potential anti-calcific effect of vitamin K as levels of dp-ucMGP and PIVKA-II are modifiable with vitamin K supplementation, as recently demonstrated [25], unlike the effects of ageing, smoking and loss of renal function which are more difficult to reverse.
Limitations and strengths
Quantification of aortic calcification based on CT-scans with subsequent Agatston scoring can detect lower levels of calcification than the lateral X-rays used for the widely applied Kauppila calcification scoring [39]. Some patients that would be graded as non-calcified using the Kaupplia method therefore likely have a positive Agatston score in our study. This higher sensitivity may lead to relatively higher estimates of calcification [20]. However, it is reasonable to assume that the higher calcification estimates apply to all levels of calcification and therefore does not introduce any systematic bias. Furthermore, compared to other modalities of aortic calcification grading, CT based Agatston scoring is more objective and allows for much more nuanced calcification grading and has even been described as the gold standard of arterial calcification grading [40].
To be included in the study, participants had to be referred for coronary angiography, which could entail a selection bias towards patients with more vascular disease; particularly so in the non-CKD group. Nonetheless, we found a lower degree of aortic calcification in the control group than in the CKD group and the presence of aortic calcification has been described as highly prevalent at all levels of kidney function in studies of patients outside the CAG setting [41]. The exact difference between the control group and general population in terms of aortic calcification is unknown. However, a higher degree of aortic calcification in the control group would likely dilute rather than strengthen the associations between CKD, vitamin K-related biomarkers and aortic calcification. The study design allows for considerable variation in both the degree of calcification as well as kidney function which allows for better adjustment for kidney function as a confounder in our analysis.
Furthermore, the cross-sectional study design limits conclusions regarding causality. While calcification is a slowly developing process, the biochemical measurements used in our regression models are snapshots. In light of these reservations, we find it likely that the significant association between PIVKA and aortic calcification is substantially smaller than what could be found in a prospective cohort study aimed at investigating the association between long term PIVKA-II-levels and aortic calcification. Moreover, it is possible that a better estimate of vitamin K deficiency would be attained using the ratio between MGP and dp-ucMGP.
Conclusion
Among markers of vitamin K deficiency, we found PIVKA-II, but not dp-ucMGP, to be significantly associated with aortic calcification in a cohort of non-VKA treated patients representing the entire spectrum of kidney function. This association remained significant after adjustment for a range of covariates including smoking, age and eGFR. Beyond PIVKA-II, our findings show eGFR, smoking and age to be independent factors related to aortic calcification. Age appears to be of particular importance, outweighing both VKDPs in predicting aortic calcification. However, even in a multivariable regression model including several factors known to be associated with arterial calcification, much variation remains unexplained underlining the need for studies investigating biochemical as well as genetic factors explaining vascular calcification in CKD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our appreciation for our research nurses for their invaluable work during the conduction of this study in addition to the laboratory technicians for technical assistance in performing the laboratory analysis.
Abbreviations
- CKD
Chronic kidney disease
- MGP
Matrix gla protein
- dp-ucMGP
dephosphorylated uncarboxylated MGP
- PIVKA-II
protein induced by vitamin K absence
- eGFR
Estimated glomerular filtration rate
- VKDP
Vitamin K dependent protein
- VKA
Vitamin K antagonist
- BSA
Body surface area
- CAG
Coronary angiography
- CT
Computed tomography
- ELICA
electrochemiluminescence immunoassay
- BSA
Body surface area
- BP
Blood pressure
- LDL
low-density lipoprotein
- CI
Confidence interval
- VIF
variance inflation factor
Author contributions
JN: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization, Project administration, Supervision. KLC: Conceptualization, Methodology, Writing – review & editing, Supervision. GA: Conceptualization, Methodology, Writing – review & editing, Supervision, Resources. MR: Conceptualization, Methodology, Writing – review & editing, Supervision. BLN: Conceptualization, Methodology, Writing – review & editing, Resources. JSM: Conceptualization, Methodology, Writing – review & editing, Resources.SN: Formal analysis, Investigation, Writing – review & editing, Resources. MBT: Formal analysis, Investigation, Writing – review & editing, Resources. JMJ: Conceptualization, Methodology, Writing – review & editing, Resources. Christian Daugaard Peters: Conceptualization, Methodology, Writing – review & editing, Resources.Niels Henrik Buus: Conceptualization, Methodology, Formal analysis, Writing – original draft, Supervision, Funding acquisition.
Funding
This study was funded through grants from Karen Elise Jensen Fond, Skibsredder Per Henriksens Fond, Aarhus University and the Central Denmark Region.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Declarations
Ethics approval and concent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Central Denmark Region Committee on Health Research Ethics (Journal number 1-10-72-21-19). All study participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 2.McCullough PA, Chinnaiyan KM, Agrawal V, Danielewicz E, Abela GS. Amplification of atherosclerotic calcification and Mönckeberg’s sclerosis: a spectrum of the same disease process. Adv Chronic Kidney Dis. 2008;15(4):396–412. [DOI] [PubMed] [Google Scholar]
- 3.Shioi A, Morioka T, Shoji T, Emoto M. The Inhibitory Roles of Vitamin K in progression of vascular calcification. Nutrients. 2020;12(2). [DOI] [PMC free article] [PubMed]
- 4.Stępień A, Koziarska-Rościszewska M, Rysz J, Stępień M. Biological role of vitamin K-With Particular emphasis on Cardiovascular and Renal aspects. Nutrients. 2022;14(2). [DOI] [PMC free article] [PubMed]
- 5.Rapp N, Brandenburg VM, Kaesler N, Bakker SJL, Stöhr R, Schuh A et al. Hepatic and Vascular Vitamin K Status in patients with High Cardiovascular Risk. Nutrients. 2021;13(10). [DOI] [PMC free article] [PubMed]
- 6.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, et al. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25(8):1629–33. [DOI] [PubMed] [Google Scholar]
- 7.Griffin TP, Islam MN, Wall D, Ferguson J, Griffin DG, Griffin MD, et al. Plasma dephosphorylated-uncarboxylated Matrix gla-protein (dp-ucMGP): reference intervals in caucasian adults and diabetic kidney disease biomarker potential. Sci Rep. 2019;9(1):18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puzantian H, Akers SR, Oldland G, Javaid K, Miller R, Ge Y, et al. Circulating Dephospho-Uncarboxylated Matrix Gla-protein is Associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31(9):988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuiri S, Nishizawa Y, Yamashita K, Ono K, Naito T, Tanji C, et al. Relationship of matrix Gla protein and vitamin K with vascular calcification in hemodialysis patients. Ren Fail. 2019;41(1):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Médart L, et al. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5(4):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato A, Yasuda H, Togawa A, Yamamoto T, Yonemura K, Maruyama T, et al. Measurement of des-gamma-carboxy prothrombin levels in hemodialysis patients positive for anti-hepatitis virus C antibody. Clin Nephrol. 2002;58(4):296–300. [DOI] [PubMed] [Google Scholar]
- 13.Caluwé R, Verbeke F, De Vriese AS. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transpl. 2020;35(1):23–33. [DOI] [PubMed] [Google Scholar]
- 14.Boxma PY, van den Berg E, Geleijnse JM, Laverman GD, Schurgers LJ, Vermeer C, et al. Vitamin k intake and plasma desphospho-uncarboxylated matrix gla-protein levels in kidney transplant recipients. PLoS ONE. 2012;7(10):e47991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liabeuf S, Bourron O, Olivier B, Vemeer C, Theuwissen E, Magdeleyns E, et al. Vascular calcification in patients with type 2 diabetes: the involvement of matrix gla protein. Cardiovasc Diabetol. 2014;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salam S, Gallagher O, Gossiel F, Paggiosi M, Eastell R, Khwaja A. Vascular calcification relationship to vascular biomarkers and bone metabolism in advanced chronic kidney disease. Bone. 2021;143:115699. [DOI] [PubMed] [Google Scholar]
- 17.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22(2):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meuwese CL, Olauson H, Qureshi AR, Ripsweden J, Barany P, Vermeer C, et al. Associations between thyroid hormones, calcification inhibitor levels and vascular calcification in end-stage renal disease. PLoS ONE. 2015;10(7):e0132353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theuwissen E, Teunissen KJ, Spronk HM, Hamulyák K, Ten Cate H, Shearer MJ, et al. Effect of low-dose supplements of menaquinone-7 (vitamin K2) on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteers. J Thromb Haemost. 2013;11(6):1085–92. [DOI] [PubMed] [Google Scholar]
- 20.Barrett H, O’Keeffe M, Kavanagh E, Walsh M, O’Connor EM. Is Matrix Gla Protein Associated with vascular calcification? A systematic review. Nutrients. 2018;10(4). [DOI] [PMC free article] [PubMed]
- 21.Nyvad J, Christensen KL, Andersen G, Reinhard M, Maeng M, Nielsen S, et al. Aortic calcification is Associated with the difference between Invasive Central and Cuff-measured brachial blood pressure in chronic kidney disease. Am J Hypertens. 2024;37(7):455–64. [DOI] [PubMed] [Google Scholar]
- 22.Marwan M, Mettin C, Pflederer T, Seltmann M, Schuhbäck A, Muschiol G, et al. Very low-dose coronary artery calcium scanning with high-pitch spiral acquisition mode: comparison between 120-kV and 100-kV tube voltage protocols. J Cardiovasc Comput Tomogr. 2013;7(1):32–8. [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 24.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–11. discussion 12 – 3. [PubMed] [Google Scholar]
- 25.Levy-Schousboe K, Frimodt-Møller M, Hansen D, Peters CD, Kjærgaard KD, Jensen JD, et al. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J. 2021;14(9):2114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed]
- 27.Tertti R, Harmoinen A, Leskinen Y, Metsärinne KP, Saha H. Comparison of calcium phosphate product values using measurement of plasma total calcium and serum ionized calcium. Hemodial Int. 2007;11(4):411–6. [DOI] [PubMed] [Google Scholar]
- 28.Marcoulides KM, Raykov T. Evaluation of Variance inflation factors in regression models using Latent Variable modeling methods. Educ Psychol Meas. 2019;79(5):874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhang W, Liu Y, Gong W, Sun P, Kong X, et al. Diagnostic value of prothrombin induced by the absence of vitamin K or antagonist-II (PIVKA-II) for early stage HBV related hepatocellular carcinoma. Infect Agent Cancer. 2017;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaesler N, Schurgers LJ, Floege J. Vitamin K and cardiovascular complications in chronic kidney disease patients. Kidney Int. 2021;100(5):1023–36. [DOI] [PubMed] [Google Scholar]
- 31.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386(6620):78–81. [DOI] [PubMed] [Google Scholar]
- 32.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewé RB, et al. Characterisation and potential diagnostic value of circulating matrix gla protein (MGP) species. Thromb Haemost. 2010;104(4):811–22. [DOI] [PubMed] [Google Scholar]
- 33.Park M, Yu BC, Choi SJ, #6246, PIVKA II IS A BIOMARKER FOR PREDICTING CORONARY CALCIFICATION IN HEMODIALYSIS PATIENTS WITH DIABETES. Nephrol Dialysis Transplantation. 2023;38(Supplement_1).
- 34.Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in Population-Based studies. Nutrients. 2016;8(1). [DOI] [PMC free article] [PubMed]
- 35.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810–5. [DOI] [PubMed] [Google Scholar]
- 36.Odink AE, van der Lugt A, Hofman A, Hunink MG, Breteler MM, Krestin GP, et al. Risk factors for coronary, aortic arch and carotid calcification; the Rotterdam Study. J Hum Hypertens. 2010;24(2):86–92. [DOI] [PubMed] [Google Scholar]
- 37.Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the multi-ethnic study of atherosclerosis (MESA). Am Heart J. 2008;155(4):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(6):1585–98. [DOI] [PubMed] [Google Scholar]
- 39.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–50. [DOI] [PubMed] [Google Scholar]
- 40.Disthabanchong S, Boongird S. Role of different imaging modalities of vascular calcification in predicting outcomes in chronic kidney disease. World J Nephrol. 2017;6(3):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sørensen IMH, Saurbrey SAK, Hjortkjær H, Brainin P, Carlson N, Ballegaard ELF, et al. Regional distribution and severity of arterial calcification in patients with chronic kidney disease stages 1–5: a cross-sectional study of the Copenhagen chronic kidney disease cohort. BMC Nephrol. 2020;21(1):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.