Abstract
Background
The World Health Organization (WHO) declared the end of the COVID-19 (SARS-CoV-2) global public health emergency on May 5, 2023, but its long-term consequences have still been haunting the global population. Post-acute sequelae of COVID-19 (PASC) and long-term COVID-19 are serious concerns and present with various symptoms. Intranasal chlorpheniramine (iCPM) has been shown to decrease the viral burden of SARS-COV-2. iCPM uses decreased COVID-19 disease progression and severity in Accelerating COVID-19 Clinical Recovery in an Outpatient Setting (ACROSS)-I & III randomized control trials (RCT).
Methods
This prospective survey study included 259 participants in ACROSS I and III RCTs. We compared the effect of iCPM versus placebo on the reduction of PASC symptoms. A PASC questionnaire containing 17 questions regarding the most common PASC symptoms was used in this study. T-test and Pearson chi-square statistics were performed according to continuous and categorical data using STATA 17.0 Basic Edition software.
Findings
The iCPM cohort had a lower proportion of patients with fatigue or tiredness vs. placebo (0 Vs 17, 21, p < 0.001). iCPM cohort had a lower proportion of patients with difficulty concentrating or mental confusion (0 vs. 22, 27, p < 0.001). iCPM cohort had also a lower number of patients with difficulty in the ability to perform daily activities or work vs. placebo (1 Vs 38, 48, p < 0.001). A smaller number of patients in the iCPM cohort sought medical attention for PACS symptoms compared to placebo (0 vs. 48, 68, p < 0.001).
Interpretation
The use of intranasal chlorpheniramine shows promise in preventing COVID-19 progression to the often-debilitating post-COVID-19 syndrome PASC. The association between iCPM use and a lower prevalence of PASC symptoms is strong. Further studies are needed to establish the role of ICPM in preventing PASC.
Keywords: COVID-19, SARS-CoV-2, Intranasal chlorpheniramine, Efficacy, Randomized clinical trial, Double-blind, Placebo-controlled clinical trial
Introduction
As the COVID-19 pandemic’s first wave slowed down, individuals believed to be at low risk for severe COVID-19, such as young individuals with few or no comorbidities, began to experience vague but tenacious symptoms after contracting COVID-19. This group commonly was referred to as “COVID Long Haulers” and alluded to the experience as “Long COVID” in these same social media circles [1]. Despite often not displaying even moderate symptoms of the disease, these healthy young people suddenly found themselves dealing with a wide variety of confounding symptomatology related to this newly identified disorder [1, 2]. The medical profession eventually renamed and officially recognized this combination of persisting symptomatology as Post-Acute Sequelae of COVID-19 (PASC), Post-Acute COVID Syndrome, or Post-COVID-19 Condition in some circles. PASC, by definition, occurs when at least one recognized symptom persists for 4–12 weeks following a confirmed or probable diagnosis of COVID-19 [3]. More than 100 unique symptoms have been documented for this disorder. Symptoms fall into four basic categories: cardiovascular [4], respiratory [5], somatic [6], and neuropsychiatric. Some patients have documented symptoms for as long as two years after their initial SARS-CoV-2 infection.
The existence of PASC was initially met with considerable debate and discussion in academic circles [7, 8]. However, by mid-2023, its global existence was universally accepted. Reports regarding the significant impact of PASC came from all continents, spanning various ethnicities in both developed and underdeveloped countries with different healthcare systems [9].
Chlorpheniramine, an older first-generation antihistamine that has been utilized for over five decades, has been shown to exhibit a notable decrease in the viral burden of SARS-COV-2 when administered via the nasal route and resulted in an essentially undetectable viral load [10]. These findings brought attention to chlorpheniramine as a potential drug to be repurposed for possible use as an antiviral. Initially released in the late 1940s through ACCROS trials [11], early outpatient use of intranasal chlorpheniramine was found to hinder COVID-19 disease progression and severity.
The purpose of this study was to determine the effectiveness of intranasal chlorpheniramine maleate (iCPM) in reducing the long-term symptoms of SARS-CoV-2 infection and the Post-Acute Sequelae of COVID-19 (PASC). This was accomplished using a 17-question survey administered after participation in the ACCROS I and ACCROS III studies to assess the presence of PASC symptoms (Table 1).
Table 1.
PACS questionnaire
| Questions | |||||
|---|---|---|---|---|---|
| 1 | Have you previously tested positive for COVID-19? | Yes | No | ||
| 2 | Have you fully recovered from your COVID-19 illness? | Yes | No | ||
| Are you currently experiencing any of the following | |||||
| 3 | Fatigue or tiredness that persists even after rest or sleep? | Yes | No | ||
| 4 | Difficulty breathing or shortness of breath? | Yes | No | ||
| 5 | Pain or pressure in the chest? | Yes | No | ||
| 6 | Joint or muscle pain? | Yes | No | ||
| 7 | Headaches? | Yes | No | ||
| 8 | Difficulty concentrating or mental confusion? | Yes | No | ||
| 9 | Loss of taste or smell? | Yes | No | ||
| 10 | Digestive problems such as nausea, vomiting, or diarrhea? | Yes | No | ||
| 11 | Skin rashes or lesions? | Yes | No | ||
| 12 | Mood changes such as depression, anxiety, or irritability? | Yes | No | ||
| 13 | If you answered yes to any of the above symptoms, how long have you been experiencing these symptoms? | < 1wk | 1–3 wk | 3–6 wk | > 6 wk |
| 14 | Have these symptoms affected your ability to perform daily activities or work? | Yes | No | ||
| 15 | Have you sought medical attention for these symptoms? | Yes | No | ||
| 16 | Have you received any treatment for these symptoms? | Yes | No | ||
| 17 | Is there anything else you would like to share about your symptoms or experience with COVID-19? | Yes | No | ||
Methods
Study design
We conducted this prospective cohort study on patients enrolled in ACROSS I and ACROSS III double-blinded placebo-controlled randomized clinical trials. All participants included in the study were confirmed positive for SARS-CoV-2 through reverse transcription polymerase chain reaction (RT-PCR) testing of nasopharyngeal swabs. Patients were eligible for the study if they had a laboratory-confirmed COVID-19 diagnosis. Quantitative RT-PCR was performed to measure viral loads (viremic titers) in a subset of patients at baseline, though these data were not available for all participants.
Upon enrollment, patients were assessed for COVID-19 severity and the presence of COVID-19-related pneumonia. For those presenting with respiratory symptoms, pneumonia severity was classified based on clinical criteria, including oxygen saturation levels, respiratory rate, and radiographic findings. Severity stages were defined as follows: mild pneumonia, characterized by an oxygen saturation (SpO₂) of ≥ 94% on room air, requiring no additional respiratory support; moderate pneumonia, with SpO₂ between 90% and 94%, necessitating low-flow supplemental oxygen; and severe pneumonia, indicated by an SpO₂ <90%, requiring high-flow oxygen or mechanical ventilation. The number of patients diagnosed at each severity level was documented to provide a comprehensive view of the disease spectrum within the cohort, allowing for a detailed assessment of baseline characteristics that could influence post-acute sequelae of COVID-19 (PASC) outcomes.
Treatment choices were stratified according to COVID-19 severity to provide standardized care across different levels of disease burden. Patients with mild to moderate COVID-19 symptoms were generally treated with the oral antiviral Nirmatrelvir–Ritonavir (Paxlovid), administered at a dosage of 300 mg nirmatrelvir with 100 mg ritonavir, taken twice daily for five days. This antiviral was prescribed if initiated within five days of symptom onset, primarily for patients who did not require hospitalization or advanced respiratory support. In contrast, patients diagnosed with severe COVID-19-related pneumonia who were at high risk of disease progression were treated with Remdesivir. This antiviral was administered intravenously, beginning with a 200 mg loading dose on the first day, followed by 100 mg daily for up to five days, in alignment with standard care guidelines for patients requiring supplemental oxygen.
Participants were enrolled in the study immediately after a confirmed diagnosis of COVID-19 via RT-PCR or antigen test. In ACROSS I, enrollment took place as soon as the COVID-19 diagnosis was confirmed, and the same approach was followed in ACROSS III.
This rapid enrollment timing may have influenced the onset and severity of PASC symptoms, as some studies suggest that earlier intervention could mitigate long-term sequelae. The ACROSS I and ACROSS III trials were designed to study the effect of intranasally administered Chlorpheniramine Maleate (iCPM) on the rate of clinical recovery in coronavirus disease 2019 (COVID-19).
RCT registries were created using 1:1 randomization using computer-generated sets of random allocation. ACROSS I had 101 patients enrolled from Dec 2021 to March 2022 predominantly infected with the Delta variant of SARS-CoV-2, and ACROSS III had 158 patients randomized from July 2022 to January 2023 that were mostly affected by the Omicron variant, as documented by local health authorities and sequencing data at the time. We used the same cohort for this study to determine the effectiveness of iCPM in reducing the symptoms of long-term COVID 19 also known as post-acute COVID-19 syndrome (PACS). Patients were called over the phone and were asked 17 questions post-acute COVID-19 syndrome surveys in March and April 2023, 12–13 months after ACROSS I and 2–3 months after ACROSS III (see Table 1).
Informed consent
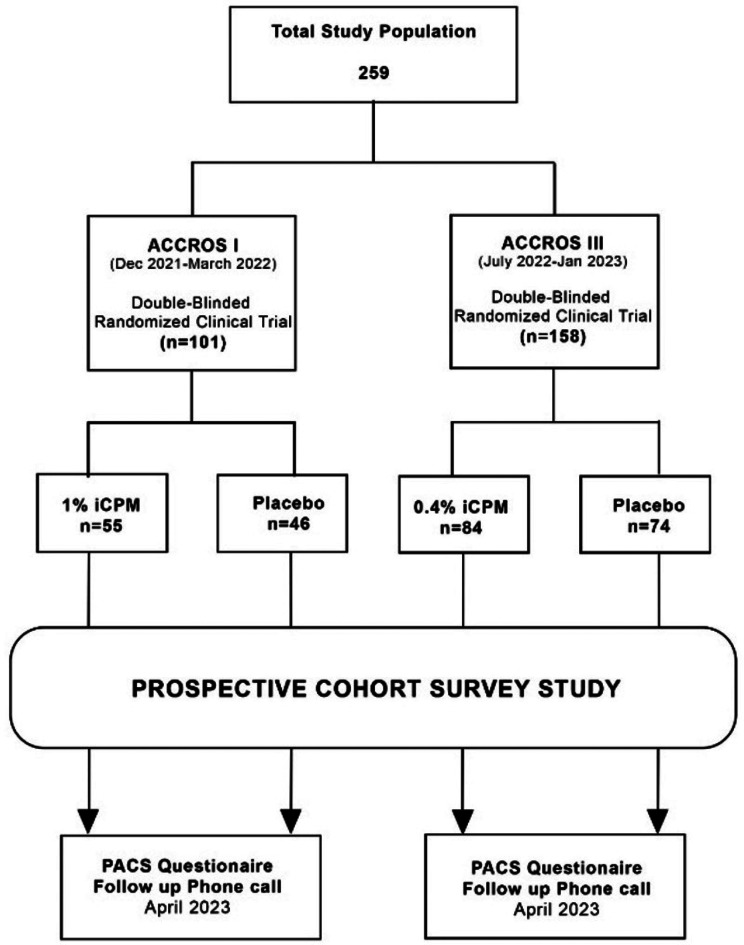
Written informed consent was obtained from all participants prior to their enrollment in the study. In cases where verbal consent was obtained due to remote contact (e.g., phone surveys), it was thoroughly documented and witnessed by study personnel. No waivers of consent were requested or provided by the ethics committee. The study was approved by the IRB of the Ethics Committee of Investigation of Infectious and Zoonotic Disease at the Universidad Nacional Autónoma de Honduras (Fig. 1).
Fig. 1.
Study flow chart
Inclusion and exclusion criteria
The inclusion criteria were adults aged ≥ 18 to ≤ 65 years of either sex, positive reverse transcriptase-polymerase chain reaction (RT-PCR) test or Rapid Antigen for SARS-CoV-2 in nasopharyngeal or oropharyngeal swabs, and mild to moderate COVID 19 infection (ranging from asymptomatic to symptoms of cough and fever, but with no oxygen desaturation (room air < 92%). Exclusion criteria included age < 18 years, hospitalized patients, patients with more than seven days of symptoms, and more than five days since COVID-19 was confirmed by a nasopharyngeal PCR test, hypoxemia (room air SpO2 < 92% plus severe polypnea), hospitalized patients, subjects with known hypersensitivity to CPM and any of the inactive ingredients, subjects receiving therapy with monoamine oxidase inhibitors (MAOIs; rasagiline, selegiline, isocarbonboxasid, phenelzine, and tranylcypromine), and those with narrow-angle glaucoma, urinary retention, sleep apnea, immunodeficiency, or receiving immunosuppressive therapy. Exclusion criteria included acute exacerbation of severe comorbidities such as chronic obstructive pulmonary disease (COPD), class 3 or 4 heart failure, a surgical procedure in the past 12 weeks, inability to provide informed consent or refuse or renounce adherence, QT interval less than 300 ms or more than 500 ms, and clinically significant arrhythmia or severe coronary artery disease.
Intervention
COVID-19 patients were assigned to either a 10-day iCPM treatment or placebo (PLB). The intervention group in ACCROS I received two spray doses in each nostril (100 µL of the solution per nostril) three times a day using a 1.0% iCPM solution (for the active drug group). The total daily dose from the CPM was 12 mg/day, approximately half (1/2) of the daily maximum recommended oral dose (24 mg). ACCROS III had a similar interval dosing, except that a lower dose of 0.4% iCPM solution was used instead of 1% iCPM solution in ACROSS I. Both groups were treated using standard-of-care medicines for early management of SARS-CoV-2.
All participants diagnosed with COVID-19 received a standardized treatment protocol based on prevailing clinical guidelines to manage symptoms and prevent progression to severe disease. The primary antiviral used was Nirmatrelvir–Ritonavir (Paxlovid), administered at 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir, taken twice daily for five days. For patients at high risk of severe outcomes, monoclonal antibody therapy (such as bebtelovimab) was provided as a single 175 mg intravenous (IV) infusion, when available. In cases requiring supplemental oxygen, dexamethasone was given at 6 mg daily for up to 10 days. To manage fever and body aches, nonsteroidal anti-inflammatory drugs (NSAIDs), typically ibuprofen (200–400 mg every 6–8 h as needed), were administered. For patients exhibiting elevated inflammatory markers or cytokine storm symptoms, anti-cytokine drugs such as tocilizumab (8 mg/kg IV, up to 800 mg) or baricitinib (4 mg orally daily for up to 14 days) were used selectively. While standardized, treatments varied in use and timing based on clinical updates, patient severity, and medication availability during the study period.For more information, please refer to ACROSS I and ACROSS III trials [11].
Survey and statistical analysis
A Post-COVID Syndrome Screening questionnaire (Version 0.28) was used for this prospective study. This questionnaire comprised 17 questions and was administered over the phone (see Table 1). The questionnaire included the most reported symptoms of long-COVID to assess the presence of post-COVID symptoms. All except one question were dichotomous (yes or no questions). Question 17 allowed the participants to comment further on their symptoms [12]. Our null hypothesis was that there was no significant difference in long-COVID symptoms between patients who received iCPM versus Placebo in ACROSS I and ACROSS III trials respectively. For statistical analysis, we used STATA 17.0 BE edition (StataCorp LLC). We used the t-test statistics for the mean comparison of continuous/integral variables. Pearson Chi-square testing was used for categorical data.
Results
In total, 259 participants were enrolled in this prospective cohort study (see Table 2). 101 patients were enrolled in the ACROSS I patient registry and 158 patients were enrolled in the ACROSS III patient registry. Participants in ACCROS I were randomized to 1% iCPM (n = 55) vs. placebo (n = 46), whereas ACROSS III participants were randomized to 0.4% iCPM (n = 84) vs. placebo (n = 74). In total, 139 patients were surveyed in the iCPM group and 120 patients were surveyed in the placebo group. There was no significant difference in the mean age between the intranasal CPM cohort and the placebo cohort (51 vs. 50 years, p = 0.72). There was no significant difference in the sex proportion among patients receiving intranasal CPM versus placebo (0.27, p = 0.59). More than 99% of patients in both iCPM and placebo groups were vaccinated, with no significant differences in vaccination status or the number of vaccine doses between the two groups (p = 0.94). The majority of patients (95%) received two or more doses of a COVID-19 vaccine. We conducted subgroup analyses to explore whether vaccination timing or dose number influenced PASC outcomes; no significant differences were found between those who were vaccinated pre-infection versus post-infection. Similarly, there was no significant difference in vaccination status between the two groups (greater than 99% of patients were vaccinated in both groups). There was no difference in the number of vaccine doses between the iCPM and placebo groups (1.21, p = 0.94), and more than 95% of patients received two or more vaccine doses. In addition, there was no statistical difference between the two groups regarding the mean number of days of intervention (11 vs. 11 days, p = 0.61). The rest of the baseline variables, including Asthma, COPD, Diabetes, Sleep Apnea, Rhinitis, Sinusitis, Polyps, AHT, and smoking status, also did not differ significantly. In the subgroup analysis, the mean age of the patients in the ACROSS I group was 46 years, with no significant difference between the 1% iCPM and placebo groups (45 vs. 48 years, p = 0.37). The mean age of the patients in the ACROSS III group was 53 years, with no significant difference between the 0.4% iCPM and placebo groups (55 vs. 51 years, p = 0.28). In addition, there was no sex difference between the iCPM and placebo groups within individual ACROSS I and ACROSS III registries, suggesting good randomization.
Table 2.
Baseline characteristics
| Trial dataset | Across I | Across III | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1% iCPM | Placebo | P value | 0.4% iCPM | Placebo | P value | iCPM | Placebo | P value | |
| N (no of patients) | 55 | 46 | 84 | 74 | 139 | 120 | |||
| Mean Age (Yrs.) | 45 | 48 | 0.37 | 55 | 51 | 0.28 | 51 | 50 | 0.727 |
| Sex (M/F) | 27/28 | 25/21 | 0.59 | 38/46 | 35/39 | 0.79 | 65/74 | 60/60 | 0.603 |
| Vaccination status (%) | 55 (100%) | 45 (98%) | 0.28 | 83 (99%) | 74 (100%) | 0.34 | 138 (99%) | 119 (99%) | 0.917 |
| Asthma | 0 | 2 | 0.12 | 4 | 6 | 0.37 | 4 | 8 | 0.140 |
| COPD | 0 | 1 | 0.28 | 4 | 1 | 0.23 | 4 | 2 | 0.525 |
| Smoking History | 2 | 3 | 0.51 | 3 | 4 | 0.58 | 5 | 7 | 0.393 |
| Diabetes | 6 | 5 | 0.99 | 9 | 10 | 0.57 | 15 | 15 | 0.651 |
| Sleep Apnea | 0 | 1 | 0.27 | 2 | 6 | 0.10 | 2 | 7 | 0.052 |
| Rhinitis | 5 | 2 | 0.35 | 8 | 6 | 0.76 | 13 | 8 | 0.430 |
| Sinusitis | 0 | 0 | NA | 1 | 1 | 0.91 | 1 | 1 | 0.912 |
| Nasal Polyps | 0 | 0 | NA | 0 | 1 | 0.28 | 0 | 1 | 0.279 |
| AHT | 17 | 13 | 0.78 | 23 | 26 | 0.27 | 40 | 39 | 0.488 |
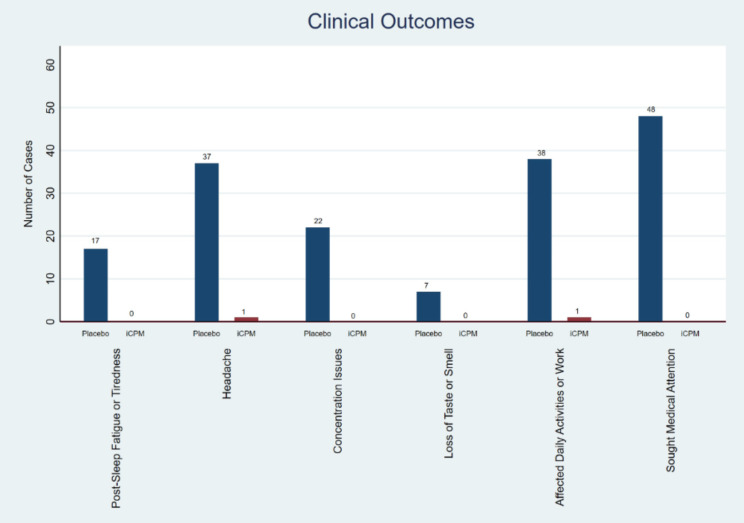
Among PACS symptoms, the iCPM cohort had a significantly lower proportion of patients with fatigue or tiredness (that persisted even after rest or sleep) vs. placebo (0 out of 139 vs. 17 out of 120, 21, p < 0.001). Joint or muscle pain did not differ significantly between the iCPM and placebo groups (0 out of 139 vs. 3 out of 120, 3.5, p = 0.06). The detailed results and comparative analysis of these clinical outcomes are visually represented in Fig. 2. Headaches were significantly less common in the iCPM cohort than in the placebo group (1 of 139 vs. 37 of 120, 46, p < 0.001). The iCPM cohort had a significantly lower proportion of patients with difficulty concentrating or mental confusion compared to the placebo (0 out of 139 vs. 22 out of 120, 27, p < 0.001). Loss of taste or smell was also significantly lower in the iCPM cohort than in the placebo group (0 of 139 vs. 7 of 120, 8, p = 0.004). The iCPM cohort had a significantly lower number of patients with difficulty in performing daily activities or work than the placebo group (1 out of 139 vs. 38 out of 120, 48, p < 0.001). A smaller number of patients in the iCPM cohort sought medical attention for PACS symptoms compared to placebo (0 out of 139 vs. 48 out of 120, 68, p < 0.001). However, the proportion of patients receiving any treatment for these symptoms did not significantly differ between the iCPM and placebo groups (0 of 139 vs. 3 of 120, 3.5, p = 0.06). A total of 86/120 (72%) patients in the placebo group had at least one symptom of PACS versus only one out of 139 (0.7%) in the iCPM group. This difference was statistically significant (145 Cramer V 0.8, p < 0.001). The average composite scores of the PACS survey for the iCPM cohort were significantly lower than those of the placebo cohort (0.14 vs. 1.45, Difference − 1.44; 95 CI -1.63 to -1.25, p < 0.001). None of the patients reported difficulty breathing or shortness of breath, chest pain or pressure, digestive problems (nausea, vomiting, or diarrhea), skin rash or lesions, or mood changes (such as depression, anxiety, or irritability) (Table 3). In the subgroup analysis, among ACCROS I patients, only 27 reported symptoms (26 in the placebo group and one in the iCPM group). Of the 26 patients in the placebo group, one patient had symptoms lasting less than 1 week, three had symptoms lasting between 3–6 weeks and 22 had symptoms that lasted more than 6 weeks. One patient in the iCPM group reported symptoms that lasted > 6 weeks. Among ACCROS III patients, 60 reported symptoms in the placebo group. 38 had symptoms lasting between 3–6 weeks and 22 had symptoms for more than 6 weeks. Our findings indicate a strong association between iCPM use and reduced PASC symptoms. However, patients treated earlier in the disease course appeared to benefit more, with significantly fewer long-term sequelae. This suggests that early intervention with iCPM may be crucial in mitigating PASC. Further research is needed to establish the optimal timing for iCPM administration to maximize its protective effects
Fig. 2.
Clinical outcomes, Placebo vs., iCPM
Table 3.
Results stratified according to ACROSS I and ACROSS III patient registries
| Trial dataset | Across I | Across III | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1% iCPM | Placebo | P value | 0.4% iCPM | Placebo | P value | i CPM | Placebo | P value | |
| N (no of patients) | 55 | 46 | 84 | 74 | 139 | 120 | |||
| Fatigue/Tiredness | 0 | 4 | 0.026 | 0 | 13 | < 0.001 | 0 | 17 | < 0.001 |
| Difficulty breathing or shortness of breath | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
| Pain or pressure in the chest | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
| Joint or muscle pain | 0 | 0 | NA | 0 | 3 | 0.062 | 0 | 3 | 0.062 |
| Headaches | 1 | 9 | 0.003 | 0 | 28 | < 0.001 | 1 | 37 | < 0.001 |
| Difficulty concentrating or mental confusion | 0 | 13 | < 0.001 | 0 | 9 | 0.001 | 0 | 22 | < 0.001 |
| Loss of taste or smell | 0 | 0 | NA | 0 | 7 | 0.004 | 0 | 7 | 0.004 |
| Digestive problems such as nausea, vomiting or diarrhea | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
| Skin rashes or lesions | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
| Mood changes such as depression, anxiety, or irritability | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
| Symptoms affected your ability to perform daily activities or work | 1 | 25 | < 0.001 | 0 | 13 | < 0.001 | 1 | 38 | < 0.001 |
| Have you sought medical attention for these symptoms? | 0 | 11 | < 0.001 | 0 | 37 | < 0.001 | 0 | 48 | < 0.001 |
| Have you received any treatment for these symptoms? | 0 | 1 | 0.272 | 0 | 2 | 0.129 | 0 | 3 | 0.129 |
| Average composite score | 0.36 | 1.36 | < 0.001 | 0 | 1.51 | < 0.001 | 0.14 | 1.46 | < 0.001 |
Discussion
Post-acute sequelae of COVID-19 (PASC) is an additional condition that occurs when at least one symptom persists for 4 to 12 weeks after a confirmed or probable diagnosis of COVID-19 [13, 14]. The prevalence of PASC varies between 10% infected with the omicron variant and 63% among individuals with acute infection during the initial Sars-CoV2 variants [15, 16]. Certain groups such as older individuals, females, unvaccinated individuals, smokers, obese individuals, those with recurrent infections, preexisting comorbidities, and hospitalized patients have higher rates of PASC [17, 18]. In a recent systematic review, Wolff et al. revealed that pre-existing asthma measured in hospital-based populations and pre-existing rhinitis were significantly associated with increased Long-COVID incidences, they recommend further studies in this area [19].
Fatigue, memory loss, insomnia, and chronic musculoskeletal pain are some of the most frequently reported symptoms associated with PASC. However, there are over a hundred different symptoms that have been described in post-COVID-19 cases which affect various systems within the body [20]. It is worth noting that some patients continue to experience these symptoms more than two years after their initial infection [21]. The long-term consequences resulting from these debilitating sequelae associated with COVID-19 have had a profound impact on productivity and overall quality of life for affected individuals, as well as substantial ramifications on economies around the globe [22].
From a cellular point of view, mast cells and their primary molecule, histamine, play a large role in the inflammation seen in severe acute COVID-19. Tan et al. [23] showed that levels of chymase, a well-known serum surrogate indicating mast cell degranulation, were more significantly elevated in hospitalized patients than in community cases. Given these data, antihistamines such as chlorpheniramine could potentially mitigate the disease by blocking histamine and mast cell degranulation. Inhaled CPM may act against SARS-CoV-2 through several different mechanisms: direct virucidal effects, inhibition of adsorption, and inhibition of viral replication [24, 25]. Chlorpheniramine maleate has been hypothesized to exert antiviral effects through its ability to block histamine release and inhibit mast cell degranulation, which plays a role in the inflammatory response seen in viral infections [26, 27]. Studies have shown its virucidal activity against influenza and SARS-CoV-2 in vitro, suggesting potential for broader antiviral use [28, 29]. However, further studies are needed to conclusively establish its efficacy as an antiviral agent in clinical settings.
Chlorpheniramine is also a potent bitter taste receptor (T2R) agonist [26], and it may stimulate sinonasal innate immunity, triggering host defense mechanisms while blocking Ig-E-mediated mast cell activation and cytokine expression. In addition, chlorpheniramine displays anticholinergic [27], bronchodilatory, and decongestant activities [28]. These actions may contribute to its effectiveness in resolving COVID-19 and preventing PASC.
Two randomized clinical trials, known as the ACCROS trials, were conducted on mildly symptomatic COVID-19 outpatients. These trials demonstrated that intranasal chlorpheniramine maleate (iCPM) was more effective than placebo in accelerating clinical recovery and reducing upper respiratory symptoms to less than three days, with conversion to negative SARS-CoV-2 PCR test results in four days [11, 29]. Here, our findings support those participants who received the Chlorpheniramine Nasal Spray had significantly fewer long-term post-COVID symptoms. This suggests the potential benefit of CPM in preventing or reducing the occurrence of these symptoms.
This study was prospective in that the data were collected in that manner. The initial premise of ACCROS studies was to assess the impact on acute disease and abating its symptoms. The patients remained blinded to the randomization at the time of the queries, favoring less-biased reporting. The callers were also blinded to patient status. The numbers are small, but the magnitude of the effect is such that this study merits close consideration. Therefore, reproducing the results in randomized controlled trials should be a priority in the future. Given the high vaccination rate in both groups, it is possible that vaccination could have influenced the incidence or severity of PASC. However, our subgroup analysis did not reveal significant differences based on the number of vaccine doses or timing of vaccination relative to infection. Nevertheless, the potential confounding effect of vaccination should be considered in future studies with larger cohorts and varying vaccination statuses.
This study naturally has limitations. Though the data was collected prospectively, it was not part of the original ACCROS trial design. The timing between initial COVID-19 diagnosis and iCPM treatment initiation varied between patients, potentially influencing the manifestation of PASC. Patients treated closer to the onset of infection may have experienced fewer long-term symptoms compared to those treated later, as has been suggested by previous studies of post-viral syndromes. This variability may partially explain the differences observed between the iCPM and placebo groups. The timing of calls after the original exposure was not unformed, though this issue was partly addressed by the randomized nature of the subjects surveyed, and their continued blinded status.
After some initial enthusiasm [30], most recent trials of antiviral medications to treat/prevent PACS have been unsuccessful. According to a study conducted on 191,057 veterans who tested positive for SARS-CoV-2 between January and July 2022, nirmatrelvir–ritonavir (Paxlovid), an antiviral drug, did not show any significant difference in long-COVID rates between the group that took the drug and the group that did not. The study compared the outcomes of 9,593 non-hospitalized patients who were treated with Paxlovid with their untreated counterparts for 31 post-COVID conditions (PCCs) [31].
In this study, our primary objective was to evaluate the effectiveness of intranasal chlorpheniramine (iCPM) in mitigating post-acute sequelae of SARS-CoV-2 infection (PASC). We recognize, however, that some participants may have received various antiviral treatments during their initial COVID-19 infection. As antiviral administration was not standardized across the cohort and was given at different points in the disease course, we did not account for antiviral effects as a baseline characteristic. This introduces potential variability in PASC outcomes, as the role of antivirals in influencing the development or severity of PASC remains a topic of ongoing investigation.
Based on the findings of this trial, intranasal chlorpheniramine may increase recovery from COVID-19 and reduce global PASC rates through a simple and safe prophylactic treatment, which has been proven to be cost-effective. Unlike other medications used to reactively treat PASC, chlorpheniramine has very few medication interactions including those commonly used to treat COVID-19 and its symptoms. Also, unlike some of the medications used to treat PASC, iCPM does not require laboratory monitoring.
Conclusions
Our study provides strong evidence supporting the use of intranasal chlorpheniramine in reducing the incidence of PASC symptoms. Given chlorpheniramine’s paucity of medication interactions, its historically low cost, and its lack of deleterious effect on the kidneys and liver, the use of intranasal chlorpheniramine shows distinct promise in treating COVID-19 and preventing progression to the often-debilitating post-COVID-19 syndrome PASC. However, the timing of treatment initiation may significantly influence these outcomes. Future studies should aim to investigate the optimal timing for iCPM administration to enhance its efficacy in preventing long-term sequelae of COVID-19.
Author contributions
FVP: Investigation, Conceptualization, Data Collection, Methodology, Writing – Original Draft, Funding Acquisition, Supervision. FB: Writing – Review & Editing, Literature Search. AKS: Literature Search, Data Interpretation, Writing – Review & Editing. MLT: Project Administration, Resources, Supervision. EJPM: Investigation, Conceptualization, Data Collection, Writing – Original Draft. SAAR: Software, Data Curation, Formal Analysis. JS: Literature Search, Data Interpretation, Validation, Writing – Review & Editing. DACB: Literature Search, Data Interpretation, Writing – Review & Editing. ACM: Literature Search, Data Interpretation, Writing – Review & Editing. JR: Literature Search, Data Interpretation, Writing – Review & Editing. ASL: Literature Search, Data Interpretation, Writing – Review & Editing. MSH: Literature Search, Data Interpretation, Writing – Review & Editing. MLS: Literature Search, Data Interpretation, Writing – Review & Editing. FFR: Literature Search, Data Interpretation, Writing – Review & Editing, supervision.
Funding
This research was funded by Dr. Ferrer Biopharma.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author, Dr. Franck F. Rahaghi, upon reasonable request. Contact details: Email: RAHAGHF@ccf.org.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of the Ethics Committee of Investigation of Infectious and Zoonotic Disease at the Universidad Nacional Autónoma de Honduras. Written informed consent was obtained from all participants prior to their involvement in the study. In cases where verbal consent was required (e.g., remote participation), this consent was documented and witnessed by study personnel. No waivers of consent were requested or provided by the ethics committee, and no minors were included in the study.
Consent for publication
N/A-Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rubin R. As their numbers grow, COVID-19 long haulers stump experts. JAMA. 2020;324(14):1381. https://jamanetwork.com/journals/jama/fullarticle/2771111 [DOI] [PubMed]
- 2.Carfì A, Bernabei R, Landi F, Gemelli Against, COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603. https://jamanetwork.com/journals/jama/fullarticle/2768351 [DOI] [PMC free article] [PubMed]
- 3.Clinical Services and Systems, Diseases C, Technical Advisory Group on SARS-CoV-2 Virus Evolution. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. World Health Organization; 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed 27 Sep 2023.
- 4.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157–72. 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis HE, McCorkell L, Vogel JM, Topol EJ, Long COVID. major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133 – 46. https://www.nature.com/articles/s41579-022-00846-2. Accessed 27 Sep 2023. [DOI] [PMC free article] [PubMed]
- 6.Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–61. 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. Cluster of pneumonia cases caused by a novel coronavirus, Wuhan, China; – 17 January 2020. Volume ECDC. Stockholm; 2020.
- 9.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593–607. 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westover JB, Ferrer G, Vazquez H, Bethencourt-Mirael A, Go CC. In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2. Cureus. 2020;12(9). 10.7759/cureus.10501. [DOI] [PMC free article] [PubMed]
- 11.Valerio-Pascua F, Mejia EJP, Tesch ML, Godoy J, Fuentes CL, Erazo GB et al. Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials. Research Square. 2022. https://www.researchsquare.com/article/rs-2167465/v1. Accessed 29 Sep 2023.
- 12.Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl JP, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28(7):955–72. 10.1016/j.cmi.2022.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370 https://www.bmj.com/content/370/bmj.m3026. Accessed 29 Sep 2023. [DOI] [PubMed]
- 14.Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12). 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed]
- 15.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934. https://jamanetwork.com/journals/jama/fullarticle/2805540. Accessed 31 Oct 2023. [DOI] [PMC free article] [PubMed]
- 16.Alvarez-Moreno CA, Pineda J, Bareño A, Espitia R, Rengifo P. Long COVID-19 in Latin America: low prevalence, high resilience or low surveillance and difficulties accessing health care? Travel Med Infect Dis. 2023;51:102492. 10.1016/j.tmaid.2022.102492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):1–11. 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–14. 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff D, Drewitz KP, Ulrich A, et al. Allergic diseases as risk factors for long-COVID symptoms: systematic review of prospective cohort studies. Clin Exp Allergy. 2023;53(9):961–75. 10.1111/cea.14391. [DOI] [PubMed] [Google Scholar]
- 20.Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among healthcare workers. JAMA. 2021;325(19):2015. 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Bae S, Chang H-H, Kim S-W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. 2023;13(1):36995–4. 10.1038/s41598-023-36995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med. 2023;29(9):2347–57. 10.1038/s41591-023-02521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan J, Anderson DE, Rathore APS, O’Neill A, Mantri CK, Saron WAA et al. Signatures of mast cell activation are associated with severe COVID-19. bioRxiv. 2021. 10.1101/2021.05.31.21255594
- 24.Elshaier YAMM, Mostafa A, Valerio-Pascua F, Tesch ML, Costin JM, Rahaghi FF. Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study. bioRxiv. 2023. 10.1101/2023.08.28.554806. [Google Scholar]
- 25.Black SD. Molecular modeling and preliminary Clinical Data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19. Cureus. 2022;14(1):e20980. 10.7759/cureus.20980. PMID: 35154957; PMCID: PMC8820487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar SA, Cheng W. A hypothesis: bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2. Pharmazie. 2021;76(2):43–54. 10.1691/pharmazie.2021.2. [DOI] [PubMed] [Google Scholar]
- 27.Fang S-Y, Druce HM, Baraniuk JN. Anticholinergic properties of brompheniramine, chlorpheniramine, and atropine in human nasal mucosa in vitro. Am J Rhinol. 1998;12(2):131–4. 10.2500/105065898781390271. [DOI] [PubMed] [Google Scholar]
- 28.Ennis M, Tiligada K. Histamine receptors and COVID-19. Inflamm Res. 2021;70(1):67–75. 10.1007/s00011-020-01422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres J, Go CC, Chohan F, Genesis Camacho L, Sanchez-Gonzalez MA, Ferrer G. Chlorpheniramine maleate nasal spray in COVID-19Patients: Case Series. J Clin Exp Pharmacol. 2021;10:275. 10.35248/2161-1459.21.10.275. [Google Scholar]
- 30.Wang Y, Zhao D, Xiao W, Shi J, Chen W, Jia Q, et al. Paxlovid reduces the risk of long COVID in patients six months after hospital discharge. J Med Virol. 2023;95(8). 10.1002/jmv.29014. [DOI] [PubMed]
- 31.Ioannou GN, Berry K, Rajeevan N, Li Y, Mutalik P, Yan L, et al. Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans: a target trial emulation. Ann Intern Med. 2023;176(11):1486–97. 10.7326/M23-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author, Dr. Franck F. Rahaghi, upon reasonable request. Contact details: Email: RAHAGHF@ccf.org.