Abstract
Sepsis represents a profound challenge in critical care, characterized by a severe systemic inflammatory response which can lead to multi-organ failure and death. The intricate pathophysiology of sepsis involves an overwhelming immune reaction that disrupts normal host defense mechanisms, necessitating innovative approaches to modulation. Nanoscale immunomodulators, with their precision targeting and controlled release capabilities, have emerged as a potent solution to recalibrate immune responses in sepsis. This review explores the recent advancements in nanotechnology for sepsis management, emphasizing the integration of nanoparticulate systems to modulate immune function and inflammatory pathways. Discussions detail the development of the immune system, the distinct inflammatory responses triggered by sepsis, and the scientific principles underpinning nanoscale immunomodulation, including specific targeting mechanisms and delivery systems. The review highlights nanoformulation designs aimed at enhancing bioavailability, stability, and therapeutic efficacy, which shows promise in clinical settings by modulating key inflammatory pathways. Ultimately, this review synthesizes the current state of knowledge and projects future directions for research, underscoring the transformative potential of nanolevel immunomodulators for sepsis treatment through innovative technologies and therapeutic strategies.
Keywords: Nanomedicine, immunomodulator, sepsis, extracellular vesicles, review
Introduction
Sepsis remains a critical and life-threatening condition. It is characterized by a dysregulated immune response to infection, leading to widespread inflammation and multi-organ dysfunction.1 Despite significant medical advancements, the complex pathophysiology of sepsis and the limited efficacy of current treatments pose ongoing challenges, contributing to high mortality rates and long-term complications for survivors. This underscores the urgent need for more effective, targeted therapeutic strategies.2
Traditional sepsis management relies on broad-spectrum antibiotics, fluid resuscitation, and supportive care; these often fail to address the underlying immune dysregulation and precise inflammatory modulation. Although systemic use of immunosuppressants or cytokine antagonists can reduce excessive inflammation, they frequently cause severe side effects. Consequently, there is increasing interest in innovative therapeutic approaches, with nanotechnology emerging as a transformative tool. Nanomedicine offers promising alternatives by enhancing targeting, immunocompatibility, and therapeutic efficacy.
This review explores the role of nanotechnology in sepsis treatment, emphasizing the potential benefits and applications of nano-immunomodulators. It summarizes recent research and discusses future directions, providing a comprehensive overview of current perspectives and emerging opportunities in this field.
Immune Response Characteristics in Sepsis
Advancements in sepsis therapy critically depend on a comprehensive understanding of its complex pathophysiology, which involves multiple interacting pathways. Sepsis, a clinical syndrome resulting from immune dysfunction, leads to a cascade of biological, biochemical, and physiological disruptions. Central to this condition are systemic inflammatory response syndrome (SIRS), microcirculatory disturbances, and multi-organ dysfunction.3
The immune response in sepsis initiates when immune cells recognize pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), leading to the activation of inflammatory pathways.3,4 Upon pathogen invasion, the complement system is activated, recruiting and activating innate immune cells such as monocytes, macrophages, and neutrophils, thereby initiating early inflammatory responses.5,6 Tissue-resident macrophages and circulating neutrophils respond to DAMPs, releasing mediators that recruit additional immune cells to the site of injury.7 Monocytes then differentiate into macrophages, secreting pro-inflammatory cytokines and engaging in mechanotransduction through cellular surface interactions.8,9 During sepsis, endothelial cells increase the expression of adhesion molecules, including E-selectin and intercellular adhesion molecule-1 (ICAM-1), in response to cytokines, as well as PAMPs and DAMPs. This upregulation of adhesion molecules enhances the recruitment of leukocytes to sites of inflammation. Specifically, it facilitates interactions between these endothelial adhesion molecules and leukocyte integrins, such as Leukocyte function-associated antigen 1 (LFA-1), Macrophage-1 antigen (Mac-1), and P-selectin glycoprotein ligand-1 (PSGL-1). Additionally, lipopolysaccharide (LPS) activates Toll-like receptor 4 (TLR4) on endothelial and immune cells, initiating inflammatory responses characterized by the release of cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 (IL-1). This response is further amplified by NOD-like receptors (NLRs) and the inflammasome, affecting pathways such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPK), and protein kinase C (PKC) pathways, which are crucial for managing sepsis and restoring homeostasis.10
In sepsis, cytokines serve dual roles: they are essential for infection clearance but can also be harmful when excessively released, leading to severe organ damage through a phenomenon known as cytokine storm.11 This cytokine dysregulation often escalates into a cytokine storm, causing significant tissue damage.12 Understanding this intricate cytokine activity is crucial for developing nanolevel immunomodulatory therapies aimed at recalibrating immune responses, reducing hyperinflammation, and preventing organ failure in sepsis. The immune response mechanisms in sepsis are illustrated in Figure 1.
Figure 1.
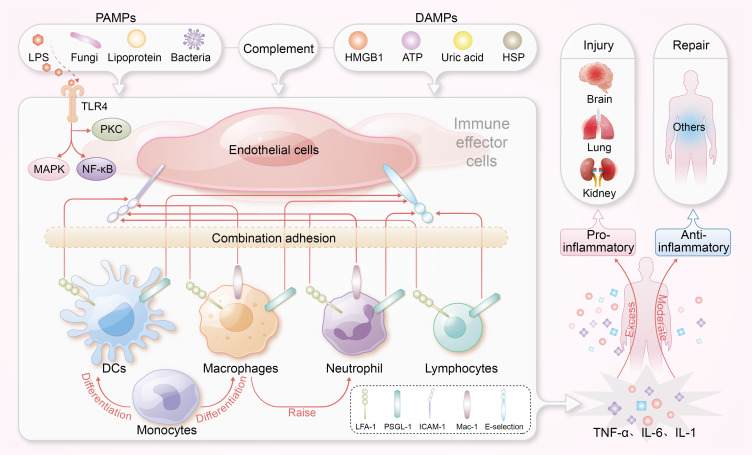
The immune response mechanisms in sepsis. Endothelial cells upregulate adhesion molecules, such as E-selectin and ICAM-1, in response to PAMPs, and DAMPs, promoting leukocyte recruitment. LPS activates TLR4, triggering the release of cytokines. This inflammatory response is amplified via NOD-like receptors and the inflammasome, engaging critical pathways such as NF-κB and MAPK.
Abbreviation: ICAM-1, intercellular adhesion molecule-1; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; TLR4, Toll-like receptor 4; DCs, dendritic cells; HMGB1, High Mobility Group Box 1; HSP, heat shock protein; ATP, adenosine triphosphate.
In addition to these immune mechanisms, several critical pathways contribute to the pathophysiology of sepsis, interacting with immune dysfunction and exacerbating organ failure. For instance, the activation of the coagulation cascade by pro-inflammatory mediators leads to a hypercoagulable state characterized by disseminated intravascular coagulation (DIC), which worsens organ dysfunction by impairing blood flow and oxygen delivery.1 Endothelial dysfunction is another crucial factor; as the endothelial barrier becomes permeable, it results in fluid extravasation, causing tissue edema and organ failure. Furthermore, metabolic dysregulation during sepsis, characterized by alterations in glucose and lipid metabolism, leads to mitochondrial dysfunction and impaired energy production, further intensifying cellular injury and systemic inflammation.3 Collectively, these interconnected pathways underscore the complexity of sepsis and highlight the urgent need for novel therapeutic strategies targeting these diverse mechanisms.
Nanotechnology in Immunomodulator Design
In the evolving field of sepsis treatment, nanotechnology plays a crucial role, particularly through synthetically engineered nanomaterials, which are more common than their naturally occurring counterparts. These nanomaterials are produced using methods such as chemical reduction, wet chemical, ligand-mediated self-assembly, electrostatic assembly, polymer encapsulation, and nanoprecipitation.13,14 Their advantages over conventional drugs include tunable properties like size, composition, surface charge, and chemical characteristics. Additionally, functional surface features, including targeting ligands (eg, antibodies or peptides), enhance specific binding to sepsis-related biomarkers and significantly improve targeting accuracy.15,16 For instance, Fan et al designed S-thanatin (Ts)-functionalized liposomes that encapsulate levofloxacin (LEV). These liposomes target and eliminate multidrug-resistant Klebsiella pneumoniae strains in both in vitro and in vivo settings. The use of these liposomes as drug carriers substantially increased LEV accumulation at the target site. In a murine model of septic shock, incorporating Ts led to a marked increase in bacterial clearance from the bloodstream and significantly improved mouse survival rates. These findings underscore the potential of nanotechnology to improve therapeutic outcomes in sepsis treatment.17
Nanoparticles, ranging from 1 nm to 100 nm, exhibit size-dependent properties that influence their cellular interactions and therapeutic efficacy. They are categorized into types including polymer nanoparticles, liposomes, biomimetic nanoparticles, exosomes, and metallic or inorganic nanoparticles.14,18 Variations in material composition, surface coatings, and geometric shapes influence the cellular interactions and mechanisms of action of nanoscale immunomodulators, enhancing their clinical potential in sepsis management. These nanomodulators utilize materials like superparamagnetic iron oxide nanoparticles, metals, and organic compounds. Surface modifications, such as charged or polyethylene glycol (PEG) coatings, enhance biological compatibility and functionality.14,19 Geometric shapes-from nanospheres and nanorods to nanostars and nanodisks-are crucial in determining cellular uptake and distribution. Targeted delivery to specific immune cells, such as immature T cells and macrophages, is achieved using surface modifications and ligands that selectively bind to cell surface markers, enhancing therapeutic efficacy and minimizing off-target effects. Their small size allows for controlled, sustained drug release and improved biocompatibility, reducing adverse reactions. Overall, these features highlight the transformative potential of nanotechnology in optimizing immunomodulatory strategies for sepsis, aligning with both current and future directions in critical care medicine. Figure 2 illustrates the diverse types of nanoparticles employed in the treatment of sepsis, highlighting their specific therapeutic advantages.
Figure 2.
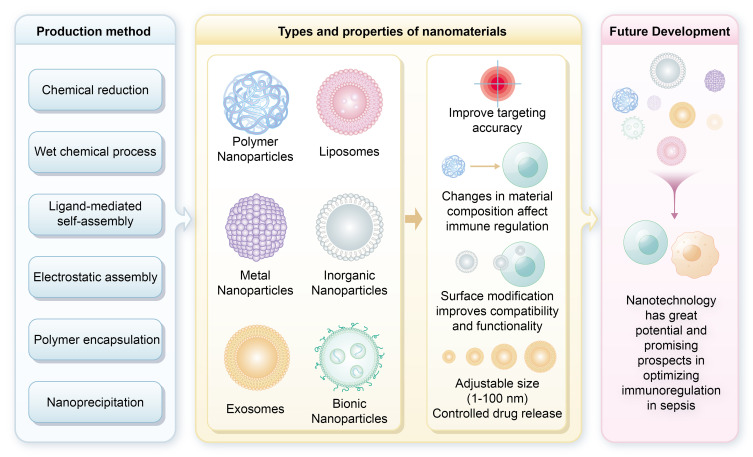
Types of nanoparticles for sepsis treatment and their therapeutic associated advantages. This figure illustrates various nanoparticle materials commonly utilized in sepsis therapy, including lipid nanoparticles, polymer nanoparticles, and metal nanoparticles. These materials offer a range of potential strategies for nanomedicine-based interventions in sepsis management.
Nanoparticle-Based Modulation of TLR Activation in Sepsis Management
TLR activation, triggered by cell and tissue damage as well as pathogen recognition, is a crucial defense mechanism against invading pathogens and endogenous danger signals.20 However, dysregulated TLR activation can lead to immune system imbalance, resulting in excessive release of both pro-inflammatory and anti-inflammatory cytokines and chemokines, contributing to pathological conditions.21 TLRs are activated upon recognition of PAMPs, such as LPS from Gram-negative bacteria or lipoteichoic acids (LTA) from Gram-positive bacteria. This recognition activates downstream signaling intermediates, including MAPK, Janus kinases (JAKs), and NF-κB. These intermediates translocate to the nucleus, initiating the transcription of genes encoding key cytokines, such as TNF-α, IL-1, IL-12, IL-18, and type I interferons.22 This signaling cascade results in further cytokine and chemokine production, leading to a cytokine storm. TNF-α and IL-1, central to these cascades, are critical targets for therapeutic strategies.23 Given that TLRs recognize various PAMPs and DAMPs, modulating their activity is essential.24 Nanomaterial-based TLR receptor antagonists are emerging as promising tools for modulating TLR signaling. These materials can specifically target and inhibit TLR-mediated pathways, potentially providing novel therapeutic strategies for sepsis where TLRs are critically involved.25–27
Cationic lipids, including positively charged liposomes, are capable of modulating TLR4 activity. For example, C14-amine liposomes can induce cytokine secretion similarly to TLR4 activation by LPS, engaging signaling pathways such as Myeloid differentiation factor 88 (MyD88)/NF-κB/JNK and Toll-like receptor adaptor molecule (TRAM) / TIR-domain-containing adaptor inducing interferon-β (TRIF).28,29 Other cationic lipids enhance cytokine production through NF-κB-independent and TRIF-dependent pathways involving CD14.30 Piazza et al demonstrated that aminoglycosides and aromatic ammonium salts specifically bind to CD14, inhibiting LPS-induced TLR4-dependent cytokine production in cellular and animal models.31–33
Leveraging natural compounds like lipid A, Lavado et al developed a cationic glycosphingolipid-coated gold nanoparticle (GNP) system that interacts with CD14 and myeloid differential protein-2 (MD-2) receptors to modulate TLR4 activity.34 Peptide-gold nanoparticle (Peptide-AuNP) hybrids designed as anti-inflammatory agents exhibit potent TLR inhibition in vitro.35,36 Yang et al identified peptide-AuNP hybrids with specific amino acid sequences that effectively inhibit TLR4 signaling and excessive cytokine release. Their immunomodulatory activity is linked to the hydrophobic properties and aromatic structures of the amino acids. Yang et al also extended these hybrids’ application to modulate TLR4 and TLR3 signaling.37 These advanced nanoplatforms control various TLR pathways and suppress pro-inflammatory cytokines; however, the non-degradable core and bioaccumulation potential of gold nanoparticles limit their in vivo use.37
Foit et al explored LPS sequestration using HDL-like nanoparticles functionalized with apolipoprotein A1.38 The most effective construct, combining 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate] (PDP PE 16:0), cardiolipin, and 1,2-dilinoleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (18:2 PG), significantly reduced NF-κB/activator protein-1 (AP-1) signaling induced by LPS.38 This approach leverages the natural properties of HDL to sequester LPS and modulate inflammatory responses. In a rat sepsis model, cerium dioxide nanoparticles also inhibited pro-inflammatory cytokine signaling in a rat sepsis model.39 Wang et al reported that solid lipid nanoparticles loaded with curcumin (Cur-SLN) inhibit pro-inflammatory cytokines through NF-κB signaling.40
Enhancing anti-inflammatory cytokines like IL-4, IL-10, and IL-13 represents a novel approach for sepsis management. Studies have demonstrated that boosting these cytokines can help counteract the pro-inflammatory responses characteristic of sepsis, improving outcomes in both cellular and animal models. Nanoparticles composed of antimicrobial peptides (AMPs) synthesized synthesized with chitosan derivatives show efficacy in cellular studies and sepsis models.41 Xu et al demonstrated that γ-Fe2O3 superparamagnetic iron oxide nanoparticles (SPION) promote macrophage autophagy and IL-10 production through the Cav1-Notch1/HES1 pathway.42 Siglec-targeted platforms, such as poly(lactic-co-glycolic acid) (PLGA) nanoparticles conjugated with natural Siglec ligands, enhance IL-10 production and mitigate inflammation in various models.43 Self-assembled lipid-modified heparin nanoparticles also serve as TLR4 antagonists, suppressing LPS-induced inflammation and cytokine production.44,45 Table 1 summarizes the mechanisms of TLR signaling modulation by these nanomaterials.34,38–41 Figure 3 delineates the modulation of TLR signaling pathways by various nanoparticles, illustrating their pivotal roles in immunoregulatory mechanisms during sepsis.
Table 1.
Mechanisms of TLR Signaling Modulation by Nanomaterials
| Type | Size | Mechanism | Results | References |
|---|---|---|---|---|
| Peptide decorated-gold NPs | 13–14nm | 1.Modulate endosomal pH 2.Blockade of endosomal acidification Inhibits downstream TLR4 signalling pathways, leading to the reduction of NF-κB, IRF3 and MAPK activation |
1.Improve the disease activity index 2.Ameliorate colonic inflammation in vivo |
[34] |
| APS NPs | 105–115nm | Inhibite the activation of TLR4/ NF-κB pathway | Decrease myocardial inflammatory cytokine expression | [41] |
| HDL-like NPs | Similar to hHDL | Decrease TLR4 signalling | LPS toxin scavenging and neutralizing | [38] |
| Cur-SLN | 40–80nm | Suppressions of NF-κB activation and IkBa degradation levels | Decrease expression of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1b) | [40] |
| Cerium oxide NPs | Not provide | Decrease transcriptional action of ROS, iNOS, COX-2, and nuclear factor-kappa light chain, the triggered B cells (NF-κB) | Decrease hepatic damage, serum cytokines/ chemokines, and swelling indicators in vivo | [39] |
| Trehalose- and glucose-derived glycoamphiphiles incorporated in Au NPs | Not provide | Interference with TLR4 activation and signalling in vitro and in vivo | Inhibite LPS-triggered IL-6 production in mice | [34] |
Abbreviations: APS, astragalus polysaccharides; HDL, high-density lipoprotein; Cur-SLN, solid lipid nanoparticles loaded with curcumin; iNOS, inducible nitric oxide synthase; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase 2.
Figure 3.
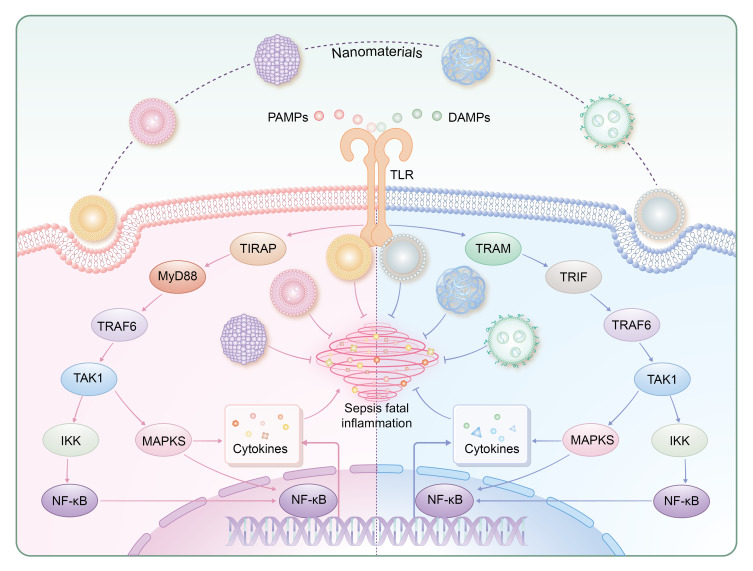
Regulation of TLR signaling pathways by nanoparticles. TLR-mediated responses are primarily governed by the MyD88-dependent pathway (utilized by all TLRs except TLR3) and the TRIF-dependent pathway (employed by TLR3 and TLR4). TIRAP and TRAM serve as adaptor proteins for TLR2-4 and TLR4, respectively, with a focus on TLR4 in this review. MyD88 recruits TRAF6, activating NF-κB and MAPK pathways to induce inflammatory responses. Similarly, TRIF recruits TRAF6, leading to TAK1 activation and subsequent MAPK and NF-κB activation. Ultimately, these pathways modulate the expression of genes encoding pro-inflammatory cytokines such as IL-6 and TNF.
Abbreviation: MyD88, Myeloid differentiation factor 88; TRIF, Toll-interleukin 1 receptor domain-containing adaptor inducing interferon-β; TIRAP, Toll-interleukin 1 receptor domain-containing adapter protein; TRAM, Toll-like receptor adaptor molecule; TRAF6, Tumor necrosis factor receptor associated factor 6; TAK1, Transforming growth factor-beta-activated kinase 1; IKK, IκB kinase.
Nanoparticle Strategies for LPS Neutralization and Cytokine Removal in Sepsis Management
Nanoparticle-based strategies for neutralizing LPS have emerged as innovative adjunctive therapies in sepsis management. LPS, an endotoxin derived from the outer membrane of Gram-negative bacteria, triggers inflammatory cytokine dysregulation upon prolonged exposure.46 Hence, targeting LPS presents a promising avenue for sepsis management. Herrmann et al engineered polymyxin B-functionalized metal alloy magnetic nanoparticles, enabling rapid LPS removal from circulation under magnetic separation guidance.47 The nanoparticles were constructed by conjugating polymyxin B to carbon-coated cobalt/iron alloy nanoparticles via an NHS-dPEG 24-MAL linker (C/CoFe-PEG-polymyxin B). Upon incubation with LPS-spiked blood, the polymyxin B motifs on the nanoparticles effectively captured LPS, which was subsequently removed via magnetic separation. Subsequent plasma infusion into endothelial cells did not upregulate pro-inflammatory cytokines such as C-X-C motif chemokine ligand 1(CXCL-1) and IL-6, indicating the potential of these polymyxin B-functionalized magnetic nanoparticles for blood purification in sepsis management. Liao et al introduced sub-nanometer gold clusters (SAuNCs) with short alkyl chain coatings, which reduced LPS-induced pro-inflammatory cytokine production in a murine sepsis model by disrupting LPS assembly.48 Mishra developed polymer-encapsulated nanoparticles loaded with ciprofloxacin, specifically designed to specifically target LPS in vivo, significantly reducing cytokine production (TNF-α and NO) in a LPS-induced sepsis model.49 Mas-Moruno et al developed acylated peptide nanostructures with longer acyl chains (C16) to enhance LPS neutralization.50 TEM analysis showed that long-chain N-acylation promoted micellar and fibrous nanostructure formation, which correlated with increased anti-LPS activity.
Another approach to managing sepsis is the removal of cytokines from plasma. During sepsis, excessive pro-inflammatory and anti-inflammatory cytokines such as TNF-α and IL-6 are released, and their removal can help alleviate symptoms.49 Eliminating inflammatory cytokines from the bloodstream of sepsis patients has been proposed as an effective method to alleviate disease symptoms. Porous and magnetic nanoparticles with high adsorption capabilities have been extensively studied for efficient cytokine removal.51,52 For instance, Yachamaneni et al developed porous carbon-derived carbons (CDCs) that demonstrated effective cytokine adsorption from plasma samples.51 This research underscores the importance of synthesizing and characterizing NPs for effective blood purification, leading to the development of other carbon-based NPs, such as graphene nanosheets with cytokine adsorption capabilities.51,53–55 The use of magnetic nanoparticles represents another prevalent strategy for purifying inflammatory cytokines from the bloodstream.47,52,56,57 While nanomaterials with high adsorption capabilities, such as graphene nanosheets and CDCs, demonstrate significant potential for cytokine removal from plasma, they also present challenges related to biosafety and metabolic clearance.58,59 Research indicated that graphene-based nanomaterials showed minimal toxicity at low concentrations; however, at higher doses, they may pose risks. The toxicity is largely influenced by their physicochemical properties, including size, surface charge, and shape.60 Notably, these materials tend to accumulate in the lungs, liver, and spleen, posing a risk of chronic toxicity if not effectively cleared.61 Studies indicated that smaller particles were rapidly eliminated via the renal pathway, while larger particles were retained in the lungs.62 Therefore, a thorough evaluation of the safety and metabolic pathways of graphene nanomaterials is essential before clinical application, since traditional cytotoxicity tests may not fully meet the rigorous standards required for safe clinical use.
Intracellular Immunomodulation by Nanoparticles in Sepsis
NPs provide a versatile approach to managing sepsis, not only by eliminating and attenuating inflammatory factors but also by modulating immune responses to enhance pathogen clearance and control inflammation. NPs can enhance the host immune system’s ability to eradicate pathogens through targeted delivery of immunomodulatory agents or by directly engaging immune cells to suppress cytokine production and modulate inflammatory responses.63 In sepsis, uncontrolled activation of immune cells can result in severe immune dysregulation. Targeting these inflammatory cells for apoptosis is a promising therapeutic strategy that NPs can facilitate through targeted delivery of pro-apoptotic agents or by enhancing apoptotic signaling pathways.64 However, complete depletion of inflammatory cells using antibodies may inadvertently exacerbate inflammation and impair both innate and adaptive immune responses by removing cells essential for resolving inflammation and coordinating immune reactions. Thus, there is a critical need for advanced platforms that are capable of selectively targeting cytokine-producing immune cells while minimizing off-target effects. Nanotechnology holds potential in addressing these challenges by enabling precise targeting and modulation of immune cell activity. Nanotechnology has emerged as a promising avenue for the development of advanced intracellular immunomodulation systems.65,66
Nanoparticles Targeting Macrophages
Macrophages (MΦ) play a crucial role in in combating bacterial infections, thanks to their abundant pattern recognition receptors (PRRs) and cytokine receptors, which mediate both pathogen recognition and cytokine-driven signaling pathways. These features enable macrophages to concentrate at sites of inflammation and effectively neutralize any existing inflammatory cytokines.67 Monocytes/macrophages are key players in the pathogenesis of sepsis.68,69 Under the influence of environmental pathogens and cytokines, macrophages differentiate into various functional phenotypes and perform diverse roles, including pathogen killing, cytokine production, and chemotactic factor release.70 However, pathogens can induce macrophage apoptosis, pyroptosis, necroptosis, and monocyte death, impairing the proliferation of immune cells and compromising the host’s ability to mount an effective immune response.71,72 In sepsis, M1 and M2 macrophage phenotypes exhibit complex dynamics. Initially, M1 macrophages dominate, producing pro-inflammatory cytokines like TNF-α and IL-6. As sepsis progresses, M2 macrophages increase, maintaining a critical balance for effective immune response throughout the infection stages. The dysregulation of macrophage functions, including overactivation, phenotype reprogramming, and programmed cell death, presents a therapeutic target that can be addressed with nanomaterials. The recognition and phagocytosis of NPs by macrophages are mediated through interactions with PRRs, similar to the interactions between PAMPs and PRRs.73 NPs delivery platforms that target PAMPs or their synthetic analogs, such as CpG oligodeoxynucleotides, have shown considerable promise in immunomodulatory therapies by enhancing immune activation and targeting specific immune pathways.74 NPs loaded with immunomodulatory drugs can enter macrophages and modulate their function by influencing the NF-κB/MAPK pathways.75,76 Moreover, PRRs assemble into inflammasomes upon detecting pathogens or DAMPs in the host cell cytosol.77 Silica nanoparticles (SiO₂NPs) induce the generation of reactive oxygen species (ROS) upon cellular entry, activating inflammasomes such as caspase-1 and Apoptosis-associated Speck-like Protein Containing a CARD (ASC), subsequently leading to increased expression of pro-inflammatory cytokines IL-1β and IL-18, thereby modulating the inflammatory response in macrophages.78,79 Multiwalled carbon nanotubes (MWCNTs) and asbestos induce NLRs family pyrin domain-containing 3 (NLRP3) inflammasome activation in macrophages, relying on ROS production, histone B activity, P2X7 receptors, and Src and Syk tyrosine kinases.80 Furthermore, imaging techniques can monitor the specific deposition of imaging agents within macrophages, enabling precise spatiotemporal tracking of macrophage responses.
Polymeric nanoparticles are advantageous for targeted drug delivery due to their ease of surface modification, biodegradability, non-toxicity, and lack of immunogenicity.81–83 In inflammatory conditions, macrophages generally polarize into pro-inflammatory M1 or anti-inflammatory M2 phenotypes. In the context of sepsis, M1 macrophages are associated with heightened inflammatory responses and tissue damage, while M2 macrophages are involved in tissue repair and resolution of inflammation.84 Modulating macrophage polarization can alter the inflammatory milieu and potentially treat sepsis.85 Jiang et al engineered chitosan-based nanoparticles (CNs) loaded with trimetaphosphate to dynamically reprogram M1 macrophages to M2 macrophages.86 In M1-like macrophages, CN treatment resulted in a reduction of CD86 and iNOS expression, which are markers of pro-inflammatory activity, while promoting the expression of Arginase-1 (Arg-1) and IL-10, both associated with anti-inflammatory responses. Conversely, in M2-like macrophages, CN treatment decreased Arg-1 levels and increased the expression of CD86, iNOS, and TNF-α, indicating a shift towards a more pro-inflammatory phenotype. This bidirectional polarization was mediated through the signal transducer and activator of transcription-1 (STAT-1)/STAT-6 signaling pathway, indicating CN’s potential in sepsis therapy.86
Pyroptosis, a caspase-mediated cell death mechanism, involves gasdermin D (GSDMD) forming pores in the cell membrane, leading to cell lysis and the release of pro-inflammatory cytokines such as IL-1β, which can result in aberrant immune activation.87 To counteract this, Ou et al developed disulfiram-lactoferrin nanoparticle complexes (DSF-LF NPs) that inhibit GSDMD-induced pyroptosis. Lactoferrin (LF) specifically binds to low-density lipoprotein receptor-related protein-1 (LRP-1), enhancing nanoparticle uptake by macrophages and imparting immunomodulatory effects. DSF-LF NPs have demonstrated significant efficacy in suppressing macrophage pyroptosis and the release of pro-inflammatory cytokine in LPS-induced sepsis models.88
Additionally, small interfering RNA targeting High Mobility Group Box 1 (siHMGB1)-lipid nanoparticles are internalized by macrophages via the mannose receptor, forming endolysosomes that release active agents to inhibit HMGB1 transcription, thereby mitigating pyroptosis.89 During the hyper-inflammatory phase of sepsis, polymeric nanoparticles have been shown to enhance macrophage anti-inflammatory responses. For example, Hongsa et al designed a biotin-azide-quaternary ammonium salt 188-chitosan (Bi-QCS) and a collagen-based nanocarrier (Bi-QCS-AuNPS@collagen) that encapsulates drugs on AuNPs. Bi-QCS significantly improves drug uptake by macrophages, while chitosan enhances physicochemical stability and controls drug release, leading to superior anti-inflammatory activity compared to conventional AuNPs.90
Furthermore, curcumin-loaded ROS and pH-sensitive chitosan/alginate hydrogel nanoparticles effectively resist gastrointestinal hydrolysis and target macrophages via the TLR4-MAPK/NF-κB signaling pathways. Chondroitin sulfate promotes targeted delivery to macrophages, while the chitosan/alginate hydrogel protects the nanoparticles from digestive degradation.76 Nanocomposites of chitosan and AMPs also significantly inhibit LPS-induced NF-κB/MAPK signaling in RAW264.7 macrophages.91 Additionally, chitosan has been used to deliver NF-κB/p65 antisense oligonucleotides, effectively suppressing NF-κB/p65 signaling and downstream inflammatory cytokines (eg, IL-1, IL-6, TNF-α) in LPS-stimulated RAW264.7 macrophages.92
Rajendrakumar et al developed a mannose-functionalized disulfide-crosslinked polyethyleneimine nanoparticle complex (MSPAM) incorporating bovine serum albumin-reduced manganese dioxide.93 This complex mitigates organ damage in sepsis models by scavenging H2O2, inhibiting HIF-1α expression, and reducing serum TNF-α and IL-6 levels. These actions collectively contribute to the alleviation of sepsis-induced organ damage. In the immune suppression phase of sepsis, nanoparticles can induce pro-inflammatory responses in macrophages, thereby improving survival rates.94 For instance, iE-DAP, a nucleotide-binding oligomerization domain-containing protein 1 (NOD1) agonist, encapsulated in poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), facilitates macrophage internalization, activating NOD1 signaling and the NF-κB pathway, and promoting IL-6 and TNF-α secretion.70 Zhao et al combined monophosphoryl lipid A (MPLA) and muramyl dipeptide (MDP) in poly (lactic-co-glycolic acid) (PLGA) nanoparticles that were cross-linked with alginate to create a dual-phase immune-stimulatory nanoparticle complex (MDP+PM@ALG).95 This formulation enhanced macrophage phagocytosis and bacterial killing, improving survival and resistance to secondary infections in CLP-induced sepsis models, thus providing long-term protection against sepsis.95
Liposomes offer several advantages, such as high drug encapsulation efficiency, reduced systemic toxicity, targeted delivery to specific tissues or cells, excellent biocompatibility, biodegradability, and optimized pharmacokinetics. Upon intravenous administration, liposomes are efficiently phagocytosed by macrophages, leading to natural accumulation in these cells and enhanced targeted delivery.96 During the hyper-inflammatory phase of sepsis, liposomes can enhance the anti-inflammatory activity of macrophages. For instance, liposomes loaded with guanidinobenzoic acid modulate macrophage anti-inflammatory responses through eIF2α-dependent signaling pathways, downregulating IL-6 and cyclooxygenase 2 (COX-2). Additionally, eIF2α-independent pathways reduce IL-1β and TNF-α, markedly decreasing the secretion of pro-inflammatory cytokines by macrophages.97 Conversely, during the immune suppression phase of sepsis, liposomes can promote pro-inflammatory activity in macrophages. Hou et al developed an AMP encoding mRNA for Cat-B, encapsulated within vitamin-liposomes. These vitamin liposomes enhance the accumulation of nanoparticles within macrophage lysosomes.98 The AMP-cat-B@VLMP-laden macrophages effectively eliminate MDR bacteria in septic mice, offering an alternative strategy to tackle multidrug-resistant sepsis. Furthermore, liposomes can modulate macrophage reprogramming. PEGylated liposomes containing IFN-γ induce an increase in NO and a decrease in arginase levels in M2 macrophages, indicating enhanced drug targeting and promotion of M2 to M1 polarization.99
Bionically engineered macrophage-membrane-coated nanoparticles possess the ability to traverse biological barriers, such as endothelial cell layers, thereby allowing precise targeting of disease sites while minimizing immune system detection and clearance.100–102 These bionic nanoparticles also facilitate macrophage reprogramming. Nanodrugs carrying oxaliplatin prodrugs and photosensitizers induce the conversion of M2 to M1 macrophages, characterized by increased inducible nitric oxide synthase (iNOS) (an M1 marker) and decreased Arg-1 (an M2 marker). This process is mediated by macrophage-mimetic nanoparticles through photo-triggered, precise drug delivery.100 Bionic nanoparticles can modulate macrophage phagocytic activity both positively and negatively. During the hyper-inflammatory phase of sepsis, liposomes can enhance macrophage anti-inflammatory activity. Lu et al developed a bionic nanomedicine (MM-CEP/NLCs) incorporating cephalosporin hydroxyampicillin (CEP) nanolipid carriers (NLCs) and externally coated with MM. This formulation ensures effective accumulation in the context of pulmonary inflammation, achieving sustained drug release and therapeutic effects against lung inflammation.103 Additionally, bionic nanoparticles can neutralize PAMPs. Macrophage-mimetic nanoparticles (MΦ-NP) combine polymeric cores with macrophage membranes, possessing LPS-binding sites (eg, CD126, CD14, and TLR4). These macrophage-mimetic nanoparticles demonstrate prolonged circulation times, low toxicity, and the ability to bind and neutralize LPS, thereby mitigating excessive immune activation associated with LPS-induced sepsis in murine models.104–106
Additionally, exosomes, known for their low immunogenicity and excellent biocompatibility, serve as effective carriers for various therapeutic agents while also modulating macrophage reprogramming. Notably, long non-coding RNAs (lncRNAs) exhibit distinct expression patterns in M1 and M2 macrophages.107 Among these, the antisense lncRNA of DNA methyltransferase 3A (Dnmt3aos), predominantly expressed in M2 macrophages, regulates Dnmt3a expression, highlighting its potential role in therapeutic strategies targeting macrophage polarization during sepsis. This lncRNA comprises three small interfering RNAs (siRNAs) and three antisense oligonucleotides (ASOs), which play critical roles in sequence-specific silencing of target genes. When drugs encapsulated in exosomes derived from M2 macrophages are administered to allergic asthma mouse models, they effectively target M2 macrophages in the lungs and significantly suppress pro-inflammatory cytokine production.107 ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) are expressed only in LPS-activated macrophages, while LFA-1 and Very Late Antigen-4 (VLA-4) are upregulated in exosomes from M2 macrophages, facilitating their recognition by LPS-activated macrophages.108 Encapsulation of plasmid DNA encoding IL-10 in M2-derived exosomes protects the DNA from nuclease degradation and adverse reactions, achieving targeted delivery to M1 macrophages and enhancing M1-to-M2 reprogramming, as evidenced by increased IL-10 and IL-4 levels and decreased IL-1β and TNF-α levels.108
Inorganic NPs can also modulate macrophage reprogramming. For instance, mangosteen-functionalized gold nanoparticles (MGF-AuNPs) target the NF-κB pathway in splenic macrophages, promoting M2 to M1 polarization with a tenfold increase in IL-12, a fiftyfold increase in TNF-α, and a twofold decrease in IL-6 and IL-10.109 In sepsis, SPIO nanoparticles, used as antimicrobial agents, modulate macrophage reprogramming dependent on the expression of TNF receptor-associated factor 1 (TRAF1) protein by mesenchymal stem cells, aiding in the treatment of septic liver injury.110 During excessive sepsis activation, macrophage anti-inflammatory activity can be enhanced. Studies have shown that GNP conjugated with ginsenoside compound K (CK) and peptide CopA3 (GNP-CK-CopA3) target RAW264.7 macrophages, reducing LPS-induced NF-κB/MAPK pathway activation. Pre-treatment of RAW264.7 cells with GNP-CK-CopA3 for one hour followed by LPS stimulation for 2 hours significantly inhibits phosphorylation and degradation of IκBα and p38 MAPK, indicating the suppressive effect of GNP-CK-CopA3 on macrophage anti-inflammatory activity.111
NPs can function not only as drug delivery systems but also as modulators of macrophage immune responses. For instance, Peled et al developed amphiphilic block copolymer NPs self-assembled from hydrolyzed galactomannan (hGM). These NPs are recognized by macrophage surface receptors such as lectin-like receptors, leading to the downregulation of M1 markers (CD80) and the upregulation of M2 markers (CD163 and CD206), thus facilitating M1-to-M2 macrophage polarization.94 In contrast, Zhao et al engineered Fe3O4@C/MnO2 NPs, which exhibit excellent photothermal, magnetic, and catalytic properties, inducing M2 macrophages to polarize into M1 phenotypes.112 Additionally, NPs can suppress macrophage phagocytic activity. Kodali et al demonstrated in 2013 that silica and SPIO NPs reduced macrophage phagocytosis of Streptococcus pneumoniae.113 SPIO binds to the class A scavenger receptor (SR-A) on macrophages through electrostatic interactions between the nanoparticle’s anionic groups and the receptor’s collagen-like domain. This binding results in transcriptional reprogramming that diminishes pathogen uptake. Similarly, Palomba et al created stable spherical NPs by conjugating natural fatty acid methyl palmitate with albumin, which induces macrophage quiescence and reduces phagocytic activity.114
NPs also modulate inflammatory pathways. CeO2 NPs, known for their antioxidant properties and biosafety, can effectively intervene in disease processes by reducing the mitochondrial electron transport chain (METC) and NADPH oxidase (NOX)-mediated superoxide flux, thereby regulating macrophage oxidative activity.115 Furthermore, CeO2 NPs mitigate LPS-induced IKB-α degradation and NF-κB/p65 nuclear translocation, attenuating MAPK/NF-κB signaling and decreasing LPS-induced cytokine release (IL-1β, IL-6, TNF-α, HMGB1).39,116,117 Conversely, some NPs promote inflammatory pathways. Silica NPs, iron oxide NPs (IONPs), and PLGA NPs can stimulate TNF-α secretion by macrophages, enhancing inflammatory responses.118,119 Pro-inflammatory cytokines can enhance the immune response in early sepsis. During the immunosuppressive phase, moderate inflammatory stimulation helps reverse “immune paralysis”, allowing the immune system to effectively recognize and eliminate pathogens. Emerging carbon dots (CDs) target lung macrophages, inducing endoplasmic reticulum stress and activating the NLRP3 inflammasome, which is evidenced by increased secretion of IL-1β and IL-8.120 This mechanism enhances localized inflammatory responses, facilitating rapid pathogen clearance, particularly in sepsis resulting from respiratory infections. Additionally, amine-treated 60 nm polystyrene spheres induce ROS production in macrophages at 20 μg/mL.121 ROS have direct bactericidal effects and enhance macrophage phagocytosis and antimicrobial functions while activating immune signaling pathways through moderate oxidative stress, thereby improving infection resistance. Immune-modulatory nanoparticles (iNPs) interact with macrophages to regulate inflammation, reducing LPS-induced NF-κB p65 and MAPK p38 activation.122–124 Furthermore, MWCNTs enhance macrophage activation by upregulating CD40 and CD80, stimulating phagocytosis through NLRP3 inflammasome activation via Tim4 receptor recognition.125,126 Collectively, these NPs contribute to sepsis treatment by modulating inflammatory pathways, enhancing pathogen clearance and maintaining inflammatory balance. Table 2 provides a detailed classification and mechanism overview of macrophage-targeted nanoparticles in sepsis research, highlighting various NP types and their specific roles in modulating macrophage functions.36,39,42,88,93,94,98,110,116,117,122,123,127–130 Figure 4 depicts the immunomodulatory effects exerted by nanoparticles on macrophage function, highlighting their integral roles in modulating immune responses in sepsis.
Table 2.
Classification and Mechanisms of Macrophage-Targeted Nanoparticles in Sepsis Research
| Nanoparticle Type | Mechanism | Function | References |
|---|---|---|---|
| Dextran NPs | Quantitative noninvasive assessment for spatiotemporal macrophage dynamics | Nanotracer for macrophage | [127] |
| SPION | Quantitative susceptibility mapping magnetic resonance for NP phagocytosis by macrophages | Monitoring tools based on macrophage phagocytosis | [128] |
| Liposomes | Promote the accumulation of NPs in macrophage lysosomes to kill multidrug-resistant bacteria | Drug-delivery system targeted for macrophages | [98] |
| MDP+PM@ALG | Enhance the phagocytic and bactericidal function of macrophages. | Macrophage activation and phagocytosis | [94] |
| Biomimic macrophage NPs | Capture and eliminate LPS and inflammatory factors | Macrophage activation and phagocytosis | [129] |
| Cerium oxide NPs | Reduce the superoxide flux of METC and plasma membrane NOX, and downregulate proinflammatory cytokines release | Induce antioxidant and anti-inflammatory activity | [39,117,130] |
| Metal and polymeric NPs | Decompose toxic H2O2 to oxygen and water, prevent proinflammatory cytokines secretion | Induce antioxidant and anti-inflammatory activity | [93] |
| Cerium oxide NPs | Reduce MAPK/NF-κB mediated pathways activation | Target inflammatory pathways | [116,117,130] |
| PLA iNPs | Elimination of NF-κB p65 and MAPK p38 activation | Target inflammatory pathways. | [122,123] |
| Au NPs | Demonstrated by the lower supernatant TNF-α and IL-1β and higher Arginase 1 | Mediate macrophage polarization | [36] |
| SPION of γ-Fe2O3 NPs | Induce TRAF1-dependent polarization | Mediate macrophage polarization | [110] |
| SPIONs of γ-Fe2O3 NPs | Induce Cav1-Notch1/HES1-mediated autophagy | Target inflammatory pathways | [42] |
| LF NPs | Inhibit GSDMD-induced pyroptosis | Target inflammatory pathways | [88] |
Abbreviations: SPION, superparamagnetic iron oxide nanoparticles; MDP+PM@ALG, muramyl dipeptide (MDP) in poly (lactic-co-glycolic acid) (PLGA) nanoparticles, cross-linked with alginate to create a dual-phase immune-stimulatory nanoparticle complex; METC, mitochondrial electron transport chain; NOX, NADPH oxidase; PLA, phoryl lipid A immune-modulatory; TRAF1, TNF receptor associated factor 1; LF, lactoferrin.
Figure 4.
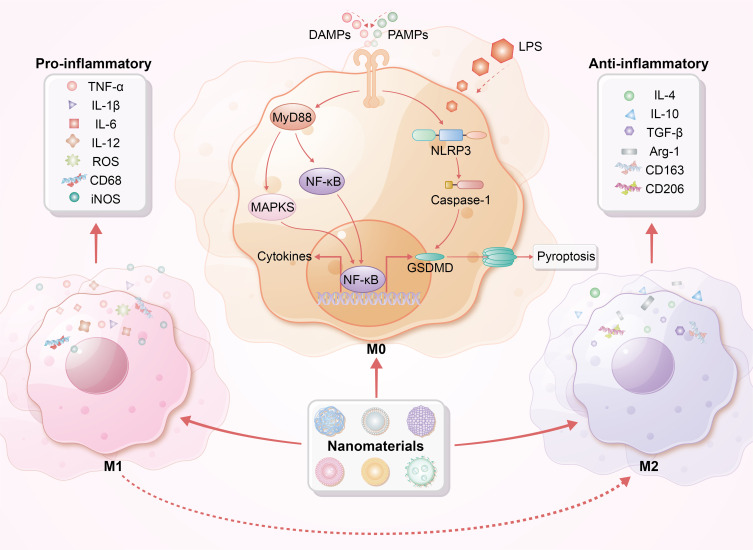
Immunomodulatory effects of macrophages by nanoparticles. This figure illustrates the distinct phenotypes of macrophages, specifically M1 (pro-inflammatory) and M2 (anti-inflammatory). DAMPs, PAMPs, and LPS can trigger M1 polarization, resulting in the release of pro-inflammatory cytokines. Nanoparticles interact with cell surface receptors and enter macrophages via endocytosis or phagocytosis, influencing MyD88, MAPK, and NF-κB signaling pathways. This interaction alters macrophage polarization, leading to cytokine production and release, inflammasome activation, and potentially pyroptosis. Notably, nanoparticles promote the transition from pro-inflammatory M1 to anti-inflammatory M2 macrophages, enhancing the production of anti-inflammatory cytokines, reducing inflammatory responses, and improving tissue repair, ultimately facilitating recovery from sepsis.
Abbreviation: MyD88, Myeloid differentiation factor 88; Arg-1, Arginase-1; NLRP3, NOD-like receptor family pyrin domain-containing 3; GSDMD, gasdermin D.
Nanoparticles Targeting Other Immune Cells
In an intriguing study, Zhang et al synthesized pH-sensitive albumin NPs conjugated with doxorubicin (DOX) to selectively target and activate neutrophils, inducing programmed cell death following the in vivo release of DOX.65 DOX was conjugated to albumin NPs through hydrazone bonds that degrade specifically in the acidic environment of activated neutrophils, allowing selective drug release.65 Once internalized, the hydrazone bonds degrade in the acidic environment of the neutrophils, leading to DOX leakage and subsequent neutrophil apoptosis. In an LPS-induced sepsis mouse model, 70% of mice treated with DOX-albumin NPs survived for 72 hours, compared to only 10% survival in mice treated with free DOX. Mice treated with DOX-albumin NPs exhibited a significant reduction in neutrophil and cytokine levels in their blood compared to those receiving conventional treatments, indicating that the designed NP platform promoted apoptosis of inflammatory neutrophils and suppressed systemic inflammation. Notably, neutrophil counts and cytokine levels in NP-treated mice returned to normal within 72 hours, suggesting no permanent damage to the immune system or bone marrow function. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is known to induce apoptosis in inflammatory cells. Encapsulation of TRAIL in antimicrobial peptide-crosslinked nanogels has been used to inhibit Klebsiella pneumoniae infection and overexpression of membrane attack complex (MAC).66 The targeting capability of such nanogels arises from their cationic fibrillar assemblies, which interact with bacterial LPS through electrostatic interactions. Subsequently, the nanogels were internalized by LPS-activated MAC and release TRAIL, promoting cell apoptosis. In a Klebsiella pneumoniae-induced sepsis mouse model, treatment with TRAIL nanogels significantly reduced serum creatinine levels, bacterial loads, several cytokines (TNF-α and IL-6), kidney injury markers, and LPS-induced lung polymorphonuclear leukocytes compared to free TRAIL, nanogel, and saline controls. Additionally, TRAIL nanogel-treated mice demonstrated a notable increase in survival rates, with nearly 70% surviving for 12 days post-treatment.
Endothelial cells play a crucial role in maintaining homeostasis through the coordination of anti-inflammatory, anticoagulant, and anti-adhesive states.131 However, excessive inflammation and reactive oxygen and nitrogen species (RONS) can impair endothelial cells, leading to dysfunction and structural damage in septic conditions.132 Thus, modulating inflammation of endothelial cells is a significant aspect of sepsis treatment. Notably, recombinant activated protein C (APC), a component of the natural anticoagulant system with potent anti-inflammatory effects on endothelial cells, has been used in the treatment of sepsis.133,134 However, the clinical application of APC is hindered by its severe bleeding risk due to the degradation of pro-coagulant factors Va and VIIIa. Recently, Lee et al designed a protein nanocage composed of short ferritin (sFn), γ-carboxyglutamic acid from protein C (PC-Gla), PAR-1 activating peptide (TRAP), and matrix metalloproteinase (MMP)-2 cleavage sites, with sFn serving as its scaffold.135 PC-Gla is inserted at the C-terminus and TRAP at the N-terminus of sFn, while MMP-2 cleavage sites are inserted between sFn and PC-Gla to enable PC-Gla to escape the nanocomposite upon reaching MMP activation sites. Notably, TRAP activates PAR-1, enhancing Gla-ERPC-PAR-1 signaling, which shifts endothelial cells from a pro-inflammatory state to a cell-protective state.133,136,137 To address endothelial inflammation, bovine serum albumin NPs have been utilized as carriers for dansylamide (a spleen tyrosine kinase inhibitor).138 Researchers also used albumin NPs to deliver dansylamide to inhibit β2 integrin signaling mediated by spleen tyrosine kinase, thereby reversing TNF-α activated neutrophil adhesion. In an LPS-induced mouse model of lung inflammation, which mimics sepsis-associated acute lung injury, albumin NPs loaded with dansylamide significantly reduced neutrophil adhesion and lung tissue myeloperoxidase activity, indicating reduced neutrophil sequestration.139 Reduced drug content in albumin NPs decreased neutrophil and monocyte infiltration into the lungs. These results underscore the potential value of NPs in treating the inflammatory cells responsible for tissue damage.
Extracellular Vesicles (EVs) in Sepsis Immunomodulation
EVs are lipid bilayer-enclosed vesicles ranging in size from 30 nm to several micrometers, secreted by various mammalian cells under both physiological and pathological conditions.140 They are classified into three main subtypes based on their biogenesis and size: exosomes (Exos), microvesicles (MVs), and apoptotic bodies.141 EVs contain a variety of bioactive substances, including intracellular proteins, nucleic acids (DNA and RNA), lipids, and metabolites, that mediate intercellular communication and affect the biological functions of recipient cells.142 Recent studies have elucidated a dual role of EVs in sepsis-related inflammatory dysregulation. Numerous reports have highlighted the pro-inflammatory role of EVs derived from tissue cells in various sepsis models. For example, in a rat model of sepsis-associated encephalopathy (SAE) induced by CLP, Xi et al observed that exosomes from intestinal epithelial cells (IECs) induce M1 polarization in mesenteric lymph nodes (MLNs), which contributes to elevated circulating levels of IL-1β and exacerbates neuronal damage in the hippocampus.143 Similarly, Balusu et al found that microRNAs (miR-146a and miR-155) from choroid plexus epithelium (CPE) cell-derived EVs could enhance the transcription of inflammatory genes such as IL-1β, TNF, IL-6, NOS2, and NF-κB, consequently increasing the secretion of IL-6, IL-1β, and TNF in cerebrospinal fluid (CSF).144 Lin et al reported that brain-derived EVs could elevate the production of pro-inflammatory mediators, leading to damage in the lungs, liver, and kidneys.145 Liu et al demonstrated that exosomes from alveolar epithelial cells (AECs) containing miR-92a-3p could activate alveolar macrophages (AMs) by inhibiting phosphatase and tensin homolog deleted on chromosome ten (PTEN) expression, thus activating the NF-κB signaling pathway in AMs and intensifying lung injury through increased expression of pro-inflammatory cytokines.146 Additionally, exosomes from hepatocytes contain high levels of HMGB1, a key mediator of late-stage inflammation.147,148
EVs derived from immune cells play a crucial role in sepsis-associated inflammation. Monocytes and macrophages, which can non-specifically kill pathogens, present antigens, and secrete cytokines, release EVs that significantly influence sepsis. Research by Li et al demonstrated that macrophage-released exosomes are internalized by neighboring macrophages, enhancing TNF-α release.149 Recent studies indicate that macrophage-derived EVs, which express high levels of CXCL2, facilitate neutrophil recruitment to the liver and activate neutrophils via the CXCR2/PKC/NOX4 pathway, thereby amplifying inflammation.149 Sui et al showed that macrophage exosomes exacerbate TNF-α, IL-1β, and IL-6 release in a sepsis-induced acute lung injury mouse model.150 Dendritic cell-derived exosomes, modified with brain-targeting peptides, cross the blood-brain barrier and enhance immune responses in the brain.151,152 Wang et al reported that monocyte-derived exosomes promote inflammatory responses in sepsis-induced myocardial dysfunction by delivering the TXNIP-NLRP3 complex to local macrophages, leading to the maturation of IL-1β and IL-18.153 Additionally, EVs from plasma, serum, or other bodily fluids exhibit pro-inflammatory effects. Xu et al identified plasma-derived EVs, containing miR-126-3p, miR-122-5p, miR-146a-5p, miR-145-5p, miR-26a-5p, miR-150-5p, miR-222-3p, and miR-181a-5p, induce inflammation by promoting the release of IL-6, TNF-α, IL-1β, and MIP-2 and by facilitating neutrophil migration. Li et al reported that plasma EVs enriched with miR-210-3p exacerbate inflammation and apoptosis in THP-1 macrophages and the human bronchial epithelium cell line (BEAS-2B) cells by targeting autophagy-related 7 (ATG7), which inhibits autophagy and contributes to enhanced inflammatory responses.154 Plasma-derived exosomes also indicate that miR-1-3p increases IL-1β and iNOS levels by downregulating the stress-related ER protein stress-associated endoplasmic reticulum protein 1 (SERP1).155 Jiang et al found that miR-155 promotes inflammation in a sepsis-associated acute lung injury mouse model by activating macrophages.156 In vitro, miR-155 targets SHIP1, enhancing M1 macrophage proliferation, and it also targets suppressor of cytokine signaling 1 (SOCS1), increasing pro-inflammatory cytokines such as IL-6 and TNF-α.157 Furthermore, Murao et al demonstrated that exosomes in sepsis serum express high levels of extracellular cold-inducible RNA-binding protein (eCIRP), which induces IL-6 and TNF-α production and neutrophil migration.156
EVs play a dual role in sepsis, exhibiting both pro-inflammatory and anti-inflammatory effects. Recent studies, such as those by Gao et al, have shown that serum-derived exosomes from septic mice not only enhance Th1/Th2 cell differentiation but also promote lymphocyte proliferation and migration.158 Notably, pre-treatment with septic serum exosomes led to a decrease in TNF-α and IL-10, with a more pronounced reduction in TNF-α, suggesting an anti-inflammatory effect of these exosomes.157 Similarly, Appiah et al reported that EVs derived from intestinal epithelial cells in septic mice alleviate mucosal inflammation by inhibiting TNF-α and IL-17A expression.159 Due to their low toxicity, reduced immunogenicity compared to stem cells, and exhibiting good circulatory stability, EVs are being explored as biomimetic drug delivery platforms and bioactive nanomedicines for treating conditions like cancer, acute lung injury (ALI), and sepsis.160,161
EVs derived from mesenchymal stem cells (MSCs) are a major focus in sepsis treatment. Exosomes from bone marrow-derived MSCs (BMMSCs) mitigate inflammation by suppressing hypoxia-inducible factor 1α (HIF-1α) and inhibiting M1 polarization while promoting M2 polarization.162 Liu et al demonstrated that miR-191 in BMMSC-EVs diminishes macrophage inflammatory responses by targeting death-associated protein kinase 1 (DAPK1).163 Chen et al reported that small EVs from human umbilical MSCs (huMSCs) increase antioxidant enzyme IκB levels and inhibit the MAPK/NF-κB pathway, thereby reducing microvascular permeability and neutrophil infiltration in the lungs, thereby mitigating inflammation.164 Deng et al found that exosomes from adipose tissue-derived MSCs (ADMSCs), BMMSCs, and huMSCs all suppress macrophage glycolysis, decrease pro-inflammatory factor synthesis, and alleviate lung damage.165 Among these, ADMSC-derived exosomes showed the most pronounced protective effects. Zhou et al confirmed in a CLP sepsis model that exosomes from endothelial progenitor cells (EPCs) lower plasma cytokine and chemokine levels by promoting the release of miR-126-3p and miR-126-5p.161 These miRNAs also inhibit HMGB1 and VCAM1 delivery, thereby reducing inflammation and pulmonary vascular permeability.166 Inhibition of alveolar epithelial cell apoptosis, a critical mechanism in ALI, aids in lung function recovery.167 Jiang et al showed that miR-125b-5p in exosomes from brain microvascular endothelial cells inhibits topo IIα, reducing lung tissue inflammation and apoptosis.168 Mizuta et al found that ADMSC-derived exosomes activate the PI3K/Akt pathway through miR-126 transport, thereby reducing endothelial cell apoptosis.169 Similarly, lncRNA-p21 in BMMSC-derived exosomes suppresses alveolar epithelial cell apoptosis by upregulating sirtuin 1 (SIRT1) and downregulating miR-181.170 Shen et al reported that circRNA-Fryl in ADMSC-derived exosomes regulates the miR-490-3p/SIRT3 pathway to inhibit inflammatory factor expression and alveolar epithelial cell apoptosis.171
Myocardial injury is a severe complication of sepsis, closely associated with poor patient outcomes.172,173 The mortality rate significantly increases when patients with sepsis experience myocardial damage.174 Thus, mitigating myocardial injury and promoting cardiac function recovery are crucial for reducing sepsis-related mortality. Wang et al demonstrated that BMMSC-derived exosomes (BMMSC-Exos) alleviate myocardial inflammation and apoptosis by transferring miR-223, which downregulates Sema3A and Stat3.175 Similarly, Pei et al found that miR-141 in BMMSC-Exos exerts cardioprotective effects by regulating the PTEN/β-catenin axis.176 Sun et al recently observed elevated levels of miR-24-3p in M2 macrophage-derived exosomes, which protect myocardial cells by downregulating tumor necrosis factor superfamily member 10 (Tnfsf10), thereby improving cardiac function in damage caused by sepsis.177 Tu et al reported that heat shock protein A12B, predominantly expressed in human umbilical vein endothelial cell (HUVEC)-derived exosomes, inhibits NF-κB activation and translocation in macrophages, reducing their pro-inflammatory activity.178
Acute kidney injury (AKI) affects approximately 60% of sepsis patients, significantly impacting mortality rates and hospital stays.179,180 Adipose-derived mesenchymal stem cell-derived exosomes (ADMSC-Exos) have been shown to mitigate sepsis-induced AKI by activating the SIRT1 pathway, which reduces inflammation, apoptosis, and improves microcirculation.181 Sun et al demonstrated that miR-27b in BMMSC-Exos modulates the JMJD3/NFκB/p65 axis, suppressing pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.182 Moreover, miR-146b in huMSC-Exos alleviates renal injury by downregulating IRAK1 and inhibiting NF-κB activation.183 Zhang et al compared ADMSC-Exos and BMMSC-Exos in a sepsis rat model, finding that ADMSC-Exos were more effective in reducing inflammation and oxidative stress.184 He et al identified that miR-93-5p in endothelial progenitor cell-derived exosomes (EPC-EVs) inhibits renal inflammation, apoptosis, and vascular leakage via the KDM6B/H3K27me3/TNF-α axis, while also reducing histological damage to the kidneys, liver, and lungs.185 Table 3 summarizes the nanotherapeutic potential of natural EVs in sepsis.162–166,168–171,175–178,181–186
Table 3.
Nanotherapeutic Potential of Natural EVs in Sepsis
| Target Organ | Source of EVs/Exosomes/MVs | Delivered Molecules | Signaling Pathways/Target Molecules | Mechanism | References |
|---|---|---|---|---|---|
| Lung | BMMSC-Exos | - | HIF-1α↑ | Reduce the inflammatory response | [162] |
| Lung | BMMSC-EVs | miR-191 | DAPK1↓ | Attenuate macrophages inflammatory response | [163] |
| Lung | huMSC-EVs | Not provide | Anti-oxidative enzymes↑, MAPK/NF-κB pathway↓ | Improve pulmonary microvascular permeability, inhibit neutrophil infiltration in lung tissue | [164] |
| Lung | ADMSC-Exos, BMMSC-Exos, huMSC-Exos | Not provide | Not provide | Inhibit glycolysis of macrophages and reduce the synthesis of pro-inflammatory factors | [165] |
| Lung | EPC-Exos | miR-126-3p, miR-126-5p | IL-6, IFNγ, TNF-α, HMGB1 and VCAM1↓ | Reduce the inflammatory response, attenuate vascular permeability | [166] |
| Lung | Cerebral microvascular endothelial cell-Exos | miR-125b-5p | Topoisomerase II alpha↓ | Inhibit inflammatory factors infiltration and alleviate apoptosis | [168] |
| Lung | ADMSC-Exos | miR-126 | PI3K/Akt pathway | Suppress endothelial apoptosis | [169] |
| Lung | BMMSC-Exos | lncRNA-p21 | SIRT1↑, miR-181↓ | Inhibit apoptosis of pulmonary epithelial cells | [170] |
| Lung | ADMSC-Exos | circ-Fryl | miR-490-3p/ SIRT3 pathway | Inhibit the expression of inflammatory factors and apoptosis of alveolar epithelial cells | [171] |
| Lung and kidney | Granulocyte-MVs | Not provide | uPAR↑ | Reduce clot formation | [186] |
| Myocardium | BMMSC-Exos | miR-223 | Sema3A and Stat3↓ | Reduce the inflammatory response and suppress cardiomyocyte apoptosis | [175] |
| Myocardium | BMMSC-Exos | miR-141 | PTEN/β-catenin axis | Reduce the inflammatory response and cardiomyocyte apoptosis | [176] |
| Myocardium | M2 macrophages-Exos | miR-24-3p | Tnfsf10↓ | Reduce the inflammatory response and cardiomyocyte apoptosis | [177] |
| Myocardium | HUVEC-Exos | HSPA12B | NF-κB activation and nuclear translocation↓ | Attenuate macrophages inflammatory response | [178] |
| Kidney | ADMSC-Exos | Not provide | SIRT1 pathway | Inhibit inflammation, apoptosis and improve microcirculation in kidney | [181] |
| Kidney | BMMSC-Exos | miR-27b | JMJD3/NFκB/p65 axis | Reduce pro-inflammatory cytokines | [182] |
| Kidney | huMSC-Exos | miR-146b | IRAK1↓, NF-κB↓ | Inhibit inflammation, renal tubular cells apoptosis, and improve kidney function | [183] |
| Kidney | ADMSC-Exos, BMMSC-Exos | Not provide | Not provide | Inhibit inflammation, oxidative stress and apoptosis | [184] |
| Kidney | EPC-EVs | miR-93-5p | KDM6B/H3K27me3/TNF-α axis | Inhibit inflammation, apoptosis, vascular leakage in kidney, and reduce organ damage | [185] |
Abbreviations: BMMSC-Exos, BMMSC-derived exosomes; huMSC, human umbilical MSC; ADMSC, adipose tissue-derived MSC; EPC, endothelial progenitor cell; Tnfsf10, tumor necrosis factor superfamily member 10; HUVEC, human umbilical vein endothelial cell.
Engineered EVs are gaining traction due to their enhanced targeting capabilities. Genetic modifications in donor cells have emerged as a promising sepsis therapy. Engineered EVs, through gene or protein modifications, exhibit anti-inflammatory and anti-apoptotic effects. Zhou et al enhanced PTEN-induced kinase 1 (PINK1) expression in huMSC-Exos by transfecting Pink1 siRNA, providing cardioprotection via the PINK1-PKA-NCLX axis.187 Li et al engineered MSC-Exos to overexpress CircRTN4, which modulates the miR-497-5p/MG53 axis to reduce inflammation and cardiomyocyte apoptosis.188 Ding et al used siCCR2 in macrophage-derived EVs to silence C-C receptor 2 (CCR2), reducing monocyte mobilization and chemotaxis.189 Sun et al demonstrated that EVs can deliver drugs like curcumin specifically to inflamed tissues, reducing lung inflammation by downregulating CD11b+Gr-1+ cells.190 Gao et al used nitrogen cavitation to prepare EVs, showing that nitrogen cavitated neutrophil-derived EVs can deliver paclitaxel and inhibit neutrophil infiltration.191 Choi et al applied EXPLOR technology, a method for loading super-suppressor IkB (srIkB) into exosomes from 293T cells, to suppress inflammation and renal tubular apoptosis.192 Preconditioning strategies, such as LL-37 for neutrophils and IL-1β for MSCs, enhance EV secretion and therapeutic efficacy.193,194 Pan et al observed that remote ischemic preconditioning significantly increased miR-21 in Exos, reducing renal cell apoptosis and inflammation by modulating the PDCD4/NF-κB and PTEN/AKT pathways.195 Zhu et al found that miR-142-5p reduced pro-inflammatory factors and neutrophil infiltration, alleviating pulmonary edema.196 Additionally, hypoxia-preconditioned ADMSC-Exos promoted mmu_circ_0001295 expression, improving renal function and reducing inflammation.197 Figure 5 illustrates the roles of EVs in modulating immune responses during sepsis, underscoring their potential as therapeutic agents in the management of this condition.
Figure 5.
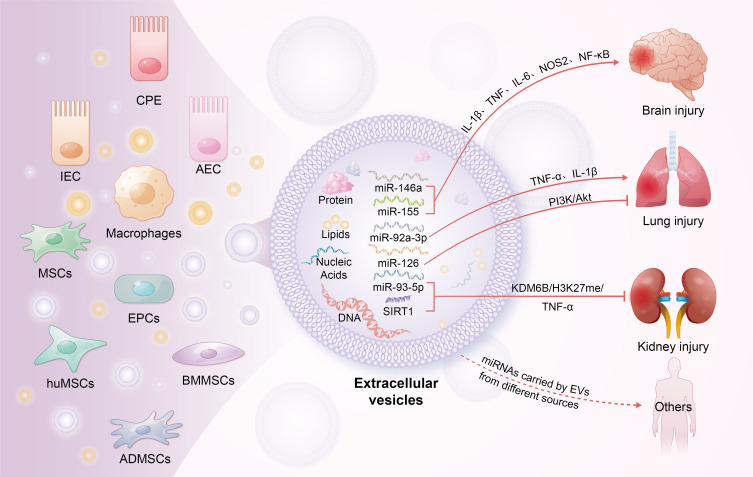
Roles of EVs in immune modulation during sepsis. This figure highlights the critical functions of EVs originating from diverse cell types in modulating immune responses during sepsis. EVs transmit signals between cells by transporting specific proteins, lipids, and nucleic acids, thereby regulating the activity of T cells, B cells, and endothelial cells. They modulate both pro-inflammatory and anti-inflammatory signaling pathways and are implicated in the damage or protective mechanisms of multiple organs in sepsis. By modulating intercellular communication, EVs affect organ function, vascular permeability, and tissue repair processes.
Abbreviation: CPE, choroid plexus epithelium; IECs, intestinal epithelial cells; AECs, alveolar epithelial cells; MSCs, mesenchymal stem cells; EPCs, endothelial progenitor cells; huMSCs, human mesenchymal stem cells; BMMSCs, bone marrow mesenchymal stem cells; ADMSCs, adipose-derived mesenchymal stem cells.
Nanopeptides in Sepsis Therapy
Peptides, which are chains of up to 50 amino acids linked by amide bonds, are increasingly utilized as nanomaterials in therapeutic applications due to their unique properties at the nanolevel.198–200 AMPs are especially effective against sepsis-related infections due to their diverse mechanisms, including direct bacterial membrane disruption, modulation of host immune responses, and a minimal propensity for resistance development. These features make AMPs a compelling alternative to traditional antibiotics.201 AMPs target bacterial pathways often bypassed by conventional treatments, thereby mitigating resistance.202 Despite the cataloging of more than 3000 AMPs (eg, http://aps.unmc.edu/AP/), their clinical application remains limited due to challenges such as stability, delivery, and cost. Anti-inflammatory peptides (AIPs) also hold significant promise in sepsis therapy by targeting specific inflammatory pathways to reduce tissue and organ damage, thereby improving outcomes.203–207 Recent advancements in AIP-based nanostructures have led to significant improvements in therapeutic properties, including enhanced neutralization of pro-inflammatory molecules, improved biodegradability, and more efficient delivery systems.208–211 For instance, Tram et al developed a dual-active peptide nanostructure that assembles into amyloid-like networks in response to endotoxins.209 This structure effectively neutralizes cytokines such as TNF-α and IL-6, thereby mitigating cytokine storms and enhancing both stability and circulation time. The integration of AIPs into nanostructures markedly boosts their efficacy in sepsis management. The current research on nanopeptides in sepsis models is summarized in Table 4.198–211
Table 4.
Summary of Current Research on Nanopeptides in Sepsis Models
| Nanomaterial | Mechanism | Sepsis Model | Beneficial Effects | Detrimental Effects | References |
|---|---|---|---|---|---|
| AMPs | Direct bacterial membrane disruption; modulation of host immune responses | Sepsis-related infections | Effective against infections; mitigate antibiotic resistance | Limited clinical application due to stability, delivery, and cost challenges | [182–198] |
| AIPs | Target specific inflammatory pathways; enhance neutralization of pro-inflammatory molecules | General sepsis models | Reduce tissue and organ damage; improve outcomes | Data not available | [203–211] |
| Dual-active peptide nanostructure | Assembles into amyloid-like networks; neutralizes cytokines (TNF-α, IL-6) | Endotoxin-induced sepsis models | Mitigates cytokine storms; enhances stability and circulation time | Data not available | [209] |
Abbreviations: AMPs, antimicrobial peptides; AIPs, anti-inflammatory peptides.
Cell-Free DNA Modulation in Sepsis and Nanoparticle-Based Gene Therapy
Studies have highlighted the critical role of cell-free DNA (cfDNA) in modulating TLR9-mediated pro-inflammatory cascades in severe sepsis. cfDNA can activate TLR9, leading to the production of pro-inflammatory cytokines and the exacerbation of sepsis. Therefore, neutralizing cfDNA could potentially attenuate excessive immune responses and improve the treatment of sepsis.212 Dawulieti et al synthesized polyethyleneimine (PEI)-functionalized mesoporous silica nanoparticles (MSNPs, 150 nm), which bind to and clear cfDNA.213 The cfDNA clearance activity of PEI-MSNPs can suppress cfDNA-induced inflammation, reduce serum cytokines (TNF-α, IL-6, and MCP-1), and mitigate organ damage. In another study, He et al designed an α-helical peptide, PPABLG, capable of encapsulating TNF-α siRNA for sepsis gene immunotherapy.214 To enhance the stability and electrostatic interactions of the nanoparticles, they incorporated an additional anionic peptide, PAOBLG-MPA, resulting in the formation of PPABLG mixed nanoparticles. The amphiphilic helical PPABLG HNPs showed enhanced membrane disruption and endosomal escape compared to non-helical PPABLG HNPs, resulting in more efficient transfection of TNF-α siRNA compared to the traditional Lipofectamine 2000 reagent. Systemic administration of helical PPABLG HNPs carrying TNF-α siRNA significantly reduced pro-inflammatory responses and rescued the 50% of animals from LPS/D-GalN-induced liver sepsis.214 This research indicates that RNA interference holds promise as an anti-inflammatory gene therapy approach, potentially circumventing the immune-related side effects commonly seen with traditional cytokine antagonists or TLR signaling inhibitors. The continued development of adaptive nanoparticle-based gene delivery systems is essential for maximizing therapeutic efficacy in sepsis, paving the way for innovative nanomedicine strategies in sepsis management.
Multimodal Nanomedicine Combination Therapy Strategies
The synergy between nano-immunomodulators and nano-antimicrobial agents represents a promising multimodal therapeutic strategy with the potential to mitigate antibiotic resistance and enhance treatment efficacy in sepsis. Nano-immunomodulators, which modulate the host immune response, can complement nano-antimicrobial agents that directly target and neutralize pathogens.
For instance, Friedman et al recently demonstrated that nanoparticles composed of chitosan and alginate exhibit direct bactericidal activity against Propionibacterium acnes, a bacterium implicated in the pathogenesis of acne. These nanoparticles also exhibit anti-inflammatory properties by suppressing cytokine production induced by Propionibacterium acnes.215 Furthermore, Ajish et al developed a hybrid peptide, Lf-KR, by combining the antimicrobial peptides LfcinB6 and KR-12-a4. This hybrid peptide significantly inhibited the expression and production of nitric oxide and TNF-α in LPS-stimulated RAW264.7 mouse macrophages while demonstrating potent eradication effects against pre-formed multidrug-resistant Pseudomonas aeruginosa biofilms. This combination enhances antibacterial, anti-inflammatory, and antibiofilm activities.216
Similarly, Shin et al utilized computational modeling to design two peptides, Ak-N’ and Ak-N’m, which exhibited significant inhibitory effects against both Gram-negative and Gram-positive bacteria.217 These peptides also downregulated pro-inflammatory mediators, including TNF-α, IL-1β, and IL-6. Recent studies have introduced multifunctional styrene-ethylene-butylene-styrene/silver nanowire (SEBS/AgNWs) composite membranes, which exhibit antibacterial, antioxidative, and anti-inflammatory properties. The SEBS/AgNWs significantly inhibited the growth of Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Escherichia coli, effectively neutralizing hydrogen peroxide (H2O2) and hydroxyl radicals and showcasing robust ROS scavenging abilities. Additionally, SEBS/AgNWs reduced the expression of IL-1β, IL-6, and TNF-α while enhancing levels of transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and CD31 in wound healing contexts.218
This combined approach addresses the critical issue of antibiotic resistance by potentially reducing reliance on conventional antibiotics, as the enhanced immune response may lessen the need for high doses of these medications. Nano-immunomodulators can bolster the host’s immune response, thereby diminishing the necessity for extensive antibiotic use and decreasing selective pressure on pathogens.219,220 Moreover, integrating these strategies facilitates a more effective and tailored treatment approach, simultaneously targeting pathogen eradication through nano-antimicrobial agents and enhancing immune system support via nano-immunomodulators. This dual approach promises to improve patient outcomes in sepsis management by providing a more comprehensive treatment regimen.
Safety and Clinical Translation Research
The safety of nano-immunomodulators is a crucial aspect to consider as these novel agents advance through clinical evaluation. Recent studies have highlighted that, while nano-immunomodulators offer promising therapeutic potential, their safety profiles require thorough assessment due to unique properties inherent in nanomaterials. Nanoparticles can exhibit altered biodistribution, such as increased accumulation in specific organs, such as the liver and spleen, and may have prolonged clearance times compared to conventional drugs, potentially leading to unexpected adverse effects. For instance, the potential for organ-specific toxicity, such as liver and kidney damage, has been reported in several preclinical models.221,222 Additionally, the long-term biocompatibility of these agents remains a concern, with studies indicating possible chronic inflammatory responses or autoimmune reactions resulting from prolonged exposure to certain nanomaterials.223 The risk of nanoparticle-induced cytotoxicity, immunogenicity, and potential for bioaccumulation necessitates comprehensive risk assessment and ongoing surveillance in clinical settings. To establish safety profiles, rigorous preclinical testing, including in vivo toxicity studies and biodistribution analysis, is essential before advancing to human trials. Balancing the therapeutic benefits with these safety considerations is paramount for the successful integration of nano-immunomodulators into clinical practice.
The translation of nano-immunomodulators from basic research to clinical practice involves a complex interplay of scientific innovation, regulatory approval, and practical application. Initial studies often focus on the mechanistic understanding and efficacy of these agents in preclinical models, demonstrating their potential to modulate immune responses and improve outcomes in sepsis.224
Historically, nanoparticles have been primarily utilized in cancer treatment, with limited applications in sepsis. However, recent advancements have led to several Food and Drug Administration (FDA)-approved antibiotic nanoparticles entering clinical use. Notably, MGF-AuNPs, which are among the most widely used metal nanoparticles approved by the FDA, exhibit antibacterial activity through various mechanisms, including the modulation of gene expression, alteration of cellular signaling (via dephosphorylation of tyrosine residues in proteins), generation of ROS, and membrane damage leading to microbial death.225
Encouragingly, nanoparticles have made significant strides in both clinical applications and the FDA approval processes in recent years. For example, Cimzia® effectively reduces autoimmune responses by blocking TNF-α, while Copaxone® suppresses inflammation by altering T cell phenotype.226 Many nanoformulations based on existing patents have successfully obtained authorization and are now available on the market.226
However, translating these findings into clinical practice requires overcoming significant challenges, including the challenges of scaling up production such as maintaining uniform nanoparticle size and surface characteristics, ensuring consistent quality, and addressing regulatory hurdles. For instance, the development of standardized manufacturing processes and rigorous quality control measures are critical for maintaining the reproducibility and safety of nano-immunomodulators.227 Furthermore, navigating the regulatory landscape poses a substantial challenge, as regulatory bodies such as FDA and European Medicines Agency (EMA) require extensive data on safety, efficacy, long-term impacts, and adherence to specific guidelines such as Good Manufacturing Practices (GMP), which often necessitates extensive clinical trials and complex documentation.221 Additionally, integrating these novel therapies into existing clinical workflows can be achieved through pilot studies, collaboration with clinical centers, and demonstrating clear, quantifiable advantages over current treatments in terms of efficacy, safety, and cost-effectiveness. Bridging the gap between laboratory discoveries and clinical applications requires interdisciplinary collaboration, continued research efforts, and a strategic approach to addressing these multifaceted challenges.
Future Directions
The development of nanolevel immunomodulators for sepsis treatment demonstrates considerable potential to reshape therapeutic strategies. However, the transition of these technologies from experimental models to clinical settings encounters significant challenges. One primary limitation is the complexity of nanoparticle design needed to achieve precise targeting specificity while minimizing off-target effects. Although recent advancements have been promising, the heterogeneous nature of sepsis in patient populations poses a considerable barrier to the universal application of these technologies.228,229 Additionally, while ligand-functionalized nanoparticles that target TLRs offer an innovative approach to modulate immune responses selectively, the variability in patient immunology may reduce the efficacy of such targeted treatments in broader clinical applications.227
Moving forward, addressing these limitations will require a focused research agenda. Future studies should prioritize the evolution of intelligent nanomaterials capable of adapting in real-time response to the dynamic biomarkers of sepsis, such as cytokines and PAMPs. This approach involves designing nanoparticles that not only detect these biomarkers but also respond by releasing therapeutic agents specifically when and where they are needed, potentially enhancing the effectiveness of the intervention.230 Additionally, the integration of artificial intelligence (AI) and machine learning algorithms to analyze patient-specific data, such as genomics and proteomics, offers a pathway to develop personalized treatment regimens tailored to individual immunological profiles. This could significantly enhance the precision of sepsis management.
Furthermore, a major focus should be on ensuring the safety and biocompatibility of these advanced nanomaterials. Robust preclinical testing followed by phased clinical trials is crucial to validate the clinical utility of these innovations. Successfully addressing these challenges will be pivotal for advancing nanolevel immunomodulators from theoretical constructs to practical and effective tools in sepsis treatment and ultimately improving outcomes in this complex clinical area.
Conclusion
In conclusion, this review emphasizes the transformative potential of nanolevel immunomodulators in sepsis, highlighting their potential as innovative therapeutic agents. By examining the unique immunological profiles of sepsis across various patient demographics and exploring various nano-immunotherapy strategies, this study identifies promising avenues for enhancing both treatment efficacy and safety. Future research should prioritize advancement in nanotechnology design to optimize bioavailability and therapeutic outcomes. Additionally, investigating the synergistic effects of nanolevel immunomodulators in multimodal therapeutic regimens could significantly advance treatment paradigms. Translating these innovations into clinical practice holds substantial promise for enhancing patient outcomes in sepsis management, representing a crucial step toward personalized and effective healthcare solutions.
Acknowledgments
The authors thank all the clinical staff contributed to the study. Liangkang Lin and Hanyou Liu are co-first authors.
Funding Statement
This research was supported by the National Key Research and Development Project (No. 2021YFC2701705).
Abbreviations
SIRS, systemic inflammatory response syndrome; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; ICAM-1, intracellular adhesion molecule 1; LFA-1, leukocyte function-associated antigen 1; Mac-1, Macrophage-1 antigen; PSGL-1, P-selectin glycoprotein ligand-1; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; IL, interleukin; TNF-α, tumor necrosis factor-α; NLRs, NOD-like receptors; MAPK, mitogen-activated protein kinases; PKC, protein kinase C; DIC, disseminated intravascular coagulation; Ts, S-thanatin; LEV, levofloxacin; PEG, polyethylene glycol; LTA, lipoteichoic acids; JAKs, Janus kinases; NF-κB, nuclear factor-kappa B; MyD88, Myeloid differentiation factor 88; TRAM, Toll-like receptor adaptor molecule; TRIF, Toll-interleukin 1 receptor-domain-containing adaptor inducing interferon-β; GNP, gold nanoparticle; MD-2, myeloid differential protein-2; Peptide-AuNP, peptide-gold nanoparticle; AP-1, activator protein-1; Cur-SLN, solid lipid nanoparticles loaded with curcumin; APS, astragalus polysaccharides; SPION, superparamagnetic iron oxide nanoparticles; PLGA, poly(lactic-co-glycolic acid); CXCL-1, C-X-C motif chemokine ligand 1; SAuNCs, sub-nanometer gold clusters; CDCs, carbon-derived carbons; PRRs, pattern recognition receptors; SiO₂NPs, Silica nanoparticles; ROS, reactive oxygen species; ASC, Apoptosis-associated Speck-like Protein Containing a CARD; MWCNTs, multiwalled carbon nanotubes; CN, chitosan-based nanoparticle; Arg-1, Arginase-1; STAT-1, signal transducer and activator of transcription-1; GSDMD, gasdermin D; DSF-LF NPs, disulfiram-lactoferrin nanoparticle complexes; LF, lactoferrin; LRP-1, low-density lipoprotein receptor-related protein-1; siHMGB1, small interfering RNA targeting High Mobility Group Box 1; Bi-QCS, biotin-azide-quaternary ammonium salt 188-chitosan; AMPs, antimicrobial peptides; MSPAM, mannose-functionalized disulfide-crosslinked polyethyleneimine nanoparticle complex; MPLA, monophosphoryl lipid A; MDP, muramyl dipeptide; NOD1, nucleotide-binding oligomerization domain-containing protein 1; PHBV, poly (3-hydroxybutyrate-co-3-hydroxyvalerate); PLGA, poly (lactic-co-glycolic acid); CLP, cecal ligation and puncture; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; CEP, cephalosporin hydroxyampicillin; NLCs, nanolipid carriers; MΦ-NP, macrophage-mimetic nanoparticles; LncRNAs, long non-coding RNAs; siRNAs, small interfering RNAs; ASOs, antisense oligonucleotides; VCAM-1, vascular cell adhesion molecule-1; MGF-AuNPs, mangosteen-functionalized gold nanoparticles; TRAF1, TNF receptor-associated factor 1; CK, compound K; GNP-CK-CopA3, peptide CopA3; Hgm, hydrolyzed galactomannan; SR-A, A scavenger receptor; METC, mitochondrial electron transport chain; IONPs, iron oxide NPs; CDs, carbon dots; INPs, immune-modulatory NPs; MWCNTs, multi-walled carbon nanotubes; NLRP3, NOD-like receptor family pyrin domain-containing 3; DOX, doxorubicin; TNF, tumor necrosis factor; MAC, membrane attack complex; RONS, reactive oxygen and nitrogen species; APC, activated protein C; SFn, short ferritin; PC-Gla, γ-carboxyglutamic acid from protein C; TRAP, thrombin receptor activating peptide; MMP, matrix metalloproteinase; EVs, extracellular vesicles; Exos, exosomes; MVs, microvesicles; SAE, sepsis-associated encephalopathy; IECs, intestinal epithelial cells; MLNs, mesenteric lymph nodes; CPE, choroid plexus epithelium; CSF, cerebrospinal fluid; AECs, alveolar epithelial cells; AMs, alveolar macrophages; PTEN, phosphatase and tensin homolog deleted on chromosome ten; BEAS-2B, human bronchial epithelium cell line; ATG7, autophagy-related 7; SERP1, stress-associated endoplasmic reticulum protein 1; SOCS1, suppressor of cytokine signaling 1; eCIRP, extracellular cold-inducible RNA-binding protein; ALI, acute lung injury; MSCs, mesenchymal stem cells; BMMSCs, bone marrow-derived MSCs; HIF-1α, hypoxia-inducible factor 1α; DAPK1, death-associated protein kinase 1; HuMSCs, human umbilical MSCs; ADMSCs, adipose tissue-derived MSCs; EPCs, endothelial progenitor cells; SIRT1, sirtuin 1; Tnfsf10, tumor necrosis factor superfamily member 10; HUVEC, human umbilical vein endothelial cell; AKI, acute kidney injury; ADMSC-Exos, adipose-derived mesenchymal stem cell-derived exosomes; EPC-EVs, endothelial progenitor cell-derived exosomes; PINK1, PTEN-induced kinase 1; CCR2, C-C receptor 2; srIkB, super-suppressor IkB; AIPs, anti-inflammatory peptides; cfDNA, cell-free DNA; PEI, polyethyleneimine; MSNPs, mesoporous Silica nanoparticles; SEBS/AgNWs, styrene-ethylene-butylene-styrene/silver nanowire; VEGF, vascular endothelial growth factor; FDA, Food and Drug Administration; EMA, European Medicines Agency; GMP, Good Manufacturing Practices; AI, artificial intelligence.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bone R. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17(2):175–181. doi: 10.1016/s0272-5231(05)70307-5 [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari N, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 3.Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist. 2017;10:204–212. doi: 10.1016/j.jgar.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 4.Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12(4):e10128. doi: 10.15252/emmm.201810128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12(6A):2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20(8):970–979. doi: 10.1038/s41590-019-0415-0 [DOI] [PubMed] [Google Scholar]
- 7.McDonald B, Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology. 2016;151(6):1087–1095. doi: 10.1053/j.gastro.2016.09.048 [DOI] [PubMed] [Google Scholar]
- 8.Assinger A, Schrottmaier WC, Salzmann M, Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front Immunol. 2019;10:1687. doi: 10.3389/fimmu.2019.01687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapik DM, Perera P, Nam J, et al. Mechanosignaling in bone health, trauma and inflammation. Antioxid Redox Signal. 2014;20(6):970–985. doi: 10.1089/ars.2013.5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376. doi: 10.3390/ijms20215376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27(6):669–684. [PMC free article] [PubMed] [Google Scholar]
- 12.Perl M, Chung C, Garber M, Huang X, Ayala A. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front Biosci. 2006;11:272–299. doi: 10.2741/1797 [DOI] [PubMed] [Google Scholar]
- 13.Boraschi D, Italiani P, Palomba R, et al. Nanoparticles and innate immunity: new perspectives on host defence. Semin Immunol. 2017;34:33–51. doi: 10.1016/j.smim.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 14.Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem Rev. 2017;117(17):11476–11521. doi: 10.1021/acs.chemrev.7b00194 [DOI] [PubMed] [Google Scholar]
- 15.Drexler K. Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc Natl Acad Sci U S A. 1981;78(9):5275–5278. doi: 10.1073/pnas.78.9.5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadikot RT. The potential role of nano- and micro-technology in the management of critical illnesses. Adv Drug Deliv Rev. 2014;77:27–31. doi: 10.1016/j.addr.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Fan J, Wang X, Wu P, Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front Pharmacol. 2015;6:249. doi: 10.3389/fphar.2015.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelaz B, Alexiou C, Alvarez-Puebla RA, et al. Diverse applications of nanomedicine. ACS Nano. 2017;11(3):2313–2381. doi: 10.1021/acsnano.6b06040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan WCW. Nanomedicine 2.0. Acc Chem Res. 2017;50(3):627–632. doi: 10.1021/acs.accounts.6b00629 [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Xiong Y, Li Q, Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beutler BA. TLRs and innate immunity. Blood. 2009;113(7):1399–1407. doi: 10.1182/blood-2008-07-019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedeva C, Menassa J, Puthalakath H. Sepsis: inflammation is a necessary evil. Front Cell Dev Biol. 2019;7:108. doi: 10.3389/fcell.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–794. doi: 10.1016/j.jconrel.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin P, Tam Y. Enhancing the pharmacokinetic/pharmacodynamic properties of therapeutic nucleotides using lipid nanoparticle systems. Future Med Chem. 2015;7(13):1751–1769. doi: 10.4155/fmc.15.108 [DOI] [PubMed] [Google Scholar]
- 28.Lonez C, Vandenbranden M, Ruysschaert JM. Cationic lipids activate intracellular signaling pathways. Adv Drug Deliv Rev. 2012;64(15):1749–1758. doi: 10.1016/j.addr.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Legat A, Adam E, et al. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through toll-like receptor 4. Eur J Immunol. 2008;38(5):1351–1357. doi: 10.1002/eji.200737998 [DOI] [PubMed] [Google Scholar]
- 30.Vangasseri DP, Cui Z, Chen W, et al. Immunostimulation of dendritic cells by cationic liposomes. Mol Member Biol. 2006;23(5):385–395. doi: 10.1080/09687860600790537 [DOI] [PubMed] [Google Scholar]
- 31.Piazza M, Calabrese V, Baruffa C, et al. The cationic amphiphile 3,4-bis(tetradecyloxy)benzylamine inhibits LPS signaling by competing with endotoxin for CD14 binding. Biochem Pharmacol. 2010;80(12):2050–2056. doi: 10.1016/j.bcp.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piazza M, Yu L, Teghanemt A, et al. Evidence of a specific interaction between new synthetic antisepsis agents and CD14. Biochemistry. 2009;48(51):12337–12344. doi: 10.1021/bi901601b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piazza M, Rossini C, Della Fiorentina S, et al. Glycolipids and benzylammonium lipids as novel antisepsis agents: synthesis and biological characterization. J Med Chem. 2009;52(4):1209–1213. doi: 10.1021/jm801333m [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez LJ, Sestito SE, Cighetti R, et al. Trehalose- and glucose-derived glycoamphiphiles: small-molecule and nanoparticle Toll-like receptor 4 (TLR4) modulators. J Med Chem. 2014;57(21):9105–9123. doi: 10.1021/jm501182w [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Fung S, Xu S, et al. Amino acid-dependent attenuation of toll-like receptor signaling by peptide-gold nanoparticle hybrids. ACS Nano. 2015;9(7):6774–6784. doi: 10.1021/nn505634h [DOI] [PubMed] [Google Scholar]
- 36.Taratummarat S, Sangphech N, Vu CTB, et al. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018;18(1):85. doi: 10.1186/s12866-018-1227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Kozicky L, Saferali A, et al. Endosomal pH modulation by peptide-gold nanoparticle hybrids enables potent anti-inflammatory activity in phagocytic immune cells. Biomaterials. 2016;111:90–102. doi: 10.1016/j.biomaterials.2016.09.032 [DOI] [PubMed] [Google Scholar]
- 38.Foit L, Thaxton CS. Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding toll-like receptor 4. Biomaterials. 2016;100:67–75. doi: 10.1016/j.biomaterials.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Xu Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male Sprague Dawley rats. Mater Sci Eng C Mater Biol Appl. 2018;83:148–153. doi: 10.1016/j.msec.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Wang H, Zhu R, et al. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1beta transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials. 2015;53:475–483. doi: 10.1016/j.biomaterials.2015.02.116 [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Rui S, Chen C, et al. Protective effects of astragalus polysaccharide nanoparticles on septic cardiac dysfunction through inhibition of TLR4/NF-kappaB signaling pathway. Int J Biol Macromol. 2020;153:977–985. doi: 10.1016/j.ijbiomac.2019.10.227 [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Li Y, Liu X, et al. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int J Nanomed. 2019;14:6779–6797. doi: 10.2147/IJN.S215055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spence S, Greene M, Fay F, et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7(303). doi: 10.1126/scitranslmed.aab3459 [DOI] [PubMed] [Google Scholar]
- 44.Babazada H, Yamashita F, Yanamoto S, Hashida M. Self-assembling lipid modified glycol-split heparin nanoparticles suppress lipopolysaccharide-induced inflammation through TLR4-NF-kappaB signaling. J Control Release. 2014;194:332–340. doi: 10.1016/j.jconrel.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 45.Ludwig R. Therapeutic use of heparin beyond anticoagulation. Curr Drug Discov Technol. 2009;6(4):281–289. doi: 10.2174/157016309789869001 [DOI] [PubMed] [Google Scholar]
- 46.Modlin R, Brightbill H, Godowski P. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340(23):1834–1835. doi: 10.1056/NEJM199906103402312 [DOI] [PubMed] [Google Scholar]
- 47.Herrmann IK, Urner M, Graf S, et al. Endotoxin removal by magnetic separation-based blood purification. Adv Healthc Mater. 2013;2(6):829–835. doi: 10.1002/adhm.201200358 [DOI] [PubMed] [Google Scholar]
- 48.Liao F, Wu T, Huang Y, et al. Subnanometer gold clusters adhere to Lipid A for protection against endotoxin-induced sepsis. Nano Lett. 2018;18(5):2864–2869. doi: 10.1021/acs.nanolett.7b05464 [DOI] [PubMed] [Google Scholar]
- 49.De Vriese A, Colardyn F, Philippé J, et al. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10(4):846–853. doi: 10.1681/ASN.V104846 [DOI] [PubMed] [Google Scholar]
- 50.Mas-Moruno C, Cascales L, Cruz LJ, et al. Nanostructure formation enhances the activity of LPS-neutralizing peptides. ChemMedChem. 2008;3(11):1748–1755. doi: 10.1002/cmdc.200800209 [DOI] [PubMed] [Google Scholar]
- 51.Yachamaneni S, Yushin G, Yeon SH, et al. Mesoporous carbide-derived carbon for cytokine removal from blood plasma. Biomaterials. 2010;31(18):4789–4794. doi: 10.1016/j.biomaterials.2010.02.054 [DOI] [PubMed] [Google Scholar]
- 52.Herrmann IK, Urner M, Koehler FM, et al. Blood purification using functionalized core/shell nanomagnets. Small. 2010;6(13):1388–1392. doi: 10.1002/smll.201000438 [DOI] [PubMed] [Google Scholar]
- 53.Presser V, Yeon SH, Vakifahmetoglu C, et al. Hierarchical porous carbide-derived carbons for the removal of cytokines from blood plasma. Adv Healthc Mater. 2012;1(6):796–800. doi: 10.1002/adhm.201200044 [DOI] [PubMed] [Google Scholar]
- 54.Tripisciano C, Kozynchenko OP, Linsberger I, et al. Activation-dependent adsorption of cytokines and toxins related to liver failure to carbon beads. Biomacromolecules. 2011;12(10):3733–3740. doi: 10.1021/bm200982g [DOI] [PubMed] [Google Scholar]
- 55.Seredych M, Haines B, Sokolova V, et al. Graphene-based materials for the fast removal of cytokines from blood plasma. ACS Appl Bio Mater. 2018;1(2):436–443. doi: 10.1021/acsabm.8b00151 [DOI] [PubMed] [Google Scholar]
- 56.Herrmann IK, Bernabei RE, Urner M, et al. Device for continuous extracorporeal blood purification using target-specific metal nanomagnets. Nephrol Dial Transplant. 2011;26(9):2948–2954. doi: 10.1093/ndt/gfq846 [DOI] [PubMed] [Google Scholar]
- 57.Herrmann IK, Schlegel AA, Graf R, Stark WJ, Beck-Schimmer B. Magnetic separation-based blood purification: a promising new approach for the removal of disease-causing compounds? J Nanobiotechnology. 2015;13(1):49. doi: 10.1186/s12951-015-0110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanakia S, Toussaint JD, Mullick Chowdhury S, et al. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials. 2014;35(25):7022–7031. doi: 10.1016/j.biomaterials.2014.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Ali SF, Dervishi E, et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano. 2010;4(6):3181–3186. doi: 10.1021/nn1007176 [DOI] [PubMed] [Google Scholar]
- 60.Mullick Chowdhury S, Lalwani G, Zhang K, Yang JY, Neville K, Sitharaman B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials. 2013;34(1):283–293. doi: 10.1016/j.biomaterials [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Zeng H, Zeng Z, Zeng Y, Xie T. Promising graphene-based nanomaterials and their biomedical applications and potential risks: a comprehensive review. ACS Biomater Sci Eng. 2021;7(12):5363–5396. doi: 10.1021/acsbiomaterials.1c00875 [DOI] [PubMed] [Google Scholar]
- 62.Ema M, Gamo M, Honda K. A review of toxicity studies on graphene-based nanomaterials in laboratory animals. Regul Toxicol Pharmacol. 2017;85:7–24. doi: 10.1016/j.yrtph.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 63.Feng X, Xu W, Li Z, et al. Immunomodulatory Nanosystems. Adv Sci. 2019;6(17):1900101. doi: 10.1002/advs.201900101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wesche-Soldato D, Swan R, Chung C, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8(4):493–500. doi: 10.2174/138945007780362764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C, Dong X, Gao J, et al. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964. doi: 10.1126/sciadv.aax7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YF, Chen GY, Chang CH, et al. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater Sci Eng C Mater Biol Appl. 2019;102:85–95. doi: 10.1016/j.msec.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 67.Lee NH, You S, Taghizadeh A, Taghizadeh M, Kim HS. Cell membrane-cloaked nanotherapeutics for targeted drug delivery. Int J Mol Sci. 2022;23(4). doi: 10.3390/ijms23042223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Poll T, Van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi: 10.1038/nri.2017.36 [DOI] [PubMed] [Google Scholar]
- 69.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15(1):123–147. doi: 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429 [DOI] [PubMed] [Google Scholar]
- 71.Robinson N, Ganesan R, Hegedus C, et al. Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26:101239. doi: 10.1016/j.redox.2019.101239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Sahoo M, Lantier L, et al. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog. 2018;14(5):e1007105. doi: 10.1371/journal.ppat.1007105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang D, Kang R, Coyne C, Zeh H, Lotze M. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–175. doi: 10.1111/j.1600-065X.2012.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malyala P, O’Hagan DT, Singh M. Enhancing the therapeutic efficacy of CpG oligonucleotides using biodegradable microparticles. Adv Drug Deliv Rev. 2009;61(3):218–225. doi: 10.1016/j.addr.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 75.Cabana-Brunod M, Herrera PA, Marquez-Miranda V, et al. Development of a PHBV nanoparticle as a peptide vehicle for NOD1 activation. Drug Deliv. 2021;28(1):1020–1030. doi: 10.1080/10717544.2021.1923862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu H, Luo R, Dong L, et al. pH/ROS dual-sensitive and chondroitin sulfate wrapped poly (beta-amino ester)-SA-PAPE copolymer nanoparticles for macrophage-targeted oral therapy for ulcerative colitis. Nanomedicine. 2022;39:102461. doi: 10.1016/j.nano.2021.102461 [DOI] [PubMed] [Google Scholar]
- 77.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39(3):229–246. doi: 10.3109/1040841X.2012.706249 [DOI] [PubMed] [Google Scholar]
- 78.Shirasuna K, Karasawa T, Takahashi M. Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes. J Cell Physiol. 2019;234(5):5436–5450. doi: 10.1002/jcp.27475 [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Lu B, Fu J, et al. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-κB signaling pathway in HUVEC cells. J Hazard Mater. 2021;404(Pt B):124050. doi: 10.1016/j.jhazmat.2020.124050 [DOI] [PubMed] [Google Scholar]
- 80.Palomäki J, Välimäki E, Sund J, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5(9):6861–6870. doi: 10.1021/nn200595c [DOI] [PubMed] [Google Scholar]
- 81.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ulbrich K, Hola K, Subr V, et al. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116(9):5338–5431. doi: 10.1021/acs.chemrev.5b00589 [DOI] [PubMed] [Google Scholar]
- 83.Ekladious I, Colson YL, Grinstaff MW. Polymer-drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18(4):273–294. doi: 10.1038/s41573-018-0005-0 [DOI] [PubMed] [Google Scholar]
- 84.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34(5):216–223. doi: 10.1016/j.it.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang X, Xing L, Wang Y, et al. Nanoengineered immunosuppressive therapeutics modulating M1/M2 macrophages into the balanced status for enhanced idiopathic pulmonary fibrosis therapy. Nanoscale. 2020;12(16):8664–8678. doi: 10.1039/d0nr00750a [DOI] [PubMed] [Google Scholar]
- 86.Jiang L, Wang Y, Wei X, et al. Improvement in phenotype homeostasis of macrophages by chitosan nanoparticles and subsequent impacts on liver injury and tumor treatment. Carbohydr Polym. 2022;277:118891. doi: 10.1016/j.carbpol.2021.118891 [DOI] [PubMed] [Google Scholar]
- 87.Sborgi L, Ruhl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ou AT, Zhang JX, Fang YF, et al. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol Sin. 2021;42(11):1913–1920. doi: 10.1038/s41401-021-00770-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou JE, Sun L, Liu L, et al. Hepatic macrophage targeted siRNA lipid nanoparticles treat non-alcoholic steatohepatitis. J Control Release. 2022;343:175–186. doi: 10.1016/j.jconrel.2022.01.038 [DOI] [PubMed] [Google Scholar]
- 90.Hongsa N, Thinbanmai T, Luesakul U, Sansanaphongpricha K, Muangsin N. A novel modified chitosan/collagen coated-gold nanoparticles for 5-fluorouracil delivery: synthesis, characterization, in vitro drug release studies, anti-inflammatory activity and in vitro cytotoxicity assay. Carbohydr Polym. 2022;277:118858. doi: 10.1016/j.carbpol.2021.118858 [DOI] [PubMed] [Google Scholar]
- 91.Haitao Y, Yifan C, Mingchao S, Shuaijuan H. A novel polymeric nanohybrid antimicrobial engineered by antimicrobial peptide MccJ25 and chitosan nanoparticles exerts strong antibacterial and anti-inflammatory activities. Front Immunol. 2021;12:811381. doi: 10.3389/fimmu.2021.811381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma L, Shen CA, Gao L, et al. Anti-inflammatory activity of chitosan nanoparticles carrying NF-kappaB/p65 antisense oligonucleotide in RAW264.7 macropghage stimulated by lipopolysaccharide. Colloids Surf B Biointerfaces. 2016;142:297–306. doi: 10.1016/j.colsurfb.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 93.Rajendrakumar SK, Revuri V, Samidurai M, et al. Peroxidase-mimicking nanoassembly mitigates lipopolysaccharide-induced endotoxemia and cognitive damage in the brain by impeding inflammatory signaling in macrophages. Nano Lett. 2018;18(10):6417–6426. doi: 10.1021/acs.nanolett.8b02785 [DOI] [PubMed] [Google Scholar]
- 94.Peled E, Sosnik A. Amphiphilic galactomannan nanoparticles trigger the alternative activation of murine macrophages. J Control Release. 2021;339:473–483. doi: 10.1016/j.jconrel.2021.10.017 [DOI] [PubMed] [Google Scholar]
- 95.Zhao H, Lv X, Huang J, et al. Two-phase releasing immune-stimulating composite orchestrates protection against microbial infections. Biomaterials. 2021;277:121106. doi: 10.1016/j.biomaterials.2021.121106 [DOI] [PubMed] [Google Scholar]
- 96.Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154-155:102–122. doi: 10.1016/j.addr.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 97.Tian M, Ticer T, Wang Q, et al. Adipose-derived biogenic nanoparticles for suppression of inflammation. Small. 2020;16(10):e1904064. doi: 10.1002/smll.201904064 [DOI] [PubMed] [Google Scholar]
- 98.Hou X, Zhang X, Zhao W, et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat Nanotechnol. 2020;15(1):41–46. doi: 10.1038/s41565-019-0600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kateh Shamshiri M, Jaafari MR, Badiee A. Preparation of liposomes containing IFN-gamma and their potentials in cancer immunotherapy: in vitro and in vivo studies in a colon cancer mouse model. Life Sci. 2021;264:118605. doi: 10.1016/j.lfs.2020.118605 [DOI] [PubMed] [Google Scholar]
- 100.Huang Y, Guan Z, Dai X, et al. Engineered macrophages as near-infrared light activated drug vectors for chemo-photodynamic therapy of primary and bone metastatic breast cancer. Nat Commun. 2021;12(1):4310. doi: 10.1038/s41467-021-24564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Z, Wang Z, Gu Z. Bioinspired and biomimetic nanomedicines. Acc Chem Res. 2019;52(5):1255–1264. doi: 10.1021/acs.accounts.9b00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khatoon N, Zhang Z, Zhou C, Chu M. Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater Sci. 2022;10(5):1193–1208. doi: 10.1039/d1bm01664d [DOI] [PubMed] [Google Scholar]
- 103.Lu C, Zheng J, Ding Y, et al. Cepharanthine loaded nanoparticles coated with macrophage membranes for lung inflammation therapy. Drug Deliv. 2021;28(1):2582–2593. doi: 10.1080/10717544.2021.2009936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gresham H, Dale B, Potter J, et al. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J Exp Med. 2000;191(3):515–528. doi: 10.1084/jem.191.3.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114(43):11488–11493. doi: 10.1073/pnas.1714267114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen S, Han F, Yuan A, et al. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. doi: 10.1016/j.biomaterials.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 107.Pei W, Li X, Bi R, et al. Exosome membrane-modified M2 macrophages targeted nanomedicine: treatment for allergic asthma. J Control Release. 2021;338:253–267. doi: 10.1016/j.jconrel.2021.08.024 [DOI] [PubMed] [Google Scholar]
- 108.Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. doi: 10.1016/j.jconrel.2021.11.019 [DOI] [PubMed] [Google Scholar]
- 109.Khoobchandani M, Khan A, Katti KK, et al. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci Rep. 2021;11(1):16797. doi: 10.1038/s41598-021-96224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Y, Liu X, Li Y, et al. SPION-MSCs enhance therapeutic efficacy in sepsis by regulating MSC-expressed TRAF1-dependent macrophage polarization. Stem Cell Res Ther. 2021;12(1):531. doi: 10.1186/s13287-021-02593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Perumalsamy H, Kang CH, et al. Intracellular synthesis of gold nanoparticles by Gluconacetobacter liquefaciens for delivery of peptide CopA3 and ginsenoside and anti-inflammatory effect on lipopolysaccharide-activated macrophages. Artif Cells Nanomed Biotechnol. 2020;48(1):777–788. doi: 10.1080/21691401.2020.1748639 [DOI] [PubMed] [Google Scholar]
- 112.Zhao X, Guo K, Zhang K, et al. Orchestrated yolk-shell nanohybrids regulate macrophage polarization and dendritic cell maturation for oncotherapy with augmented antitumor immunity. Adv Mater. 2022;34(9):e2108263. doi: 10.1002/adma.202108263 [DOI] [PubMed] [Google Scholar]
- 113.Kodali V, Littke MH, Tilton SC, et al. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano. 2013;7(8):6997–7010. doi: 10.1021/nn402145t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palomba R, Di Francesco M, Di Francesco V, et al. Boosting nanomedicine performance by conditioning macrophages with methyl palmitate nanoparticles. Mater Horiz. 2021;8(10):2726–2741. doi: 10.1039/d1mh00937k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casals E, Zeng M, Parra-Robert M, et al. Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small. 2020;16(20):e1907322. doi: 10.1002/smll.201907322 [DOI] [PubMed] [Google Scholar]
- 116.Selvaraj V, Manne ND, Arvapalli R, et al. Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-kappaB signaling in cultured macrophages. Nanomedicine (Lond). 2015;10(8):1275–1288. doi: 10.2217/nnm.14.205 [DOI] [PubMed] [Google Scholar]
- 117.Selvaraj V, Nepal N, Rogers S, et al. Inhibition of MAP kinase/NF-κB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saborano R, Wongpinyochit T, Totten JD, et al. Metabolic reprogramming of macrophages exposed to silk, Poly(lactic-co -glycolic acid), and silica nanoparticles. Adv. Healthcare Mater. 2017;6(14). doi: 10.1002/adhm.201601240 [DOI] [PubMed] [Google Scholar]
- 119.Ying H, Ruan Y, Zeng Z, et al. Iron oxide nanoparticles size-dependently activate mouse primary macrophages via oxidative stress and endoplasmic reticulum stress. Int Immunopharmacol. 2022;105:108533. doi: 10.1016/j.intimp.2022.108533 [DOI] [PubMed] [Google Scholar]
- 120.Weiss M, Fan J, Claudel M, et al. Combined in vitro and in vivo approaches to propose a putative adverse outcome pathway for acute lung inflammation induced by nanoparticles: a study on carbon dots. Nanomaterials. 2021;11(1):180. doi: 10.3390/nano11010180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiu H-W, Xia T, Lee Y-H, et al. Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale. 2015;7(2):736–746. doi: 10.1039/c4nr05509h [DOI] [PubMed] [Google Scholar]
- 122.Getts D, Terry R, Getts M, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6(219). doi: 10.1126/scitranslmed.3007563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lasola JJM, Cottingham AL, Scotland BL, et al. Immunomodulatory nanoparticles mitigate macrophage inflammation via inhibition of PAMP interactions and lactate-mediated functional reprogramming of NF-kappaB and p38 MAPK. Pharmaceutics. 2021;13(11):1841. doi: 10.3390/pharmaceutics13111841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Casey LM, Kakade S, Decker JT, et al. Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials. 2019;218:119333. doi: 10.1016/j.biomaterials.2019.119333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beyeler S, Steiner S, Wotzkow C, et al. Multi-walled carbon nanotubes activate and shift polarization of pulmonary macrophages and dendritic cells in an in vivo model of chronic obstructive lung disease. Nanotoxicology. 2020;14(1):77–96. doi: 10.1080/17435390.2019.1663954 [DOI] [PubMed] [Google Scholar]
- 126.Omori S, Tsugita M, Hoshikawa Y, et al. Tim4 recognizes carbon nanotubes and mediates phagocytosis leading to granuloma formation. Cell Rep. 2021;34(6):108734. doi: 10.1016/j.celrep.2021.108734 [DOI] [PubMed] [Google Scholar]
- 127.Nahrendorf M, Hoyer FF, Meerwaldt AE, et al. Imaging cardiovascular and lung macrophages with the positron emission tomography sensor (64)Cu-macrin in mice, rabbits, and pigs. Circ Cardiovasc Imaging. 2020;13(10):e010586. doi: 10.1161/CIRCIMAGING.120.010586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong R, Shou J, Wang Y. Probing sepsis and sepsis-like conditions using untargeted SPIO nanoparticles. Annu Int Conf IEEE Eng Med Biol Soc. 2010;3053–3056. doi: 10.1109/IEMBS.2010.5626123 [DOI] [PubMed] [Google Scholar]
- 129.Wu B, Lin L, Zhou F, Wang X. Precise engineering of neutrophil membrane coated with polymeric nanoparticles concurrently absorbing of proinflammatory cytokines and endotoxins for management of sepsis. Bioprocess Biosyst Eng. 2020;43(11):2065–2074. doi: 10.1007/s00449-020-02395-5 [DOI] [PubMed] [Google Scholar]
- 130.Li YR, Zhu H. Nanoceria potently reduce superoxide fluxes from mitochondrial electron transport chain and plasma membrane NADPH oxidase in human macrophages. Mol Cell Biochem. 2021;476(12):4461–4470. doi: 10.1007/s11010-021-04246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li M, van Esch B, Wagenaar GTM, et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 132.Cross D, Drury R, Hill J, Pollard AJ. Epigenetics in sepsis: understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front Immunol. 2019;10:1363. doi: 10.3389/fimmu.2019.01363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281(29):20077–20084. doi: 10.1074/jbc.M600506200 [DOI] [PubMed] [Google Scholar]
- 134.Zhao XY, Wilmen A, Wang D, et al. Targeted inhibition of activated protein C by a non-active-site inhibitory antibody to treat hemophilia. Nat Commun. 2020;11(1):2992. doi: 10.1038/s41467-020-16720-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee W, Seo J, Kwak S, et al. A double-chambered protein nanocage loaded with Thrombin Receptor Agonist Peptide (TRAP) and gamma-Carboxyglutamic Acid of Protein C (PC-Gla) for sepsis treatment. Adv Mater. 2015;27(42):6637–6643. doi: 10.1002/adma.201503093 [DOI] [PubMed] [Google Scholar]
- 136.Matthay M. Severe sepsis--a new treatment with both anticoagulant and antiinflammatory properties. N Engl J Med. 2001;344(10):759–762. doi: 10.1056/NEJM200103083441009 [DOI] [PubMed] [Google Scholar]
- 137.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004 [DOI] [PubMed] [Google Scholar]
- 138.Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9(3):204–210. doi: 10.1038/nnano.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Evans R, Lellouch AC, Svensson L, McDowall A, Hogg N. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood. 2011;117(12):3331–3342. doi: 10.1182/blood-2010-06-289140 [DOI] [PubMed] [Google Scholar]
- 140.Murao A, Brenner M, Aziz M, Wang P. Exosomes in sepsis. Front Immunol. 2020;11:2140. doi: 10.3389/fimmu.2020.02140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Niel G, Carter DRF, Clayton A, et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369–382. doi: 10.1038/s41580-022-00460-3 [DOI] [PubMed] [Google Scholar]
- 142.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xi S, Wang Y, Wu C, et al. Intestinal epithelial cell exosome launches IL-1beta-mediated neuron injury in sepsis-associated encephalopathy. Front Cell Infect Microbiol. 2021;11:783049. doi: 10.3389/fcimb.2021.783049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Balusu S, Van Wonterghem E, De Rycke R, et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. 2016;8(10):1162–1183. doi: 10.15252/emmm.201606271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin H, Chen H, Qi B, et al. Brain-derived extracellular vesicles mediated coagulopathy, inflammation and apoptosis after sepsis. Thromb Res. 2021;207:85–95. doi: 10.1016/j.thromres.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 146.Liu F, Peng W, Chen J, et al. Exosomes derived from alveolar epithelial cells promote alveolar macrophage activation mediated by miR-92a-3p in sepsis-induced acute lung injury. Front Cell Infect Microbiol. 2021;11:646546. doi: 10.3389/fcimb.2021.646546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li W, Deng M, Loughran PA, et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front Immunol. 2020;11:229. doi: 10.3389/fimmu.2020.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deng M, Tang Y, Li W, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753e747. doi: 10.1016/j.immuni.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li ZG, Scott MJ, Brzoska T, et al. Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Mil Med Res. 2018;5(1):24. doi: 10.1186/s40779-018-0173-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sui X, Liu W, Liu Z. Exosomes derived from LPS-induced MHs cells prompted an inflammatory response in sepsis-induced acute lung injury. Respir Physiol Neurobiol. 2021;292:103711. doi: 10.1016/j.resp.2021.103711 [DOI] [PubMed] [Google Scholar]
- 151.Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Zhao H, Xu H, et al. Targeting the TXNIP-NLRP3 interaction with PSSM1443 to suppress inflammation in sepsis-induced myocardial dysfunction. J Cell Physiol. 2021;236(6):4625–4639. doi: 10.1002/jcp.30186 [DOI] [PubMed] [Google Scholar]
- 154.Li G, Wang B, Ding X, et al. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med. 2021;53(7):1180–1191. doi: 10.1038/s12276-021-00651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao M, Yu T, Liu D, et al. Sepsis plasma-derived exosomal miR-1-3p induces endothelial cell dysfunction by targeting SERP1. Clin Sci (Lond). 2021;135(2):347–365. doi: 10.1042/CS20200573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murao A, Tan C, Jha A, Wang P, Aziz M. Exosome-mediated eCIRP release from macrophages to induce inflammation in sepsis. Front Pharmacol. 2021;12:791648. doi: 10.3389/fphar.2021.791648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang K, Yang J, Guo S, et al. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27(10):1758–1771. doi: 10.1016/j.ymthe.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao K, Jin J, Huang C, et al. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front Immunol. 2019;10:1560. doi: 10.3389/fimmu.2019.01560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Appiah MG, Park EJ, Darkwah S, et al. Intestinal epithelium-derived luminally released extracellular vesicles in sepsis exhibit the ability to suppress TNF-a and IL-17A expression in mucosal inflammation. Int J Mol Sci. 2020;21(22):8445. doi: 10.3390/ijms21228445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. 2018;15(9):3625–3633. doi: 10.1021/acs.molpharmaceut.8b00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Terrasini N, Lionetti V. Exosomes in critical illness. Crit Care Med. 2017;45(6):1054–1060. doi: 10.1097/CCM.0000000000002328 [DOI] [PubMed] [Google Scholar]
- 162.Deng H, Wu L, Liu M, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020;54(6):828–843. doi: 10.1097/SHK.0000000000001549 [DOI] [PubMed] [Google Scholar]
- 163.Liu H, Zhang L, Li M, et al. Bone mesenchymal stem cell-derived extracellular vesicles inhibit DAPK1-mediated inflammation by delivering miR-191 to macrophages. Biochem Biophys Res Commun. 2022;598:32–39. doi: 10.1016/j.bbrc.2022.02.009 [DOI] [PubMed] [Google Scholar]
- 164.Chen J, Li C, Liang Z, et al. Human mesenchymal stromal cells small extracellular vesicles attenuate sepsis-induced acute lung injury in a mouse model: the role of oxidative stress and the mitogen-activated protein kinase/nuclear factor kappa B pathway. Cytotherapy. 2021;23(10):918–930. doi: 10.1016/j.jcyt.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 165.Deng H, Zhu L, Zhang Y, et al. Differential lung protective capacity of exosomes derived from human adipose tissue, bone marrow, and umbilical cord mesenchymal stem cells in sepsis-induced acute lung injury. Oxid Med Cell Longev. 2022;2022:7837837. doi: 10.1155/2022/7837837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26(5):1375–1384. doi: 10.1016/j.ymthe.2018.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Y, Xiang D, Zhang H, Yao H, Wang Y. Hypoxia-inducible factor-1: a potential target to treat acute lung injury. Oxid Med Cell Longev. 2020;2020:8871476. doi: 10.1155/2020/8871476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiang L, Ni J, Shen G, et al. Upregulation of endothelial cell-derived exosomal microRNA-125b-5p protects from sepsis-induced acute lung injury by inhibiting topoisomerase II alpha. Inflamm Res. 2021;70(2):205–216. doi: 10.1007/s00011-020-01415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mizuta Y, Akahoshi T, Guo J, et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther. 2020;11(1):508. doi: 10.1186/s13287-020-02015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sui X, Liu W, Liu Z. Exosomal lncRNA-p21 derived from mesenchymal stem cells protects epithelial cells during LPS-induced acute lung injury by sponging miR-181. Acta Biochim Biophys Sin. 2021;53(6):748–757. doi: 10.1093/abbs/gmab043 [DOI] [PubMed] [Google Scholar]
- 171.Shen W, Zhao X, Li S. Exosomes derived from ADSCs attenuate sepsis-induced lung injury by delivery of circ-Fryl and regulation of the miR-490-3p/SIRT3 pathway. Inflammation. 2022;45(1):331–342. doi: 10.1007/s10753-021-01548-2 [DOI] [PubMed] [Google Scholar]
- 172.Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424–434. doi: 10.1038/s41569-020-00492-2 [DOI] [PubMed] [Google Scholar]
- 173.Stanzani G, Duchen MR, Singer M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865(4):759–773. doi: 10.1016/j.bbadis.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 174.Ravikumar N, Sayed MA, Poonsuph CJ, et al. Septic cardiomyopathy: from basics to management choices. Curr Probl Cardiol. 2021;46(4):100767. doi: 10.1016/j.cpcardiol.2020.100767 [DOI] [PubMed] [Google Scholar]
- 175.Wang X, Gu H, Qin D, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5(1):13721. doi: 10.1038/srep13721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pei Y, Xie S, Li J, Jia B. Bone marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets PTEN and activates beta-catenin to alleviate myocardial injury in septic mice. Immunopharmacol Immunotoxicol. 2021;43(5):584–593. doi: 10.1080/08923973.2021.1955920 [DOI] [PubMed] [Google Scholar]
- 177.Sun X, Liu Y, Wang J, Zhang M, Wang M. Cardioprotection of M2 macrophages-derived exosomal microRNA-24-3p/Tnfsf10 axis against myocardial injury after sepsis. Mol Immunol. 2022;141(5):309–317. doi: 10.1016/j.molimm.2021.11.003 [DOI] [PubMed] [Google Scholar]
- 178.Tu F, Wang X, Zhang X, et al. Novel role of endothelial derived exosomal HSPA12B in regulating macrophage inflammatory responses in polymicrobial sepsis. Front Immunol. 2020;11:825. doi: 10.3389/fimmu.2020.00825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26(3):245–253. doi: 10.1097/01.shk.0000225415.5969694.ce [DOI] [PubMed] [Google Scholar]
- 181.Gao F, Zuo B, Wang Y, et al. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719 [DOI] [PubMed] [Google Scholar]
- 182.Sun J, Sun X, Chen J, et al. microRNA-27b shuttled by mesenchymal stem cell-derived exosomes prevents sepsis by targeting JMJD3 and downregulating NF-kappaB signaling pathway. Stem Cell Res Ther. 2021;12(1):14. doi: 10.1186/s13287-020-02068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang R, Zhu Y, Li Y, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 2020;42(4):669–679. doi: 10.1007/s10529-020-02831-2 [DOI] [PubMed] [Google Scholar]
- 184.Zhang W, Zhang J, Huang H. Exosomes from adipose-derived stem cells inhibit inflammation and oxidative stress in LPS-acute kidney injury. Exp Cell Res. 2022;420(1):113332. doi: 10.1016/j.yexcr.2022.113332 [DOI] [PubMed] [Google Scholar]
- 185.He Z, Wang H, Yue L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-alpha axis. Exp Cell Res. 2020;395(2):112173. doi: 10.1016/j.yexcr.2020.112173 [DOI] [PubMed] [Google Scholar]
- 186.Cointe S, Vallier L, Esnault P, et al. Delivery of sirNA to the mouse brain by systemic injection of targeted exosomes. Blood. 2022;139(15):2377–2391. doi: 10.1182/blood.2021013328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Q, Xie M, Zhu J, et al. PINK1 contained in huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium overload in sepsis via recovery of mitochondrial Ca(2+) efflux. Stem Cell Res Ther. 2021;12(1):269. doi: 10.1186/s13287-021-02325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li J, Jiang R, Hou Y, Lin A. Mesenchymal stem cells-derived exosomes prevent sepsis-induced myocardial injury by a CircRTN4/miR-497-5p/MG53 pathway. Biochem Biophys Res Commun. 2022;618:133–140. doi: 10.1016/j.bbrc.2022.05.094 [DOI] [PubMed] [Google Scholar]
- 189.Ding L, Zhou W, Zhang J, et al. Calming egress of inflammatory monocytes and related septic shock by therapeutic CCR2 silencing using macrophage-derived extracellular vesicles. Nanoscale. 2022;14(13):4935–4945. doi: 10.1039/d1nr06922e [DOI] [PubMed] [Google Scholar]
- 190.Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Choi H, Kim Y, Mirzaaghasi A, et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6(15):eaaz6980. doi: 10.1126/sciadv.aaz6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35(5):1208–1221. doi: 10.1002/stem.2564 [DOI] [PubMed] [Google Scholar]
- 194.Yao M, Cui B, Zhang W, et al. Exosomal miR-21 secreted by IL-1beta-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658. doi: 10.1016/j.lfs.2020.118658 [DOI] [PubMed] [Google Scholar]
- 195.Pan T, Jia P, Chen N, et al. Delayed remote ischemic preconditioning confersrenoprotection against septic acute kidney injury via exosomal miR-21. Theranostics. 2019;9(2):405–423. doi: 10.7150/thno.29832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhu W, Huang X, Qiu S, et al. miR-142-5p encapsulated by serum-derived extracellular vesicles protects against acute lung injury in septic rats following remote ischemic preconditioning via the PTEN/PI3K/Akt axis. J Innate Immun. 2022;14(5):532–542. doi: 10.1159/000522231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cao S, Huang Y, Dai Z, et al. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered. 2022;13(3):6323–6331. doi: 10.1080/21655979.2022.2044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li T, Lu XM, Zhang MR, Hu K, Li Z. Peptide-based nanomaterials: self-assembly, properties and applications. Bioact Mater. 2022;11:268–282. doi: 10.1016/j.bioactmat.2021.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ghosh D, Peng X, Leal J, Mohanty R. Peptides as drug delivery vehicles across biological barriers. J Pharm Investig. 2018;48(1):89–111. doi: 10.1007/s40005-017-0374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Komin A, Russell LM, Hristova KA, Searson PC. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: mechanisms and challenges. Adv Drug Deliv Rev. 2017;110-111:52–64. doi: 10.1016/j.addr.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 201.Zou P, Chen WT, Sun T, et al. Recent advances: peptides and self-assembled peptide-nanosystems for antimicrobial therapy and diagnosis. Biomater Sci. 2020;8(18):4975–4996. doi: 10.1039/d0bm00789g [DOI] [PubMed] [Google Scholar]
- 202.Yang Z, He S, Wu H, et al. Nanostructured antimicrobial peptides: crucial steps of overcoming the bottleneck for clinics. Front Microbiol. 2021;12:710199. doi: 10.3389/fmicb.2021.710199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Son H, Park SC, Kim YM, et al. Potent anti-inflammatory effects of a helix-to-helix peptide against Pseudomonas aeruginosa endotoxin-mediated sepsis. Antibiotics (Basel). 2022;11(11). doi: 10.3390/antibiotics11111675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun Y, Shang D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation. Mediators Inflamm. 2015;2015(1):167572. doi: 10.1155/2015/167572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee JK, Seo CH, Luchian T, Park Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob Agents Chemother. 2016;60(1):495–506. doi: 10.1128/AAC.01998-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Z, Datta G, Zhang Y, et al. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol. 2009;297(2):H866–873. doi: 10.1152/ajpheart.01232.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cheng N, Zhang Y, Delaney MK, et al. Targeting Galpha(13)-integrin interaction ameliorates systemic inflammation. Nat Commun. 2021;12(1):3185. doi: 10.1038/s41467-021-23409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Piktel E, Wnorowska U, Ciesluk M, et al. Inhibition of inflammatory response in human keratinocytes by magnetic nanoparticles functionalized with PBP10 peptide derived from the PIP2-binding site of human plasma gelsolin. J Nanobiotechnology. 2019;17(1):22. doi: 10.1186/s12951-019-0455-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tram NDT, Tran QTN, Xu J, et al. Multifunctional antibacterial nanonets attenuate inflammatory responses through selective trapping of endotoxins and pro-inflammatory cytokines. Adv Healthc Mater. 2023;12(20):e2203232. doi: 10.1002/adhm.202203232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wei B, Ma Y. Synergistic effect of GF9 and streptomycin on relieving gram-negative bacteria-induced sepsis. Front Bioeng Biotechnol. 2022;10:973588. doi: 10.3389/fbioe.2022.973588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sadikot RT, Rubinstein I. Long-acting, multi-targeted nanomedicine: addressing unmet medical need in acute lung injury. J Biomed Nanotechnol. 2009;5(6):614–619. doi: 10.1166/jbn.2009.1078 [DOI] [PubMed] [Google Scholar]
- 212.Dwivedi D, Toltl L, Swystun L, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16(4):R151. doi: 10.1186/cc11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dawulieti J, Sun M, Zhao Y, et al. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci Adv. 2020;6(22). doi: 10.1126/sciadv.aay7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.He H, Zheng N, Song Z, et al. Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano. 2016;10(2):1859–1870. doi: 10.1021/acsnano.5b05470 [DOI] [PubMed] [Google Scholar]
- 215.Friedman AJ, Phan J, Schairer DO, et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: a targeted therapy for cutaneous pathogens. J Invest Dermatol. 2013;133(5):1231–1239. doi: 10.1038/jid.2012.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ajish C, Yang S, Kumar SD, et al. A novel hybrid peptide composed of LfcinB6 and KR-12-a4 with enhanced antimicrobial, anti-inflammatory and anti-biofilm activities. Sci Rep. 2022;12(1):4365. doi: 10.1038/s41598-022-08247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shin MK, Lee B, Kim ST, Yoo JS, Sung JS. Designing a novel functional peptide with dual antimicrobial and anti-inflammatory activities via in silico methods. Front Immunol. 2022;13:821070. doi: 10.3389/fimmu.2022.821070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen C, Amona FM, Chen J, et al. Multifunctional SEBS/AgNWs nanocomposite films with antimicrobial, antioxidant, and anti-inflammatory properties promote infected wound healing. ACS Appl Mater Interfaces. 2024;16(45):61751–61764. doi: 10.1021/acsami.4c15649 [DOI] [PubMed] [Google Scholar]
- 219.Dong Q, Xue T, Yan H, et al. Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy. J Nanobiotechnology. 2023;21(1):395. doi: 10.1186/s12951-023-02152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Askarizadeh A, Badiee A, Khamesipour A. Development of nano-carriers for Leishmania vaccine delivery. Expert Opin Drug Deliv. 2020;17(2):167–187. doi: 10.1080/17425247.2020.1713746 [DOI] [PubMed] [Google Scholar]
- 221.Yang Y, Zheng X, Chen L, et al. Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int J Nanomed. 2022;17:2041–2067. doi: 10.2147/IJN.S355142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hattab D, Gazzali AM, Bakhtiar A. Clinical advances of siRNA-based nanotherapeutics for cancer treatment. Pharmaceutics. 2021;13(7):1009. doi: 10.3390/pharmaceutics13071009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang YL, Zheng CM, Lee YH, et al. Micro- and nanosized substances cause different autophagy-related responses. Int J Mol Sci. 2021;22(9). doi: 10.3390/ijms22094787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ramos TI, Villacis-Aguirre CA, Lopez-Aguilar KV, et al. The Hitchhiker’s guide to human therapeutic nanoparticle development. Pharmaceutics. 2022;14(2). doi: 10.3390/pharmaceutics14020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sibuyi NRS, Moabelo KL, Fadaka AO, et al. Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: a review. Nanoscale Res Lett. 2021;16(1):174. doi: 10.1186/s11671-021-03632-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chauhan A, Patil C, Jain P. Dendrimer-based marketed formulations and miscellaneous applications in cosmetics, veterinary, and agriculture. Micro Nano Tech. 2020;325–334. doi: 10.1016/B978-0-12-814527-2.00014-7 [DOI] [Google Scholar]
- 227.Shan X, Gong X, Li J, et al. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12(7):3028–3048. doi: 10.1016/j.apsb.2022.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Presutti D, Agarwal T, Zarepour A, et al. Transition Metal Dichalcogenides (TMDC)-based nanozymes for biosensing and therapeutic applications. Materials. 2022;15(1):337. doi: 10.3390/ma15010337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ikram M, Javed B, Raja NI, Mashwani ZU. Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int J Nanomed. 2021;16:249–268. doi: 10.2147/IJN.S295053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mayampurath A, Ajith A, Anderson-Smits C, et al. Early diagnosis of primary immunodeficiency disease using clinical data and machine learning. J Allergy Clin Immunol Pract. 2022;10(11):3002–3007e3005. doi: 10.1016/j.jaip.2022.08.041 [DOI] [PubMed] [Google Scholar]


