Abstract
Background
To assess the psychosocial impact of moderate-severe juvenile idiopathic arthritis (JIA) on patients and their families, among those who had been treated with at least one anti-tumor necrosis factor (anti-TNF-α), according to routine clinical practice in Spain.
Patients and methods
A 24-month observational, multicentric, cross-sectional and retrospective study was performed. Children diagnosed with JIA were enrolled at three tertiary-care Spanish hospitals. The study included children treated with biologic disease-modifying antirheumatic drugs (bDMARD) who participated in a previous study, the ITACA, and who continued follow-up in these pediatric rheumatology units. Patient health-related quality of life (HRQoL) was assessed using the Pediatric Quality of Life Inventory (PedsQL™). Caregivers completed an interview to gather information about school attendance, their children’s participation in school and social activities, its impact on their jobs and social life and perceived psychosocial support.
A descriptive statistical analysis of all the variables was performed. The Mann–Whitney-U test or Kruskall-Wallis H test were used to compare quantitative variables and Fisher’s exact tests was used for qualitative variables. Tests were two-tailed with a significance level of 5%. The data were analyzed using SPSS V18.0 statistical software.
Results
One hundred and seven patients were included. Overall, patients were on inactive disease or low disease activity according to JADAS-71 score and had very low functional disability according to CHAQ score. Up to 94.4% of patients were receiving drug treatment, mainly with bDMARD in monotherapy (84.5%). Based on PedsQL, patients and parents referred a high HRQoL. School Functioning PedsQL domain achieved the lowest score. Work and social impact due to the child´s disease was greater for mothers than for fathers. The understanding of the disease was lower at school than in the with family and friends’ environments.
Conclusion
Most of the patients had a high HRQoL and had controlled disease activity, despite having a negative psychosocial impact on some of them and their families, mainly on school functioning. Children’s disease seems to involve greater work and psychosocial impacts for mothers than for fathers of children affected by JIA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12969-024-01035-6.
Keywords: JIA, Children, Family, Work, Psychosocial
Background
Juvenile idiopathic arthritis comprises a heterogeneous group of chronic inflammatory arthritis with quite variable presentations and courses [1]. It places great burden on the affected children and their families as well as on the healthcare system [2–5]. An optimal control of JIA activity may lead to improved long-term clinical results, which could affect social function, work, quality of life, and healthcare expenses [6, 7].
JIA affects not only patients’ physical functions but also the psychological and social components of their lives [8]. JIA symptoms can range from a single swollen joint to multiple joints affected, associated with pain, fatigue, erythema, and loss of vision due to uveitis, as well as erythema or fever in systemic forms [1]. Its effect on school-age children can vary from missing a few school days each year to recurring hospitalizations requiring a tutor to keep them on track with schoolwork [9]. Frequent absences from school and lack of participation in school activities affect academic success and social relationships [10].
The impact of a chronic pediatric illness is not restricted to the child, as it affects all family members [8]. Families with children affected by JIA may have to face many difficulties, mainly related to the social, emotional and economic impact of the disease [7]. Disease negatively impacts the quality of life of caregivers [6]. The aim of the current sub-analysis of the ITACA-OR study was to provide a descriptive data on the psychosocial impact of this disease in moderate-severe JIA patients and their families in Spain.
Patients and methods
This is a subanalysis of ITACA OR (Outcomes Resources in Cohort from Immunogenicity and Treatment Efficacy in Children with JIA Treated with Anti-TNF and Calprotectin as Disease Activity Biomarker; multicentric study) a 24-month retrospective study of children who had previously participated in the ITACA study (PFI-ETA-2013–01) from 2016 to 2018 [11].
The ITACA study was a 12-month observational and prospective study that included outpatients younger than 18 years diagnosed with non-systemic JIA according to the International League of Associations for Rheumatology criteria [12] (JIA can be diagnosed if age at onset is < 16 years, disease duration is ≥ 6 weeks, and other known conditions are excluded) and previously treated with anti-TNF-α treatment (entry criteria for the ITACA cohort) [13].
Written informed consent was obtained from parents or legal tutor of all enrolled children. In addition, children over 7 years of age provided assent. In accordance with the Spanish recommendations, this 24-month retrospective study was approved by the Clinical Research Ethics Committee of La Fe Hospital apart from the 12-month ITACA study and was conducted following the principles included in the Declaration of Helsinki for studies in human subjects.
The study was conducted among children attending the pediatric rheumatology department in three tertiary-care hospitals in Spain in the context of a single routine visit. Data were recorded retrospectively over a 24-month period. A structured questionnaire was designed to include national health system resource use, family of patient out-of-pocket expenses, and parent work impact [11]. Data collection included demographics and clinical characteristics and use of healthcare resources. Other structured questionnaire was completed by parents, those questions were designed to obtain costs related to the disease in the last 24 months (work absences, caregiver, family and professional, hospital visits cost, home adaptations, assistance and mobility devices, physiotherapy, psychotherapy, and others), and its collected data to calculate direct costs from the National Health System (NHS). As well, clinical and healthcare resource usage data were obtained from NHS registry of electronic health records, which includes visits (eg, medical, nursing, public physiotherapy), hospital admissios and complementary testing (eg, analytic and image tests). Indirect costs were estimated from the number of working days lost, using the human capital approach. Unitary costs were acquired from official sources (in 2020 euros) [14].
Childhood Health Assessment Questionnaire (CHAQ) to measures functional capacity in eight aspects of daily life: dressing, grooming, getting up, eating, walking, bathing, reaching, and gripping) and discomfort (pain and general well-being) and the Pediatric Quality of Life Inventory™ (PedsQL™ [15]) were completed by parents and/or children depending on age. The 23-item PedsQL™ includes four domains: Physical Functioning (8 items), Emotional Functioning (5 items), Social Functioning (5 items), and School Functioning (5 items). The PedsQL™ Scales are composed of parallel child self-report and parent-delegated forms. Higher scores indicate a better HRQoL.
The Juvenile Arthritis Disease Activity Score (JADAS-71) [16], to assess the JIA activity, was calculated from children/parent’s well-being based on a visual analog scale (VAS), joint count, erythrocyte sedimentation rate (ESR), and physician disease activity based on VAS, higher scores are associated with more severe disease activity.
Caregivers completed a structured interview to gather detailed information about school attendance, participation of their children in school and social activities, the impact of JIA on their jobs, social impact, and psychosocial support over the last 24 months. Understanding of JIA as a disease by the social environment of patients and families was also gathered during this interview. A structured interview was designed according to the pediatric rheumatologists with wide clinical experience, over 30 years of professional practice, from centers participating in the study, and consulted with the social workers and patients experience member Group at San Joan De Deu Hospital, and with a rheumatic patient association after which a list of questions related to psychosocial aspects from parents and patients was established (Table 1 includes list of items and list of questions included in the interview are included as Supplementary Material S1).
Table 1.
Evaluation on psychosocial impact in JIA patients and parents. Items included in the interview
| Psychosocial impact in JIA patients | Psychosocial impact in JIA parents | |
|---|---|---|
| Educational/Work |
Patients’ school performance • Number of missed school days/hours due to JIA • After-school support needed due to the disease |
Parents’ missed days/hours due to the child’s JIA: • Number of missed days/hours due to the child’s JIA (father/mother/another caregiver) • Job (father/mother/another caregiver) • Professional level (father/mother/another caregiver) • Need for a job change (father/mother/another caregiver) |
| Social |
• Difficulties in participating in school and social activities • Number of activities missed (sport, trips, culture, hobbies) |
• Number of social activities missed by parents due to JIA |
| Psychological |
• Number of monthly private/public physiotherapy sessions • Number of private/public psychology sessions (children) |
• Number of private/public psychology sessions (parents) • Number of social activities missed by parents due to JIA |
Functional and psychological impact were evaluated separately at different levels of disease activity measured by JADAS 71.
Statistical methodology
A descriptive statistical analysis of all the variables was performed, including central tendency and dispersion measures for continuous variables, and absolute and relative frequencies for categorical variables. Mann–Whitney-U test or Kruskall-Wallis H test were used to compare quantitative variables and Fisher’s exact tests was used for qualitative variables. Tests were two-tailed with a significance level of 5%. Data were analyzed using SPSS V18.0 statistical software.
Results
A total of 107 patients were included in the study, all of whom were analyzed. Most of the patients were female (69.2%) with a mean (SD) age of 12.9 (4.1) years, a mean (SD) disease duration of 9.1 (3.4) years and a mean (SD) time from diagnosis to start anti-TNFα treatment of 2.9 (2.8) years.
According to JADAS-71 index, overall, 82.4% of patients had inactive disease/low disease activity, whereas 17.6% had moderate/high disease activity. Mean (SD) JADAS-71 score for all patients was 1.3(2.6)/1(1.5) in patients with inactive disease/low disease activity vs 2.5 (3.0) in those with a moderate/ high disease, respectively). Similarly, a very low functional disability was observed in the overall population [mean (SD) CHAQ score 0.2 (0.4); 0,1 (0,2) in patients with inactive disease/low disease activity vs 0.4 (0.6) in those with a moderate/ high disease, respectively]. A positive correlation between a higher disease activity and a higher CHAQ score was observed (Table 2).
Table 2.
Demographic and clinical patient characteristics
| n (%) | Mean (SD) | Min | Max | Q1-Q3 | |
|---|---|---|---|---|---|
| JIA patients (N) | 107 | ||||
| Age (mean years, SD) | 12.9 (4.1) | 5.0 | 21.0 | 10-16 | |
| Gender (female) (n, %) | 74 (69.2%) | ||||
| Residence urban (n, %) | 88 (82.2) | ||||
| Disease duration (years) | 9.1 (3.4) | 4.0 | 19.0 | 6.6-12.0 | |
| Time from diagnosis to first anti-TNF (years) | 2.9 (2.8) | 0.0 | 10.9 | 0.6-4.7 | |
| ILAR classification (n, %): | |||||
| Persistent | 36 (33.3) | ||||
| Oligoarthritis | 15 (14.0) | ||||
| Extended | 1 (0.9) | ||||
| Oligoarthritis Polyarthritis RF ( +) | 35 (32.7) | ||||
| Polyarthritis RF (-) | 3 (2.8) | ||||
| Psoriatic Arthritis | 13 (12.1) | ||||
| Arthritis Related to Enthesitis Undifferentiated Arthritis | 4 (3.7) | ||||
| History of uveitis, | 37 (34.6) | ||||
| Episodes of uveitis in the last 24 months, n (%) | 27 (25.2) | ||||
| JADAS-71 | 1.4 (2.9) | 0.0 | 17.0 | 0.0- 2.0 | |
| Oligoarticular Polyarticular | 1.0 (1.9) | 0.0 | 8.2 | 0.0 - 1.4 | |
| bJADAS-71, na (%) | 75 (71,5) | ||||
| Inactive | 12 (11,4) | ||||
| Low disease activity Moderate disease activity | 10 (9,5) | ||||
| High disease activity | 8 (7,6) | ||||
| cHAQ (mean, SD) | 0.2 (0.4) | 0.0 | 2.0 | 0.0 -1.0 | |
| c cHAQ, na (%) | 99 (92.5) | ||||
| Mild dysfunction Moderate dysfunction | 6 (5.6) | ||||
| PedsQL (0–100) Mean, (SD) | |||||
| Children | 84.8 (15.2) | 37.0 | 100.0 | 77.2-97.8 | |
| Parents | 82.0 (17.4) | 31.5 | 100.0 | 72.6-96.7 | |
JIA juvenile idiopathic arthritis, ILAR International League of Associations for Rheumatology, JADAS-71 Juvenile Arthritis Disease Activity Score, ESR erythrocyte sedimentation rate, VAS Visual analogic scale, CHAQ the Childhood Health Assessment Questionnaire, PedsQL Pediatric Quality of Life Inventory, Min Minimum, Max Maximum, SD Standard Deviation
aIn 2 patients JADAS and CHAQ results were not included in the CRF (Case report Form). Percentages are expressed as valid percentage (% Valid): Percentage of the total number of valid answers, i.e., with data in the variable
bJADAS cut-off for oligoarthritis and polyarthritis: inactive ≤ 1 (both); low 1–2 and 1–3.8; moderate > 2–4.2 and > 3.8–10.5; high > 4.2 and > 10.5 respectively
c cHAQ cut-off: mild ≤ 1; moderate 1- ≤ 2
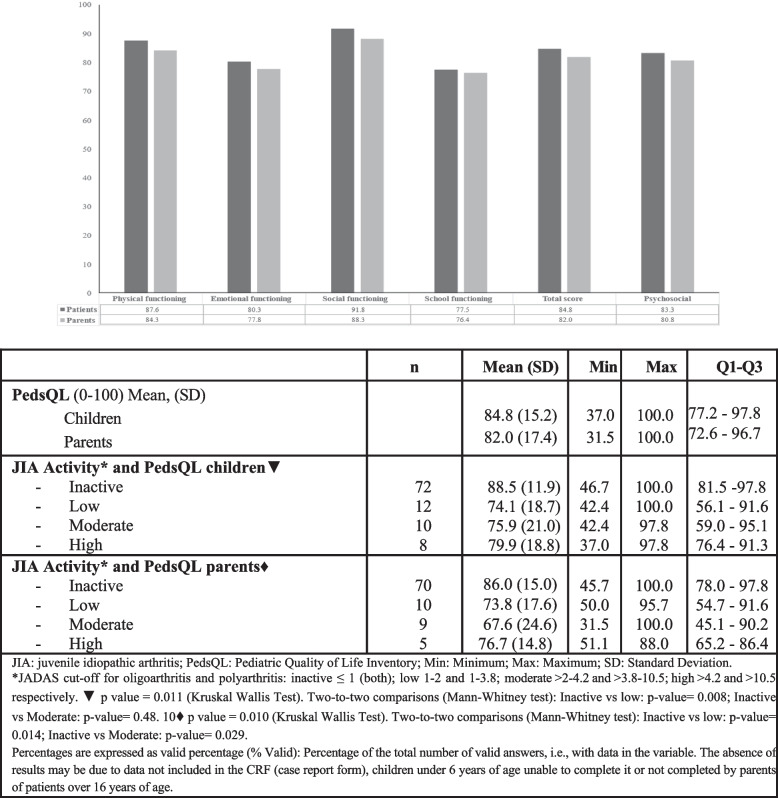
Patients and parents reported a high HRQoL (PedsQL 4.0) total scores of 84.8 and 82 out of 100, respectively. Regarding disease activity estimated with JADAS-71, in patients with inactive disease/low disease activity, the child´s and parent´s PedsQL scores were 81.3 and 79.9, respectively; in those with moderate-high disease activity, the child´s PedsQL was 77.9, and the parents´ PedsQL was 72.15 (Fig. 1). A statistically significant difference was found between PedsQL values according to disease activity for both parents and children (Fig. 1). Scale and Summary scores showed that children perceived their QoL to be better than that of their parents, the mean score for Social Functioning for children was 91.8 and that for parents 88.3, that for School Functioning mean score for children was 77.5 and that for parents was 76.4 (Fig. 1).
Fig. 1.
PedsQL results
The impact of the JIA on patient’s life is shown in Table 3. The mean (SD) number of school absences in the last 24 months because of JIA was 15.7 (25.2) days and 14.2% of patients needed additional school support. The inability to participate in school activities with peers occurred in 29.2% of patients (school outing 29%, physical education 54.8%, sports 22.6%). Similarly, 35.6% of patients with inactive disease/low disease activity and 47.9% with moderate-severe disease reported difficulty participating in school activities, with a mean number of missed school activities of 4.4 (2.4) and 7.5 (9.1), respectively. Among patients who remained inactive (n = 75), there was a significant difference between those who reported difficulties participating in school activities and those who did not [78.7% vs 21.3%, respectively (p = 0.021)]. Additionally, 9.3% had difficulties in participating in social activities with friends. Overall, 12.3% needed physiotherapy, and 34.3% reported need for psychological support.
Table 3.
Impact of JIA on patient´s lives (24 months)
| Na, n (%) | Mean (SD) | Min–Max | Q1-Q3 | ||
|---|---|---|---|---|---|
| Education impact | |||||
| N of school absences due to JIA (days) | 104 | 15.7 (25,3) | 0–182.6 | 5-16.8 | |
|
% of children who needed additional school support due to JIA - Hours per children who needed support |
106, 15 (14.2) | ||||
| 255.3 (252.4) | 30–528 | 30- -- | |||
| Functional impact | |||||
| cHAQ and disease activity▼ | |||||
| - Inactive | 0.1 (0.2) | 0.0 - 1.5 | 0.0 - 0.0 | ||
| - Low | 0.2 (0.3) | 0.0 - 1.1 | 0.0 - 0.3 | ||
| - Moderate | 0.6 (0.8) | 0.0 - 2.0 | 0.0 - 1.4 | ||
| - High | 0.3 (0.4) | 0.0 - 1.3 | 0.0 - 1.3 | ||
| Social impact | |||||
| Related to school activities | Patients with difficulties in participating in school activities | 106, 31 (29.0) | |||
| Patients with difficulties in participating in School Activities by disease activity: | |||||
| - Inactive* | 75, 16 (21.3) | ||||
| - Low | 12, 6 (50) | ||||
| - Moderate | 9, 3 (33.3) | ||||
| - High | 5 (62.5) | ||||
| N of school activities missedb per children who missed activities | 5.2 (6.0) | 1.0 - 30.0 | 2.0 - 6.0 | ||
| N of school activities missed by disease activity** | |||||
| - Inactive | 3.1 (1.1) | 1.0 - 4.5 | 2.0 - 4.0 | ||
| - Low | 5.8 (3.7) | 2.0 - 12.5 | 2.8 - 7.6 | ||
| - Moderate | 4.5 (4.9) | 1.0 - 8.0 | 1.0 - -- | ||
| - High | 10.5 (13.3) | 2.0 - 30.0 | 2.0 - 24.5 | ||
| Related to social activities | % of patients with difficulties in participating in social activities | 97, 10 (9.3) | |||
| N of social activities missed in children who refer difficulties | 3.1 (2.4) | 1.0 - 8.0 | 1.5 - 4.5 | ||
| Additional support | |||||
| Private/public psychology support (children) | 67, 23 (34.3) | ||||
▼p value = 0.047 (Kruskal Wallis Test). Two-to-two comparisons Inactive vs Moderate: p-value = 0.007 (Mann–Whitney test)
* p value = 0.021 (Fisher's Exact Test)
** p value = 0.483 (Kruskal Wallis Test)
a In some cases, data was not available at CRF. Percentages are expressed as valid percentage (% Valid): Percentage of the total number of valid answers, i.e., with data in the variable
b Number of activities missed by patients in the 23 patients who reported missing some activity
The impact of JIA on parents’ lives is shown in Table 4. Parents’ perception that JIA strongly affects their working life was 7.1% for the fathers, while this item increased to 21.8% for mothers. Over the course of the 24-month observation period, 64% of the fathers and 68.9% of the mothers had work absences due to their child`s JIA; the mean (SD) days of work missed by fathers was 6.9 (6.0), whereas those missed by mothers were of 8.0 (5.8) days. Changes in work due to their children’s disease were reported by 2% of the fathers and 5.9% of the mothers. The involvement of other family members was also collected, reporting a need for support due to JIA from grandparents during afterschool time in 34.3% of the patients and a number of hours of 214.15 (SD 252.6) per child in 24 months.
Table 4.
JIA Impact on families lives (24 months)
| N, n (%) | Days, Mean (SD) | ||
| Work impact | |||
| Father | Work absences due to child’s JIA, | 98, 64 (64.0) | 6.9 (6) |
| Change of work due to child’s JIA | 98, 2 (2) | ||
| Loss of work due to child’s JIA | 98, 1 (1) | ||
| Mother | Work absences due to the child’s JIA | 101, 71 (68.9) | 8.0 (5.8) |
| Change of work due to child’s JIA | 101, 6 (5.9) | ||
| Loss of work due to child’s JIA | 101, 2 (2) | ||
| Social impact | |||
| Father | Social activities lost due to JIA | 95, 9 (9.5) | |
| Impact on the partner relationship | 95, 11 (11.6) | ||
| Mother | Social activities lost due to JIA | 101, 14 (13.9) | |
| Impact on the partner relationship | 101, 16 (16.3) | ||
| Grandparents impact | |||
| Grandparents | N, n (%) | Mean (SD), Min-Max | |
| Need of afterschool support by grandparents | 105, 36 (34.3) | ||
| Number of hours | 214.5 (252.6); [6 - 520] | ||
| Psychosocial support | |||
| Private/public psychology support | 62, 16 (15) | ||
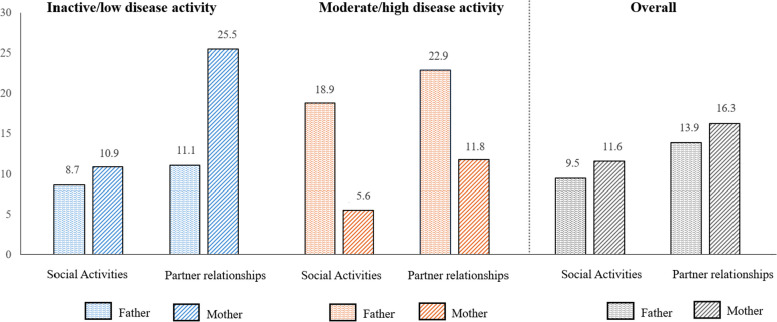
With respect to the social impact of children´s JIA on parents, 9.5% of fathers and 13.9% of mothers reported a loss of social activity, with a greater impact when the child´s disease activity was moderate/ high. Parents reported that partner relationships were affected—11.6% for fathers and 16.3% for mothers. In this case, there was a lower impact in parents of children with moderate/ high JIA (Fig. 2). Fifteen percent of parents also attended private/public psychology support.
Fig. 2.
Impact on social activities and partner relationship in fathers and mothers and JIA activity. Percentage of fathers and mothers who report impact in social activities and partner relationship by children disease activity according to JADAS-71
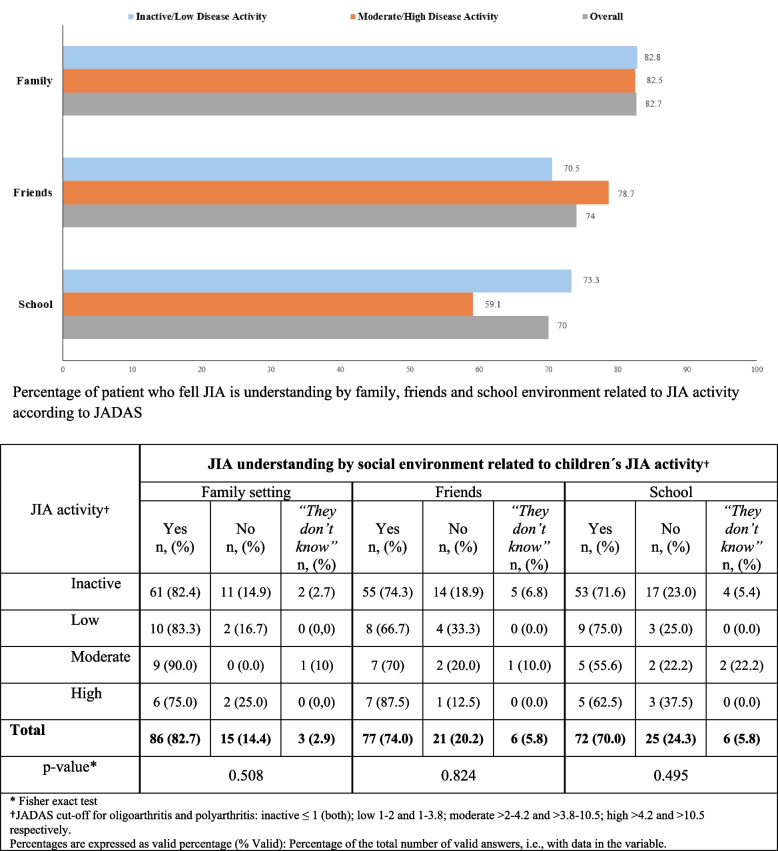
The perceived understanding of JIA by the social environment of patients and parents was greater in the family setting (83%) than among friends (74.5%) or in the school environment (70.5%), where surprisingly, JIA seems to be less understood when disease activity is moderate/ high (59%) (Fig. 3). Disease activity did not appear to influence en the perceived understanding of JIA by social environment among family settings, friends or school (p values 0.508, 0.824 and 0.495, respectively) (Fig. 3).
Fig. 3.
JIA understanding by social environment and JIA activity†
Discussion
The present study offers descriptive data regarding consequences of JIA for patient’s quality of life, impact at school setting and caregiver burden in a cohort of moderate-severe JIA patients in Spain in daily clinical practice.
In previous studies, children with JIA had a lower HRQoL than did the reference population, and the HRQoL improved within 3 years of diagnosis [17]. A better control of JIA activity may lead to improved long-term clinical results, as well as improved social function and quality of life [18]. Overall, most of our patients presented a sustained inactive disease/low disease activity state and a very low functional disability state after a mean disease duration of 9.1 years and long-term biologic treatment. In the present study patients had a high HRQoL as measured by PedsQL, both for physical functioning and for psychosocial dimension.
In a Canadian cohort [19], including 1,249 children with early (< 6 months) JIA, factors contribution to HRQoL improvement were explored. The follow up determined that disease activity, pain, and disability improve faster than HRQoL, confirming other studies results [20–22] and that higher basal disease activity heralds an unfavorable HRQoL trajectory [20]. Another German cohort compared, prospectively, HRQoL in recently diagnosed (< 6 months) JIA patients with healthy peers, founding a very small difference between groups at month 36. Worst HRQoL results were related with higher family burden, pain level and lower wellbeing at baseline. PedsQL mean for JIA children at month 36 was 93.6 (SD 6.6) and 76% of patient reach an adequate HRQoL (≥ 79.3) [23].
In this Spanish cohort, although has not a longitudinal follow up and JIA duration is longer, children and parents reported a high HRQoL, mean was 84.8 (15.2) but children in our cohort had longer disease duration with a mean of 9.1 years and the minimum value of disease duration was 4 years. Consistently with other studies also a lower HRQoL was found the group of patients with higher disease activity. Studies that include children with early JIA [20, 24] had a better HRQoL than in our study.
Disease activity, time of evolution and potentially early treatment with advanced therapy has influence in HRQoL, but it could be influenced not only by disease itself but for other social and psychological impact that should be taken in account, providing support also to families and maybe at school.
In our study the PedsQL school functioning dimension scored the lowest, and the school absenteeism and difficulties in participating in school activities were still present in a relatively high proportion of patients, as has also been observed in other studies [24].
Data from a recent study demonstrated that children with JIA are at risk of exhibiting worse behavior in school than healthy children are, as is a higher school absence rate [25]. In contrast, our study showed that the school absenteeism because of JIA was low [mean (SD) 15.7 (25.2) days/24 months]. Similarly, a recent cross-sectional study of Canadian JIA patients aged 8–17 years with a mean [interquartile range (IQR)] disease duration of 5 (3–8) years, showed that fewer days of schooling were missed (7/per year) [10]. Higher school absenteeism has been reported by other authors [26]. However, the countries in which these studies were performed vary drastically from North America or Europe regarding educational economic and health care systems [10].
Little attention has been given to characterizing which particular domains of HRQoL are affected the most and which remain most affected over time [10]. The physical limitations of JIA include associated complications that can cause considerable restrictions in daily activities [16]. In our study 29% of patients reported difficulties participating in school activities with peers, especially related to physical activities such as school outing (29%), physical education (54.8%) and sports (22.6%), even in patients with inactive/low disease activity. A Danish study also showed less physical activity than healthy children of the same age, which was not justified by JIA-related inflammation or pain [27]. In Canada similar results were reported, 32.2% of patients were unable to participate in activities within or outside the class, and 40.7% were unable to participate in the gym10. JIA patients are encouraged to be physically active to promote positive psychosocial and biological effects of exercise [28]. Therefore, any decreased in participation shown by JIA patients is of particular concern [10] and the implementation of measures that increase active healthy lifestyle in JIA schooler should be included in health plans.
Our results indicate that the school environment was the social setting in which the participants had the lowest understanding of JIA. Other studies have shown that parents with feelings of frustration and indignation from peers and teachers who are unable to understand the children`s fluctuation in physical activity and schoolwork [29]. Therefore, interventions to improve knowledge about JIA and its burdens among teachers and other schoolmates would be desirable.
Some aspects about which parents’ impact were evaluated in our study are considered as affected areas in other questionnaires or studies, as pocket expenses, family roles, impact of diagnosis, couple/mate relationships, limited time for other activities or impact at work. Several specific studies have reported different burden components of caring for a child with JIA [23] as well as other studies found worst results in them than in parents of healthy children [30–35]. In our study, parents' lives were affected in the work and psychosocial spheres, reflecting that emotionally the diagnosis of a chronic illness in a child is one of the most difficult situations a parent can face [29]. Regarding work impact, our study evaluated a period of 24 months in children with long-standing JIA, but it should be noted that previous studies showed that during the year following diagnosis, differences in the loss of work time compared to that of parents with healthy children, were particularly significant [36]. A cross-sectional and international European study assessed the subjective burden among caregivers [6], revealing variability among countries, from 16.4 (low burden) to 50.0 (moderate burden) in the case of France [6]. Changes in emotional impact over time and parents of children with JIA have been described as a mix of negative and positive emotions [9]. At the time of disease control, parents feel less overwhelmed and better equipped to process information about their child’s illness; however, this situation is sometimes mixed with fatigue and frustration about the continuing need for treatment as well as with anxiety regarding potential treatment side-effects [23]. In our study, patients had a long-term disease, and most of the children had inactive or low disease activity; and even so, an impact on work life, social activities and relationships was reported.
The mother’s impact in the study appears to be greater than that of the fathers. The disease affected working life in 21.8% of mothers, while it did so in 7.1% of fathers. Similarly, our results indicated that the prevalence of psychosocial impairment reported was greater in mothers. The proportion of mothers who considered that their social activities and partner relationships were affected by their children’s JIA was numerically greater than that of the fathers. This impact could be related not only to the social roles assumed by mothers but also to differences in maternal views and feelings about their disease [37]. In general, the involvement of caring for the child seems to fall more on the mother, and it is usually the mothers who come more frequently to the visit with the child, with or without chronic disease, in most out the countries. This might explain why most out studies had been focused on mothers, seem that mothers experienced more stress, sleep disturbances, anxiety,… than fathers and had higher anxiety and depression scores than mothers of healthy children [38]. A few studies focused on JIA fathers experience had suggested that fathers are affected as mothers, but they adopt different adaptational strategies than women, as denial and distraction [39, 40]. Although several studies indicate that women have worse stress scores compared to men when it comes to caring for sick children the literature is inconclusive about the relationship between stress rates and gender differences among caregivers resulting from the exercise of care [41, 42]. According with a social role evolution, with increasing fathers’ involvement in care we included separately issues of social and work impact on mothers and fathers. In our study perceived impact in social activities and partner relationships was slightly higher in mothers, but fathers seem have a higher impact in children with moderate/high disease activity.
Impact of children chronic diseases siblings had been described previously, in diseases with a wide range of severity. This impact can be shown as anxiety, depression, feelings of worry and social problems among others, and Quality of Life decrease [43–46]. Despite this, parents are not always very aware and could minimize this impact [47, 48]. Nevertheless, positive outcomes had been described in those siblings as ‘sense of purpose’, strengthened intra-family relationships, and resilience [49], therefore, knowing if these families have differentiating characteristics could help the design of future strategies. In a systematic review that explored parenting intervention for parents of children with chronic disease, a need to improve this approach was noted, and also to measure the intervention results [50]. Informational support to parents could help to create awareness of siblings needs. Especially in JIA, due to lack of evidence available, it is important to make visible problems related with social and family impact, as low perception about this impact at school or in siblings, it will allow improving health outcomes for the patient and families.
Limitations of the present study concern the fact that the results should only be generalized to a specific population of Spanish patients, at three national reference hospitals in pediatric rheumatology, placed in urban areas, treating JIA patients within the NHS, which has universal coverage for pathologies in children. These children had severe non-systemic JIA, have required treatment with biologic DMARDs, followed up in high complexity hospitals, with a long evolution of the disease, a long-term treatment with bDMARD, and a close follow-up. Study limitations are the long disease duration and lack of prospective follow-up of HRQoL, with a higher number of patients comparations between longer and short JIA duration and between early or late initiation of an antiTNF could bring additional information that reinforce importance of reach remission not only in physical patient limitation but also in family, economic and psychosocial impact. For future studies, this impact might be evaluated prospectively and considered in therapeutic decisions. Regarding data acquisition remember missing work or school days over the last 24 months could be difficult but medical appointments appear in the electronic registry of NHS, in addition parents usually annotate medical appointments in their agendas, and missing days are register at work, just like in schools. While there may be some inaccuracy, parents were allowed to take home the questionnaire, consult and respond later. Lack of a consensus among JIA experts on the most appropriate aspects and tools to assess disease impact in the family’s comparison and reproducibility are difficult and its future development would be desirable.
Conclusion
In this study in Spain most of the patients had a high HRQoL and had controlled disease activity, despite having a negative psychosocial impact on some of them and their families, mainly on school functioning. Children’s disease seems to involve greater work and psychosocial impacts for mothers than for fathers of children affected by JIA.
Findings from this study could help healthcare providers, organizations, researchers, and policy makers on the development of future clinical practices and research for psychosocial impact among JIA patients and families.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- anti-TNF-α
Anti-tumor necrosis factor
- bDMARD
Biologic disease-modifying antirheumatic drugs
- cHAQ
Childhood Health Assessment Questionnaire
- ESR
Erythrocyte sedimentation rate
- HRQoL
Health-related quality of life
- IQR
Interquartile range
- ITACA OR
Outcomes Resources in cohort of anti-TNF drug Immunogenicity influence on Therapeutic afficacy in juvenile idiopathic Arthritis using serum CAlprotectin as a disease activity marker
- JADAS-71
Juvenile Arthritis Disease Activity Score
- JIA
Juvenile idiopathic arthritis
- PedsQL™
Pediatric Quality of Life Inventory
- SD
Standard deviation
Authors’ contributions
List each author’s contributions using the following format with the author’s initials. MM, CP, JA conceived of the study. MM, CP, IC, JA participated in the design of the study. JA, IC, EM, MM, MV, CP, AL, participated in the statistical analysis design and interpretation. MM, CP, MV, AL, JA, IC participated in its design. MM, JA, IC, EM participated in coordination. MM, CP, AL, MV, NLl, JA, IC, EM, MLC, VB, EI, RB, JCH, JSM, SCA, CEG, MIGF, MMM, BLM, LRD, JMA, LLP, ADL participated helped draft the manuscript. JA, IC, EM, MLC, VB, EI, RB, JCH, JSM, SCA, CEG, MIGF, MMM, BLM, LRD, JMA participated acquisition of data for the work.
Funding
This study is funding by Pfizer.
Data availability
The data that support the findings of this study are available from Pfizer, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Pfizer and EC.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from parents or legal tutor of all enrolled children. In addition, children over 7 years of age provided assent. In accordance with the Spanish recommendations, this 24-month retrospective study was approved by the Clinical Research Ethics Committee of La Fe Hospital in addition to the 12-month ITACA study and was conducted following the principles included in the Declaration of Helsinki for studies in human subjects.
Consent for publication
The publication plan was included in the study protocol.
Competing interests
MV, NLl, AL, MM and CP are Pfizer employes.
JA, IC received compensation from Pfizer for their services as a member of the ITACA Steering Committee.
JA has received institutional grants from AbbVie, Amgen, AstraZeneca, Aurinia, BMS, GSK, Lilly, Novartis, Sanofi Sobi, consulting fees from Novartis, Sobi and GSK, payment honoraria from Sobi, Pfizer and Novartis, support for attending meetings and congress from Sobi, Pfizer, AbbVie and Roche, participation on Data Safety Monitoring Board or Advisory Board in STARS trial.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martini A, Lovell DJ, Albani S, et al. Juvenile idiopathic arthritis. Nat Rev Dis Primers. 2022;8:5. 10.1038/s41572-021-00332-8. [DOI] [PubMed] [Google Scholar]
- 2.Minden K, Niewerth M, Listing J, Biedermann T, Schontube M, Zink A. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63(7):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gidman W, Meacock R, Symmons D. The humanistic and economic burden of juvenile idiopathic arthritis in the era of biologic medication. Curr Rheumatol Rep. 2015;17(5):31. [DOI] [PubMed] [Google Scholar]
- 4.García-Rodríguez F, Gamboa-Alonso A, Jiménez-Hernández S, et al. Economic impact of Juvenile Idiopathic arthritis: a systematic review. Pediatr Rheumatol. 2021;19:152. 10.1186/s12969-021-00641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenoi S, Horneff G, Cidon M, Ramanan AV, Kimura Y, Quartier P, Foeldvari I, Zeft A, Lomax KG, Gregson J, Abma T, Campbell-Hill S, Weiss J, Patel D, Marinsek N, Wulffraat N. The burden of systemic juvenile idiopathic arthritis for patients and caregivers: an international survey and retrospective chart review. Clin Exp Rheumatol. 2018;36(5):920–8 Epub 2018 Mar 21. PMID: 29600940. [PubMed] [Google Scholar]
- 6.Kuhlmann A, Schmidt T, Treskova M, Lopez-Bastida J, Linertova R, Oliva-Moreno J, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. 2016;17(Suppl 1):79–87. [DOI] [PubMed] [Google Scholar]
- 7.Rasu RS, Cline SK, Shaw JW, Hayes O, Agbor BW, Cifaldi MA. Impact of JIA on parents’ work absences. Rheumatology (Oxford). 2015;54(7):1177–85. [DOI] [PubMed] [Google Scholar]
- 8.Bruns A, Hilario MO, Jennings F, Silva CA, Natour J. Quality of life and impact of the disease on primary caregivers of juvenile idiopathic arthritis patients. Joint Bone Spine. 2008;75(2):149–54. [DOI] [PubMed] [Google Scholar]
- 9.Sanzo M. The child with arthritis in the school setting. J Sch Nurs. 2008;24(4):190–6. [DOI] [PubMed] [Google Scholar]
- 10.Chomistek K, Johnson N, Stevenson R, et al. Patient-reported barriers at school for children with juvenile idiopathic arthritis. ACR Open Rheumatol. 2019;1(3):182–7. 10.1002/acr2.1023. Published 2019 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antón J, Moreno Ruzafa E, Lopez Corbeto M, et al. Real-world health care outcomes and costs among patients with juvenile idiopathic arthritis in Spain. J Health Econ Outcomes Res. 2023;10(2):141–9. 10.36469/001c.85088. PMID:38145114;PMCID:PMC10742379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 13.Calvo I, Modesto C, Montoro M, et al. Influence of anti-tumor necrosis factor (TNF) drug immunogenicity on the treatment effectiveness in juvenile idiopathic arthritis (JIA) using serum calprotectin as a disease activity marker. multicenter study. ITACA STUDY. Ann Rheum Dis. 2019;78(1):A973. 10.1136/annrheumdis-2019-eular.7963. [Google Scholar]
- 14.BotPlus. Consejo General de Colegios Oficiales de Farmacéuticos. Base de datos del Conocimiento Sanitario. Accessed November 2, 2020. https://botplusweb.farmaceuticos.com/ 29. eSalud. Oblikue eHealth. Database of economic information of the sanitary sector. 2020. http:// esalud.oblikue.com/excel.asp. Accessed 2 Nov 2020.
- 15.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual. Life Outcomes. 2007;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–66. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD, Saqib B, Lee RR, Shoop-Worrall S, Hyrich KL, McDonagh JE et al. Is time a healer? How quality of life changes over time reported by parents of children and young people with Juvenile Idiopathic Arthritis. Rheumatology (Oxford) 2022. [DOI] [PMC free article] [PubMed]
- 18.Barth S, Haas JP, Schlichtiger J, Molz J, Bisdorff B, Michels H, Hügle B, Radon K. Long-Term health-related quality of life in german patients with Juvenile idiopathic arthritis in comparison to German general population. PLoS ONE. 2016;11(4):e0153267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oen K, Guzmán J, Dufaultb B, et al. Health-related quality of life in an inceptioncohort of children with Juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res. 2018;70(1):134–44. 10.1002/acr.23236. [DOI] [PubMed] [Google Scholar]
- 20.Seid M, Opipari L, Huang B, Brunner HI, Lovell DJ. Diseasecontrol and health-related quality of life in juvenile idio-pathic arthritis. Arthritis Rheum. 2009;61:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haverman L, Grootenhuis MA, van den Berg JM, vanVeenendaal M, Dolman KM, Swart JF, et al. Predictors ofhealth-related quality of life in children and adolescentswith juvenile idiopathic arthritis: results from a web-basedsurvey. Arthritis Care Res (Hoboken). 2012;64:694–703. [DOI] [PubMed] [Google Scholar]
- 22.Brunner HI, Klein-Gitelman MS, Miller MJ, Trombley M, Baldwin N, Kress A, et al. Health of children with chronic arthritis: relationship of different measures and the quality of parent proxy reporting. Arthritis Rheum. 2004;51:763–73. [DOI] [PubMed] [Google Scholar]
- 23.Listing M, Mönkemöller K, Liedmann I, et al. The majority of patients with newly diagnosed juvenile idiopathic arthritis achieve a health-related quality of life that is similar to that of healthy peers: results of the German multicenter inception cohort (ICON). Arthritis Res Ther. 2018;20:106. 10.1186/s13075-018-1588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouaddi I, Rostom S, El BD, Hassani A, Chkirate B, Amine B, et al. Impact of juvenile idiopathic arthritis on schooling. BMC Pediatr. 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milatz F, Klotsche J, Niewerth M, et al. Participation in school sports among children and adolescents with juvenile idiopathic arthritis in the German national paediatric rheumatologic database, 2000–2015: results from a prospective observational cohort study. Pediatr Rheumatol. 2019;17:6. 10.1186/s12969-019-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordal E, Rypdal V, Arnstad ED, Aalto K, Berntson L, Ekelund M, et al. Participation in school and physical education in juvenile idiopathic arthritis in a Nordic long-term cohort study. Pediatr Rheumatol Online J. 2019;17(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohr AH, Nielsen S, Müller K, Karup Pedersen F, Andersen LB. Reduced physical activity in children and adolescents with Juvenile idiopathic arthritis despite satisfactory control of inflammation. Pediatr Rheumatol Online J. 2015;13:57. 10.1186/s12969-015-0053-5. Published 2015 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philpott JF, Houghton K, Luke A. Physical activity recommendations for children with specific chronic health conditions: juvenile idiopathic arthritis, hemophilia, asthma, and cystic fibrosis. Clin J Sport Med. 2010;20(3):167–72. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Ramirez O, Gibbon M, Berard R, Jurencak R, Green J, Tucker L, et al. A recurring rollercoaster ride: a qualitative study of the emotional experiences of parents of children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres-Made MD, Peláez-Ballestas I, García-Rodríguez F, et al. Development and validation of the CAREGIVERS questionnaire: multi-assessing the impact of juvenile idiopathic arthritis on caregivers. Pediatr Rheumatol. 2020;18:3. 10.1186/s12969-020-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan JL, Mullins LL, Ramsey RR, Bonner MS, Jarvis JN, Gillaspy SR, et al. Caregiver demand and parent distress in juvenile rheumatic disease: the mediating effect of parent attitude toward illness. J Clin Psychol Med Settings. 2013;20(3):351–60. [DOI] [PubMed] [Google Scholar]
- 32.Yuwen W, Lewis FM, Walker AJ, Ward TM. Struggling in the dark to help my child: parents’ experience in caring for a young child with Juvenile idiopathic arthritis. J Pediatr Nurs. 2017;37:e23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhlmann A, Schmidt T, Treskova M, Lopez-Bastida J, Linertova R, OlivaMoreno J, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. 2016;17(Suppl 1):79–87. [DOI] [PubMed] [Google Scholar]
- 34.Gerhardt CA, Vannatta K, McKellop JM, Zeller M, Taylor J, Passo M, et al. Comparing parental distress, family functioning, and the role of social support for caregivers with and without a child with juvenile rheumatoid arthritis. J Pediatr Psychol. 2003;28(1):5–15. [DOI] [PubMed] [Google Scholar]
- 35.Pearce C, Newman S, Mulligan K. Illness uncertainty in parents of children with Juvenile idiopathic arthritis. ACR Open Rheumatol. 2021;3(4):250–9. 10.1002/acr2.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafia S. Rasu, Stephanie K. Cline, James W. Shaw, Oscar Hayes, Walter Agbor Bawa, Mary A. Cifaldi, Impact of JIA on parents’ work absences, Rheumatology, 2015;54(7):1177–1185. 10.1093/rheumatology/keu414 [DOI] [PubMed]
- 37.Mulligan K, Etheridge A, Kassoumeri L, Wedderburn LR, Newman S. Do mothers and fathers hold similar views about their child’s arthritis? Arthritis Rheum. 2009;61:1712–8. 10.1002/art.25008. [DOI] [PubMed] [Google Scholar]
- 38.Waite-Jones JM, Swallow V, Madill A. From ‘neurotic’ to ‘managing’ mother: The ‘medical career’ experienced by mothers of a child diagnosed with Juvenile Idiopathic Arthritis. Br J Health Psychol. 2020;25(2):324–38. 10.1111/bjhp.12409. [DOI] [PubMed] [Google Scholar]
- 39.Waite-Jones JM, Madill A. Concealed concern: fathers’ experiences of having a child with juvenile idiopathic arthritis. Psychol Health. 2008;23(5):585–601. 10.1080/08870440802036911. [DOI] [PubMed] [Google Scholar]
- 40.McNeill T. Fathers’ experience of parenting a child with juvenile rheumatoid arthritis. Qual Health Res. 2004;14(4):526–45. 10.1177/1049732303262374. [DOI] [PubMed] [Google Scholar]
- 41.Toledano-Toledano F, Domínguez-Guedea MT. Psychosocial factors related with caregiver burden among families of children with chronic conditions. BioPsychoSocial Med. 2019;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penning MJ, Wu Z. Caregiver stress and mental health: impact of caregiving relationship and gender. Gerontologist. 2016;56(6):1102–13. [DOI] [PubMed] [Google Scholar]
- 43.Limbers CA, Skipper S. Health-related quality of life measurement in siblings of children with physical chronic illness: a systematic review. Fam Syst Health. 2014;32(4):408–15. 10.1037/fsh0000077. Epub 2014 Aug 18 PMID: 25133709. [DOI] [PubMed] [Google Scholar]
- 44.Quintana Mariñez MG, Chakkera M, Ravi N, Ramaraju R, Vats A, Nair AR, Bandhu AK, Koirala D, Pallapothu MR, Khan S. The other sibling: a systematic review of the mental health effects on a healthy sibling of a child with a chronic disease. Cureus. 2022;14(9):e29042. 10.7759/cureus.29042. PMID:36249634;PMCID:PMC9550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezgin S, Özkaya Y, Akbaş Y, Elbasan B. An investigation of computer-game addiction, physical activity level, quality of life and sleep of children with a sibling with a chronic condition. Child Care Health Dev. 2024;50(1):e13228. 10.1111/cch.13228. [DOI] [PubMed] [Google Scholar]
- 46.Blamires J, Foster M, Rasmussen S, Zgambo M, Mörelius E. The experiences and perceptions of healthy siblings of children with a long-term condition: Umbrella review. J Pediatr Nurs. 2024;77:191–203. 10.1016/j.pedn.2024.03.022. ISSN 0882. 5963. [DOI] [PubMed] [Google Scholar]
- 47.Dinleyici M, Çarman KB, Özdemir C, et al. Quality-of-life evaluation of healthy siblings of children with chronic illness. Balkan Med J. 2019;37(1):34–42. 10.4274/balkanmedj.galenos.2019.2019.7.142. Epub 2019 Oct 24. PMID: 31647208; PMCID: PMC6934013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Boudec, Audraine, Akre, Christina, Barrense-Dias, Yara, Berchtold, André and Suris, Joan-Carles. Siblings of youths with chronic conditions: a school-based survey. Int J Adolesc Med Health. 2021;33(3):173–180. 10.1515/ijamh-2018-0247 [DOI] [PubMed]
- 49.Lummer-Aikey S, Goldstein S. Sibling adjustment to childhood chronic illness: an integrative review. J Fam Nurs. 2021;27(2):136–53. 10.1177/1074840720977177. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell AE, Morawska A, Vickers-Jones R, et al. A systematic review of parenting interventions to support siblings of children with a chronic health condition. Clin Child Fam Psychol Rev. 2021;24:651–67. 10.1007/s10567-021-00357-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Pfizer, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Pfizer and EC.