Abstract
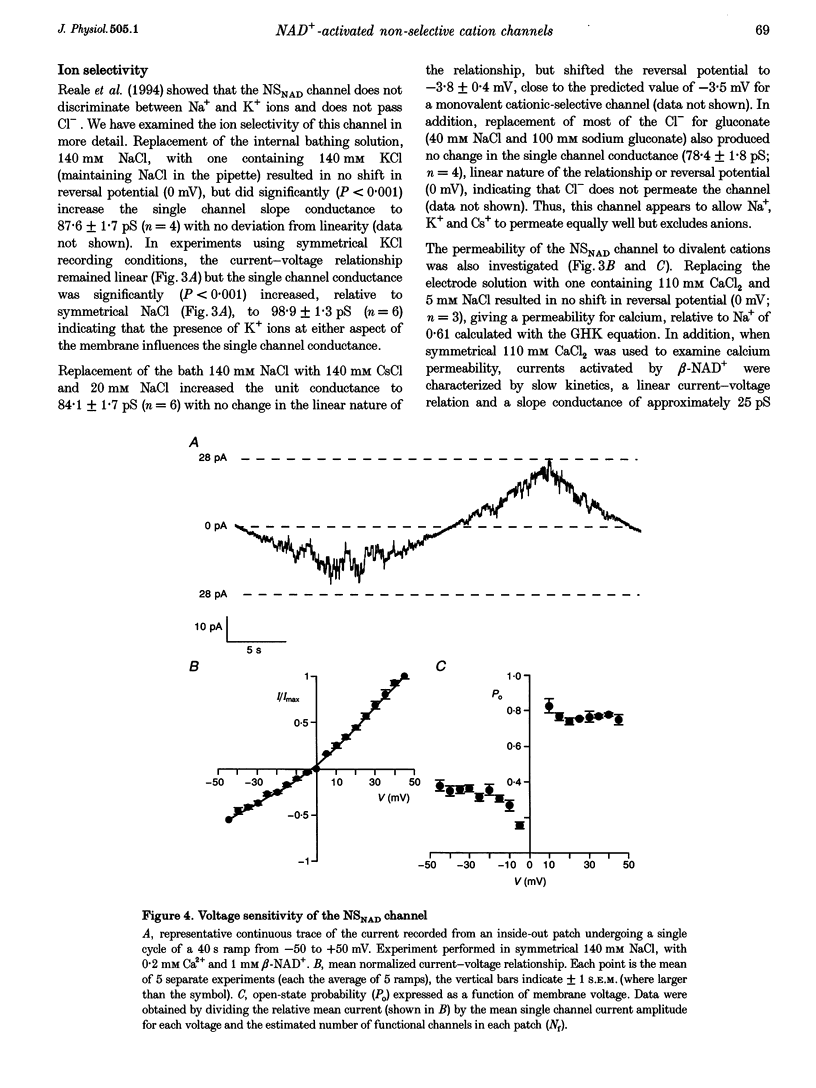
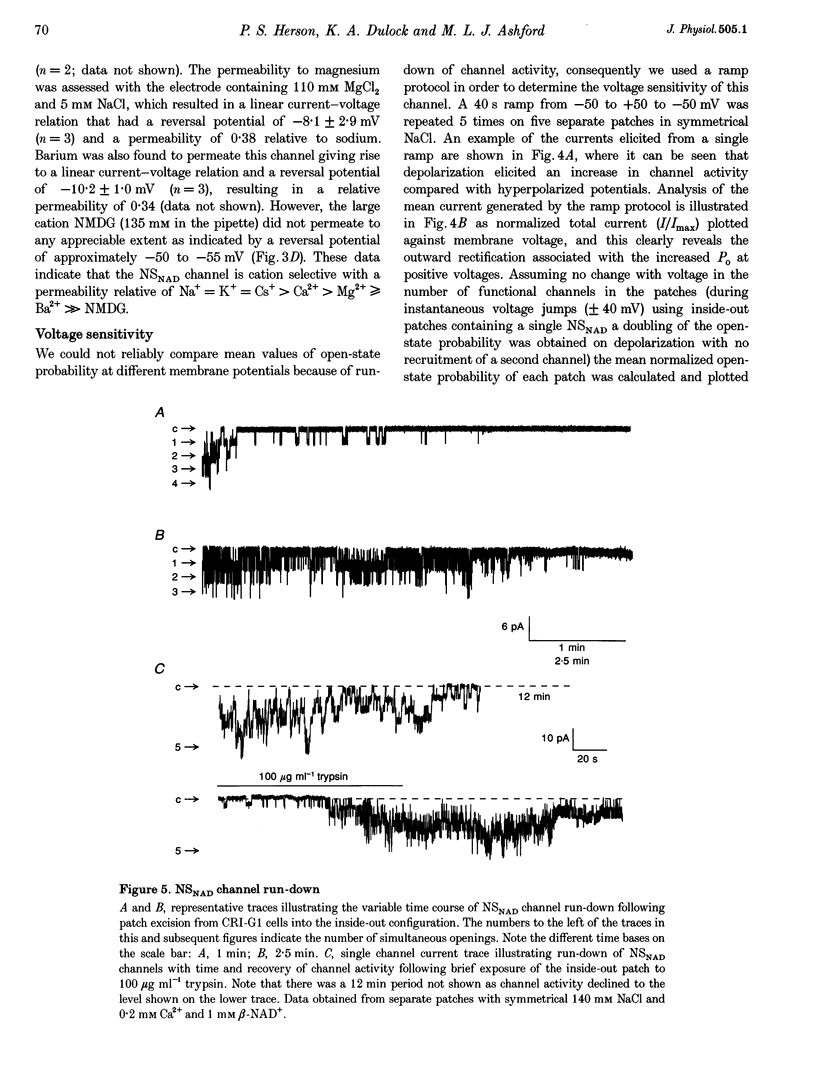
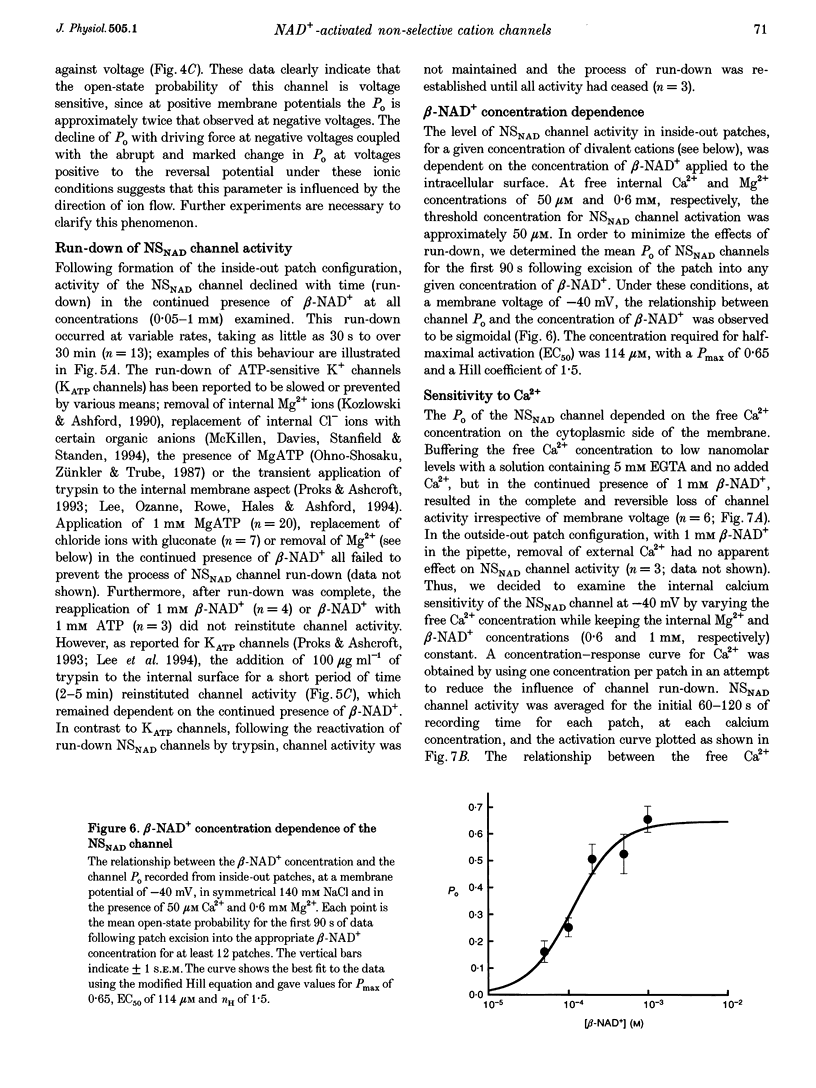
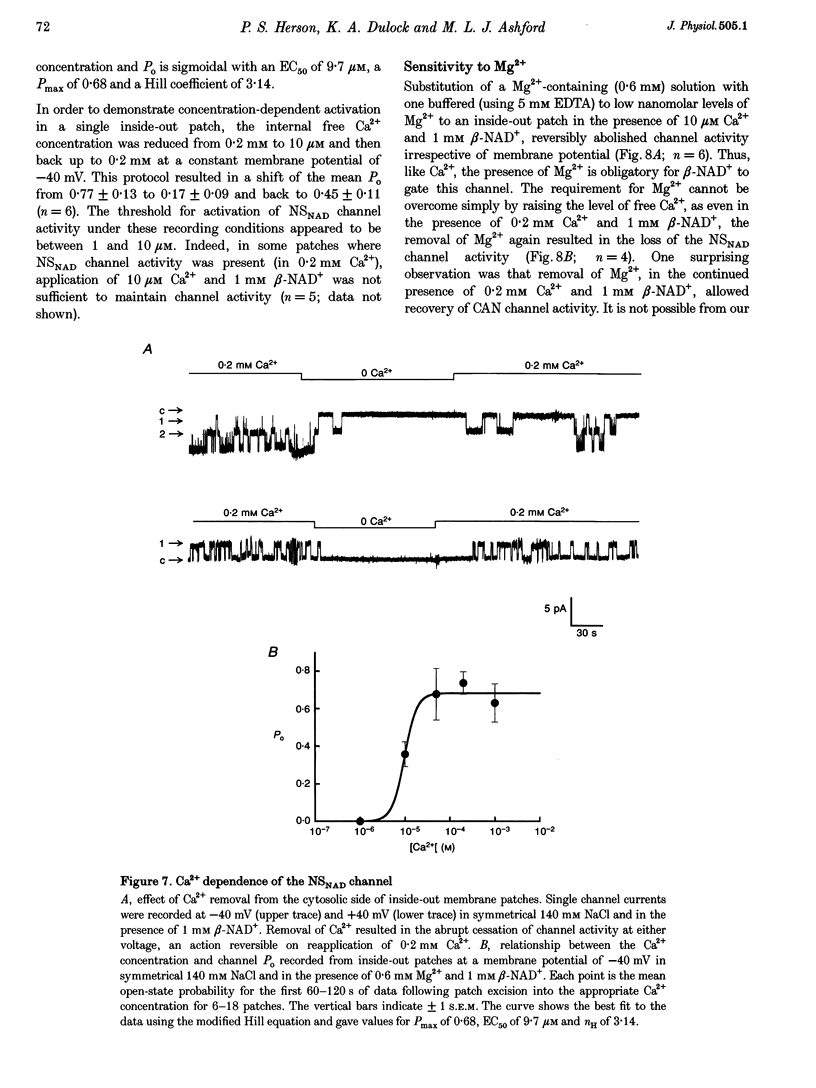
1. Cell-free excised membrane patches were used to examine the properties of a novel nicotinamide-adenine dinucleotide (beta-NAD+)-activated ion channel in the rat insulin-secreting cell line, CRI-G1. 2. In inside-out recordings, beta-NAD+ (0.05-1.0 mM) induced the appearance of a channel characterized by extremely slow kinetics, with mean open times in the range of seconds. The estimated EC50 for activation was 114 microM. Channel activity declined with time (run-down) following activation by beta-NAD+ in excised patches and this was not prevented by intracellular application of trypsin. 3. The single channel current-voltage relationship was linear with a conductance of 74 pS in symmetrical NaCl. The channel appears equally permeable to Na+, K+ and Cs+, exhibits an appreciable permeability to Ca2+, Mg2+ and Ba2+, but excludes anions. 4. The channel displays an unusual voltage sensitivity, with an abrupt increase in open-state probability at depolarized voltages. 5. Channel opening, in the presence of beta-NAD+, required both Ca2+ and Mg2+ to be present at the internal side of the membrane. Activation by Ca2+ required a concentration of at least 10 microM and was maximal at 0.1 mM. Ba2+ did not substitute for Ca2+ in inducing channel activity nor did it inhibit activation by Ca2+. Increasing the concentration of intracellular Mg2+ stabilized the open state of NAD(+)-activated channels. 6. The non-selective cation channel reported here differs in its gating and modulatory characteristics from non-selective cation channels described in other tissues. This channel may play a role in the pathophysiological responses of beta-cells to oxidative stress.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan S., Gray P. T., Ritchie J. M. A calcium-activated cation-selective channel in rat cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):349–355. doi: 10.1098/rspb.1984.0068. [DOI] [PubMed] [Google Scholar]
- Carrington C. A., Rubery E. D., Pearson E. C., Hales C. N. Five new insulin-producing cell lines with differing secretory properties. J Endocrinol. 1986 May;109(2):193–200. doi: 10.1677/joe.0.1090193. [DOI] [PubMed] [Google Scholar]
- Chernaya G., Vázquez M., Reeves J. P. Sodium-calcium exchange and store-dependent calcium influx in transfected chinese hamster ovary cells expressing the bovine cardiac sodium-calcium exchanger. Acceleration of exchange activity in thapsigargin-treated cells. J Biol Chem. 1996 Mar 8;271(10):5378–5385. doi: 10.1074/jbc.271.10.5378. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H. Effects of pyridine nucleotides on the gating of ATP-sensitive potassium channels in insulin-secreting cells. J Membr Biol. 1988 Jun;102(3):205–216. doi: 10.1007/BF01925714. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19(3):141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- Guérineau N. C., Bossu J. L., Gähwiler B. H., Gerber U. Activation of a nonselective cationic conductance by metabotropic glutamatergic and muscarinic agonists in CA3 pyramidal neurons of the rat hippocampus. J Neurosci. 1995 Jun;15(6):4395–4407. doi: 10.1523/JNEUROSCI.15-06-04395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J., Capito K., Thams P. Cytosolic ratios of free [NADPH]/[NADP+] and [NADH]/[NAD+] in mouse pancreatic islets, and nutrient-induced insulin secretion. Biochem J. 1987 Jan 1;241(1):161–167. doi: 10.1042/bj2410161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson P. S., Ashford M. L. Activation of a novel non-selective cation channel by alloxan and H2O2 in the rat insulin-secreting cell line CRI-G1. J Physiol. 1997 May 15;501(Pt 1):59–66. doi: 10.1111/j.1469-7793.1997.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz G. G., 4th, Leech C. A., Habener J. F. Activation of a cAMP-regulated Ca(2+)-signaling pathway in pancreatic beta-cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995 Jul 28;270(30):17749–17757. [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Imanaga I. Mechanism of activation of nonselective cation channels by putative M4 muscarinic receptor in guinea-pig chromaffin cells. Br J Pharmacol. 1995 Jan;114(2):419–427. doi: 10.1111/j.1476-5381.1995.tb13243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Lee K., Ozanne S. E., Hales C. N., Ashford M. L. Mg(2+)-dependent inhibition of KATP by sulphonylureas in CRI-G1 insulin-secreting cells. Br J Pharmacol. 1994 Feb;111(2):632–640. doi: 10.1111/j.1476-5381.1994.tb14783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Ozanne S. E., Rowe I. C., Hales C. N., Ashford M. L. The effects of trypsin on ATP-sensitive potassium channel properties and sulfonylurea receptors in the CRI-G1 insulin-secreting cell line. Mol Pharmacol. 1994 Jul;46(1):176–185. [PubMed] [Google Scholar]
- Lee S., Park M., So I., Earm Y. E. NADH and NAD modulates Ca(2+)-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch. 1994 Jun;427(3-4):378–380. doi: 10.1007/BF00374548. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ghosh A. K., Meglasson M. D., Prentki M., June V., von Allman D. Metabolic concomitants in pure, pancreatic beta cells during glucose-stimulated insulin secretion. J Biol Chem. 1986 Oct 25;261(30):14057–14061. [PubMed] [Google Scholar]
- McKillen H. C., Davies N. W., Stanfield P. R., Standen N. B. The effect of intracellular anions on ATP-dependent potassium channels of rat skeletal muscle. J Physiol. 1994 Sep 15;479(Pt 3):341–351. doi: 10.1113/jphysiol.1994.sp020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Olanow C. W., Arendash G. W. Metals and free radicals in neurodegeneration. Curr Opin Neurol. 1994 Dec;7(6):548–558. doi: 10.1097/00019052-199412000-00013. [DOI] [PubMed] [Google Scholar]
- Proks P., Ashcroft F. M. Modification of K-ATP channels in pancreatic beta-cells by trypsin. Pflugers Arch. 1993 Jun;424(1):63–72. doi: 10.1007/BF00375103. [DOI] [PubMed] [Google Scholar]
- Reale V., Hales C. N., Ashford M. L. The effects of pyridine nucleotides on the activity of a calcium-activated nonselective cation channel in the rat insulinoma cell line, CRI-G1. J Membr Biol. 1994 Dec;142(3):299–307. doi: 10.1007/BF00233437. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Hales C. N., Ashford M. L. Calcium and ATP regulate the activity of a non-selective cation channel in a rat insulinoma cell line. Pflugers Arch. 1987 Aug;409(6):607–615. doi: 10.1007/BF00584661. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine is a coagonist at the NMDA receptor/channel complex. Prog Neurobiol. 1990;35(1):53–74. doi: 10.1016/0301-0082(90)90040-n. [DOI] [PubMed] [Google Scholar]
- Thorn P., Petersen O. H. Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J Gen Physiol. 1992 Jul;100(1):11–25. doi: 10.1085/jgp.100.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele T., Tran Ba Huy P., Teulon J. Modulation by purines of calcium-activated non-selective cation channels in the outer hair cells of the guinea-pig cochlea. J Physiol. 1996 Jul 1;494(Pt 1):77–89. doi: 10.1113/jphysiol.1996.sp021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W. Cyclic nucleotide-gated channels: an expanding new family of ion channels. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3481–3483. doi: 10.1073/pnas.91.9.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y., Burnashev N., Papin A., Portnov V., Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Arch. 1988 May;411(5):584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]


