Abstract
Purpose
β-amyloid overload-induced neuroinflammation and neuronal loss are key pathological changes that occur during the progression of Alzheimer’s disease (AD). Dexmedetomidine (Dex) exhibits neuroprotective and anti-inflammatory effects on the nervous system. However, the effect of Dex in AD mice remains unclear, and its neuroprotective regulatory mechanism requires further investigation. This study aimed to reveal how Dex protects against Aβ induced neuropathological changes and behavior dysfunction in AD mice.
Methods
An AD mouse model was established by the injection of Aβ into the brains of mice, followed by intraperitoneal injection with Dex. CXCL2 overexpression and Yohimbine, a Dex inhibitor, were used to investigate the role of Dex and CXCL2 in the regulation of neuronal loss, cognitive decline, and anxiety-like behavior in AD mice. Behavioral tests were performed to evaluate the cognitive and anxiety status of the mice. Nissl staining and immunofluorescence experiments were conducted to evaluate the status of the hippocampal neurons and astrocytes. qRT-PCR was performed to detect the expression of CXCL2, IL-1β, INOS, SPHK1, Bcl2, IFN-γ, and Caspase 1. The malondialdehyde (MDA) level was detected using an ELISA kit. Terminal TUNEL and Fluoro-Jade C (FJC) staining were used to measure the cell apoptosis rate.
Results
In AD mice, cognitive decline and anxiety-like behaviors were significantly improved by the Dex treatment. The number of neurons was increased in mice in the Dex + AD group compared to those in the AD group, and the number of astrocytes was not significantly different between the two groups. CXCL2, IL-1β, iNOS, and SPHK1 levels were significantly lower in Dex-treated AD mice than those in AD mice. Overloading of CXCL2 or Yohimbine reversed the protective effect of Dex on neuron number and cognitive and anxiety symptoms in AD mice.
Conclusion
Our results suggest that Dex exerts neuroprotective effects by downregulating CXCL2. Dex shows potential as a therapeutic drug for AD.
Keywords: dexamethasone, Alzheimer’s disease, chemokine CXCL2, anxiety, cognitive dysfunction, behavioral Tests
Introduction
Alzheimer’s disease (AD) is the most common type of dementia in older people.1 Forty million people, mostly older than 60 years, were estimated have dementia worldwide, and this population is predicted to double at 2050.2 Patients with AD experience cognitive decline and emotional impairment. Currently, there are no effective drugs for alleviating the neuropathological damage and neurological symptoms associated with AD.1 Evidence suggests that vascular health status and many other lifestyle-related factors, such as diabetes, obesity, physical and mental inactivity, depression, smoking, low educational attainment are all have a role in AD. Genetically, APOE4 is the major genetic risk factor for AD.2 Mostly due to that APOE4 is responsible for the Aβ aggregation and transportation. It is widely accepted that Aβ plaques and tau tangles are typical pathology changes occurring in the late stage of AD. However, genetic, pathological, and biomarker-related studies have illustrated that tauopathy and abnormal behavior appear later than Aβ accumulation.3 The development of neurofibrillary tangles, inflammation-mediated neuronal dysfunction, and chronic inflammation are also consequences of Aβ overload.2 Aβ caused neuronal death in the hippocampus is the main cause of anxiety and memory loss in AD patients. Aβ has such a crucial role in AD, a rational way to treat or prevent AD is to block the accumulation of Aβ. Rescuing neuronal death to attenuate cognitive and emotional damage is crucial in AD therapy.
Dexmedetomidine (Dex) is a highly selective α2-adrenoceptor agonist with sedative, anxiolytic, and analgesic-sparing effects.4 An increasing number of studies have shown that Dex has anti-inflammatory effects, which improve cognitive decline and attenuate anxiety.5 Dex also activates anti-apoptotic signaling pathways, alleviating neuronal damage and neurological deficits,6 including apoptosis,7 pyroptosis,8 and autophagy.9 A clinical studied performed by Glumac et al demonstrated that dexamethasone, akin to dexmedetomidine, can decrease the severity and incidence of cognitive decline in patients with Postoperative cognitive decline after surgery through its anti-inflammatory effect.10 Pretreatment with Dex prior to ischemic manipulation reduced neurobehavioural scores and reduced cerebral infarct volume in mice at 1 hour of ischemia and 24 hours of reperfusion injury.11 Moreover, administration of Dex once daily for one week to mice with chronic pain-induced depression could ameliorate depression.12 However, there is limited understanding of the underlying intrinsic mechanism of the Dex-mediated protective effect on cognitive impairment in AD.
Aβ42 overload causes an increase in chronic inflammatory cytokines in the nervous system, including interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and chemokines.13 The C-X-C motif chemokine ligand CXCL2 was found to be upregulated in the brain tissues of AD patients whose major pathological characterization was depression. The aforementioned neuroinflammatory responses are highly correlated with the development of psychiatric disorders and are considered promising targets for drug development for AD therapy. The anti-inflammatory and neuroprotective effects of Dex have been widely reported. Collectively, we hypothesis that, Dex may conduct neuron protective effect, ameliorate cognitive and emotional damage in AD mice model through its anti-inflammation effect. To study how Dex protects against Aβ induced neuropathological changes and behavior dysfunction in AD mic, we conducted the following experiments. Firstly, intracerebral injection of Aβ-42 was used to establish an in vivo mouse model of AD. Following 7 days of Dex injection, behavioral tests were performed to evaluate its effect on cognitive decline and anxiety in AD mice. Subsequently, the effects of Dex on neuronal status, astrocytes, inflammatory responses, and Aβ accumulation in the CA1 region were investigated. Based on CXCL2, the potential mechanism of action of Dex in AD was analyzed. Our study suggests that Dex and CXCL2 are an effective drug and a promising target for AD therapy.
Material and Methods
Animal Model
All animal experiment were conducted at Xi’an Jiaotong University, health science center, China. All experiments were started at July, 2021 and lasted till August, 2024. Animal experimental procedures were approved by and conducted in accordance with the guidelines of the Animal Care and Use Committee of the Xi’an Jiaotong University Health Science Center. All efforts were made to minimize animal suffering.
Two months old (25–30 g) male C57BL/J6 mice was used in all the experiments. Mice were maintained in a light–dark cycle and allowed access to food and water at room temperature.
Study Design
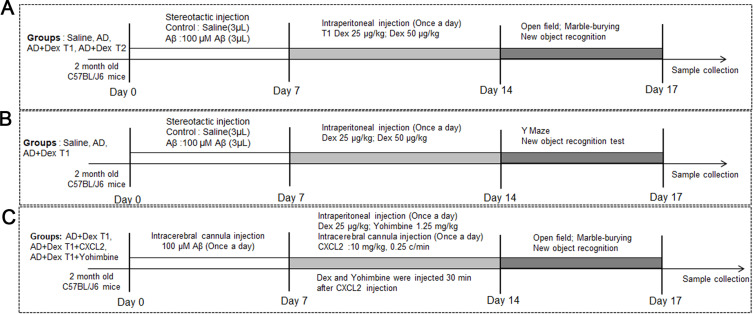
In the first part of the study, mice were randomly divided into 4 groups: control, AD, AD + Dex T1, and AD + Dex T2. In the control group, 3 μL of 0.01 M PBS was injected into the lateral ventricles (ML: 1.13 mm, AP: 1.0 mm, DV: 3.0 mm). In the AD group, 3 μL of 100 μM Aβ was injected stereotaxically. For mice in the AD + Dex T1 and AD + Dex T2 groups, 25 μg/kg and 50 μg/kg Dex, respectively, was injected for 8 days after 7 days of Aβ injection (Figure 1A). In the second part of the study. We chose a concentration of 25μg/kg Dex in this study. Mice were randomly divided into 3 groups: control, AD, AD + Dex T1 (Figure 1B). After Aβ and Dex injection, mice were subjected to Y maze and new object recognition test. Mice were randomly assigned to three groups: AD + Dex, AD + Dex + CXCL2, and AD + Dex + Yohimbine. For mice in the AD + Dex group, 25 μg/kg Dex was injected intraperitoneally (ip) for 7 days; in the AD + Dex + CXCL2 group, CXCL2 monomer was injected using two cannulas implanted in the brain (see next section for the detailed method of CXCL2 injection) before 7 days of Dex injection; and for mice in the AD + Dex + Yohimbine group, Yohimbine (1.25 mg/kg) was injected intraperitoneally for 7 days along with Dex treatment (Figure 1C). All mice were subjected to behavioral tests.
Figure 1.
Experiment design. (A) the experiment schedule of mice in the first part of the study. Mice were randomly assigned to four groups: control, AD, AD + Dex T1, and AD + Dex T2. (B) The experiment schedule of mice in the second part of the study. Mice were randomly assigned to control, AD, AD + Dex T1 groups. (C) The experiment schedule of mice in the last part of the study. Mice were randomly assigned to three groups: AD + Dex, AD + Dex + CXCL2, and AD + Dex + Yohimbine groups.
CXCL2 Injection
CXCL2 monomer was injected through cannulas previously implanted in the mouse brain. After all treatments, the animals were anesthetized with tribromoethanol (100 mg/kg, 0.25%, i.p.) and fixed in a stereotaxic apparatus. Mice were placed on a warming pad to maintain their body temperature. The skull was exposed, and cannulas were implanted at a section in the CA1 region, which was 1 mm caudal to the bregma, 1.3 mm lateral to the midline, and 2.5 mm ventral to the bregma. The cannulas and screws were fixed to the skull using stainless-steel screws and dental cement. After a recovery period (48 h) in individual metal cages, mice were anesthetized using sevoflurane for 10 min. The CXCL2 monomer (10 mg/kg) was injected slowly (0.25 c/min) using a system consisting of a 30G needle inserted into the guide cannula and connected to a Hamilton microsyringe with a polyethylene catheter. After injection, the animals were placed on a warm pad for a few minutes to recover from anesthesia and then allowed to settle in experimental cages.
Animal Behavior Test
The mice were transferred to a testing room 2 h before the behavioral tests for acclimation. The mice were given 30 min to adapt to the dark environment. Subsequently, a 1-day window was maintained between each test to avoid inter-test effects. The detailed timeline and method of testing followed those previously published. The testing room was dimly lit using a red lamp. Animal activities were recorded for 8 min and analyzed. Smart version 3.0 was used to analyze the recordings and data.
Open Field Test
The open-field test was used to examine the locomotor activity and anxious behavior of the mice. A video camera was placed in the clamp of the retort stand above the open-field box (45 cm × 45 cm × 31 cm, gray background). One mouse was placed in the center of the arena, and the operators left the testing room.14 The spontaneous behavior of the mice was recorded for 8 min. The entire area of the box was cleaned with 75% ethanol and a paper towel before proceeding to the next test.15 The traveling speed and distance in the center and total zones were recorded and calculated using Smart 3.0.
Marble-Burying Test
Burying marbles is a natural defense mechanism that occurs in mice under conditions of anxiety or stress.16 Unscented corncob bedding material was spread in the cage at a depth of 5 cm. Twenty marbles were then distributed across the bedding, evenly spaced in four rows. The testing period was 30 min. A marble with at least two-thirds of its total size covered was considered “buried.”17
New Object Recognition Test
Mice were individually exposed to an empty arena for environmental habituation (10 min). The following day, the mice were placed in the arena for 8 min. After 2 h of rest, one of the objects was exchanged, and the mice were returned to the arena for another 8 min. The time the mice spent exploring novel and familiar objects was recorded. Finally, the recognition index (RI), a value representing the learning and memory abilities of mice when exploring novel objects, was calculated: RI = Time spent on new object / (time spent on new object + time spent on original object) × 100%.
Y Maze Test
Y-maze was used to assess working memory in mice.18 Ensure that the Y-maze is clean and dry prior to testing using the 75% ethanol and paper towels. Place the camera on the retort stand above the Y-maze (30 x 5×15 cm, equal length arms). Leave the testing room and allow the mouse to explore the maze 8 min. Examine the video and record the number of all arm entries and alternations. Alternation is determined from successive entries of the three arms on overlapping triplet sets in which three different arms are entered. The number of alternations is then divided by the number of alternation opportunities namely, total arm entries minus one.
Morphology Study
Brain Tissue Preparation
Mice were deeply anesthetized with tribromoethanol and transcardially perfused with 4% paraformaldehyde in 0.1 M (pH 7.4) phosphate buffer. Their brains were dissected, immersed overnight, and transferred into 0.1 M phosphate buffer containing 30% sucrose for 3 days for cryoprotection.19 Brain samples were frozen and cut into 14-μm–thick coronal sections using a microtome (Leica CM1950).
Nissl Staining
After drying, unstained coronal sections were stained with buffered thionin for 10 min. Finally, the sections were dehydrated, cleared, and coverslipped with neutral resin.
Immunofluorescence Staining
For immunostaining, the cryostat sections were rinsed with PBS after antigen repair and permeabilization with Triton, and then blocked with 5% normal serum for 2 h. Sections were incubated for 36 h at 4°C using the following primary antibodies: Aβ (Proteintech, 1:100, rabbit), NeuN (Abcam, 1:1000, rabbit), and GFAP (Thermo Fisher, 1:400, rat). The sections were then incubated at room temperature for 2 h with 594-conjugated anti-rabbit IgG antibody (Invitrogen, 1: 400), 488-conjugated anti-mouse IgG antibody (Invitrogen, 1: 400), or 594-conjugated anti-rat IgG antibody (Invitrogen, 1: 400). Nuclear DNA was labeled with DAPI. Fluorescence signals were detected using a fluorescence microscope (Olympus).
TUNEL Staining
An in situ cell death detection kit, Fluorescein (Roche, Basel, Switzerland), was used for TUNEL staining. After antigen repair and permeabilization with Triton, sections were rinsed with PBS. A system containing 50 μL TdT buffer and 450 μL dUTP buffer was constructed, and the section samples were incubated with this system for 1 h at 37 °C. Sections were rinsed and incubated for 1–5 min at room temperature in DAPI labeling solution. Fluorescence microscopy was used to evaluate fluorescent signals.
Fluoro-Jade C Staining
Fluoro-Jade C (FJC) is a marker of degenerating neurons that specifically binds to degenerating neurons. The brain sections were incubated with NaOH and ethanol, followed by potassium permanganate to block background staining. Fluoro-Jade C (Fluoro-Jade® C RTDTM Stain Reagent, Biosensis) and DAPI were then added for 30 min of incubation in the dark.
Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
Hippocampal tissues were homogenized and resuspended in RNA lysis buffer. TRIzol reagent was used to isolate total RNA according to the manufacturer’s protocol (Thermo Fisher Scientific). A RevertAid First Strand cDNA Synthesis Kit was used to synthesize cDNA samples. The qPCR reaction was initiated with a 2 × RealStar Fast probe-based qPCR premix with added cDNA template, primers (primer sequences listed in Table 1), probe, and water. The qPCR process included pre-denaturation (95°C, 2 min), 35 cycles of denaturation (95°C, 15s), and annealing and extension (60°C, 30s).
Table 1.
mRNA Primer Sequences
| Gene Name | Sequences |
|---|---|
| Atg5-F | TGTGCTTCGAGATGTGTGGTT |
| Atg5-R | GTCAAATAGCTGACTCTTGGCAA |
| Maplic3b-F | TTATAGAGCGATACAAGGGGGAG |
| Map1lc3b-R | CGCCGTCTGATTATCTTGATGAG |
| NLRP3-F | ATTACCCGCCCGAGAAAGG |
| NLRP3-R | CATGAGTGTGGCTAGATCCAAG |
| GAPDH-F | GCCAAGGCTGTGGGCAAGGTl |
| GAPDH-R | TCTCCAGGCGGCACGTCAGA |
| iNOS-F | CACCAAGCTGAACTTGAGCG |
| iNOS-R | CGTGGCTTTGGGCTCCTC |
| IL-1β-F | TGGGAAACAACAGTGGTCAGG |
| IL-1β-R | CCATCAGAGGCAAGGAGGAA |
| IFN-γ-F | GAACTGGCAAAAGGATGGTGA |
| IFN-γ-R | TGTGGGTTGTTGACCTCAAAC |
| TNF-α-F | GACGTGGAACTGGCAGAAGAG |
| TNF-α-R | TTGGTGGTTTGTGAGTGTGAG |
| CXCL2-F | GCCCAGACAGAAGTCATAGCC |
| CXCL2-R | CTCCTCCTTTCCAGGTCAGTTA |
| caspase1-F | CGTACACGTCTTGCCCTCAT |
| caspase1-R | CTCTTTCACCATCTCCAGAGC |
Colorimetric Malondialdehyde (MDA) Assay Kit
Total malonaldehyde (MDA) were assayed using the MDA assay kit (S0131S, Beyotime, China), according to the manufacturer’s instructions. After configuring the TBA storage solution and MDA assay working solution, diluting the standards and establishing a blank control, brain tissue samples were homogenized and centrifuged. The supernatant was aspirated and the absorbance was measured at 532 nm with an enzyme marker, and then the molar concentration of MDA in the sample was calculated according to the standard curve.
Statistics
Data are presented as mean ± standard division (SD) and were analyzed by Student’s t-test or One-way ANOVA using GraphPad Prism 8.0. Normality of the data was assessed individually, and non-parametric tests were used when the P value was < 0.05, which was also regarded as statistically different.
Results
Dex Improved Cognitive Decline and Anxiety-Like Behavior in AD Mice
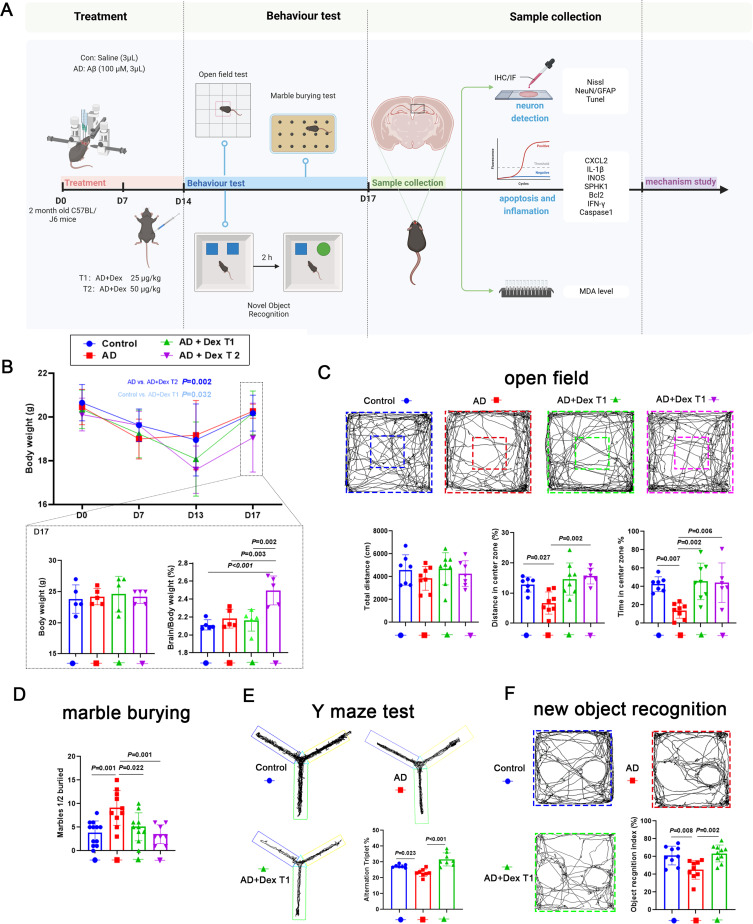
To investigate the effect of Dex on cognition and anxiety during AD progression, 25 μg/kg and 50 μg/kg Dex were injected (i.p). into AD mice. The body and brain weights of the mice were monitored and a series of behavioral tests were performed (Figure 2A). The body weights of mice in all groups decreased during the Aβ stimulation period and continuously decreased under Dex treatment, except for the AD group, which showed a slight increase (Figure 2B). However, the rates of weight loss in the AD + Dex T1 and AD + Dex T2 groups were significantly higher than those in the AD and control groups. In addition, mouse body weights in all groups increased when Dex treatment was removed, and there were no significant differences found between the final weights of the control, AD, and AD + Dex T1 groups at day 17, while the AD + Dex T2 group showed an obvious decrease in body weight compared to the other three groups. Next, the cognitive ability and anxiety levels of the mice were assessed using behavioral tests. In the open-field test, the total distance traveled by mice in the control, AD, AD+Dex-T1, and AD+Dex-T2 groups was similar, indicating that Aβ and Dex treatment had no significant effects on the mobility of the mice. AD mice traveled less distance and spent less time in the center zone compared to mice in the control group, while mice receiving Dex treatment traveled a greater distance and spent more time in the center zone (Figure 2C). Mice in the control and AD + Dex groups buried fewer marbles than those in the AD group (Figure 2D). In Y maze test, the percentage of spontaneous alternation time was of mice in AD + Dex T1 group was significantly higher than mice in the AD group (Figure 2E). The same with this, in the new object recognition test, mice in the AD + Dex T1 group spent more time on new objects than mice in the AD group (Figure 2F). Our results indicate that Dex improves cognitive impairment and anxiety in AD mice.
Figure 2.
Dex attenuates the abnormal behavior of AD mice. (A) Schematic of experimental design. Aβ was injected into mouse brains on day 0 (D0); 7 days after Aβ injection, Dex was injected for 7 days. (B) The body weight of each mouse from D0 to D17 is shown in the line chart; body weight of mice at D20 is shown in the bar chart (n = 4 in each group); the ratio of brain weight to body weight is shown in the last bar chart (n = 5 in control and AD + Dex T2 groups, n = 6 in AD and AD + Dex T1 groups). (C) Trajectory diagram in the open-field test for each group; total distance, average speed, distance in center zone, and time in center zone were calculated and are shown in the bar graph (n = 7 in control group, n = 6 in AD + Dex T1 group, n = 8 in AD and AD + Dex T1 groups). (D) Bar graph showing the number of marbles buried in each group (n = 12 in control group, n = 10 in AD and AD + Dex T1 group, n = 8 in AD + Dex T2 group). (E) The alternation triplet was calculated and shown in the bar graph (n = 7 in control group, n = 8 in AD and AD + Dex T1 groups). (F) The percentage of time exploring new objects was calculated and is shown in the bar graph (n = 9 in control and AD groups, n = 10 in AD + Dex T1 group).
Dex Rescued Neuron Damage in the Hippocampal CA1 Region of in AD Mice
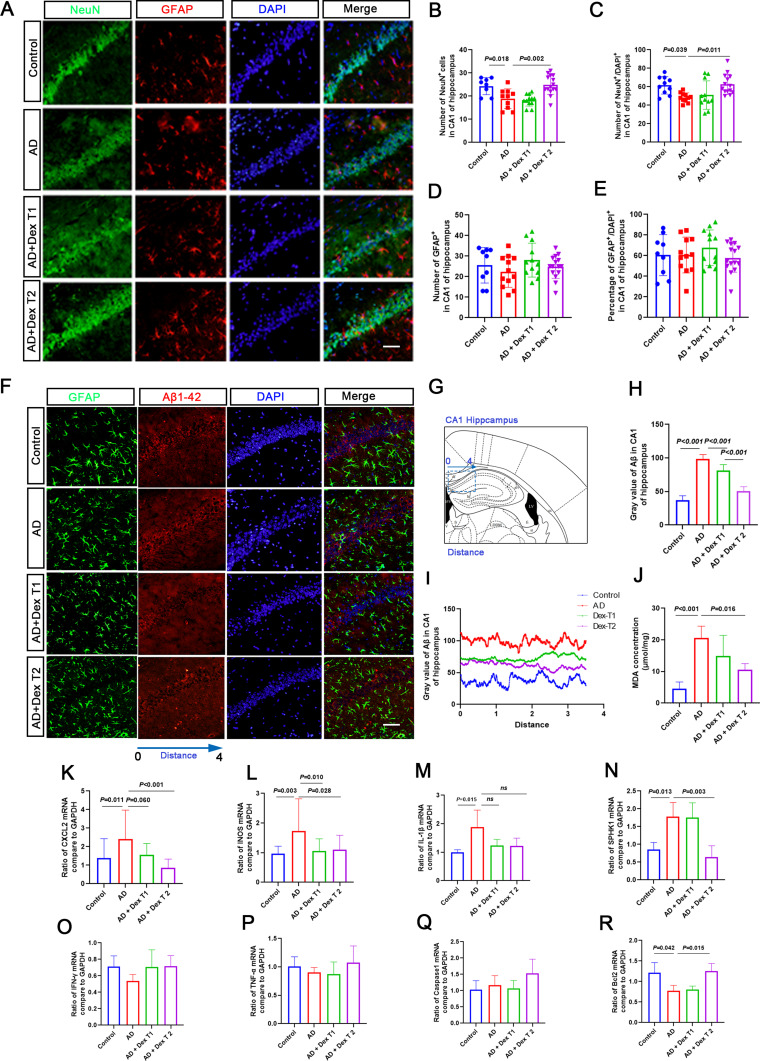
Neuron loss is a key pathological injury caused by Aβ-42, which leads to behavioral disability during AD progression. Therefore, we evaluated the effects of Dex on neuronal damage in the hippocampal CA1 region. Compared with the control group, NeuN-positive cells were reduced in the AD group and significantly increased in the AD + Dex T2 group; however, the ratio of GFAP/DAPI-positive cells showed no significant changes among the four groups (Figure 3A–E). In line with this, the intensities of Aβ-positive particles in the hippocampal CA1 region of the AD + Dex T1 and AD + Dex T2 groups were lower than those in the AD group, and the Aβ-positive particles were not co-localized with GFAP-positive cells in the CA1 region of the hippocampus, indicating that Aβ aggregates in neurons during this period (Figure 3F–I).
Figure 3.
Dex attenuates neuron reduction in the CA1 region of AD mice. (A) NeuN was used to detect neuron cells (green), GFAP was used to detect astrocytes (red), and DAPI was used to detect the nucleus (blue). (B and C) The bar chart shows the number of NeuN-positive cells in each brain slide from 3 mice, and each dot in the bar chart represents the NeuN-positive cell number or the percentage of NeuN-positive cells in the CA1 region from one brain slide. (D and E) The bar chart shows the number of GFAP-positive cells in each brain slide from 3 mice, and each dot in the bar chart represents the GFAP-positive cell number or the percentage of GFAP-positive cells in the CA1 region from one brain slide. (F) Aβ was used to stain Aβ-42 positive particles (red), and DAPI was used to stain the nucleus (blue). (G) Illustrated the observed area in the brain. (H) The fluorescence intensity is shown in the bar chart. (I) The fluorescence intensity was measured using ImageJ. The x axis of the line chart represents the distance (arrowhead line). The y-axis represents the fluorescence intensity of Aβ-42-positive particles. (J) The concentration of MDA in the hippocampus was measured using an ELISA kit (n = 4 in each group). (K–R) Inflammation and cell death-related gene expression in the hippocampus of Dex-treated AD mice. Samples were collected from the hippocampus of mouse brains in each group. qRT-PCR was performed to detect the mRNA expression of CXCL2, IL-1β, iNOS, SPHK1, Bcl2, IFN-γ, and Caspase 1. GAPDH was used as a control.
To investigate the mechanism of action of Dex in rescuing neuronal damage, the expression of genes related to cell injury and apoptosis was detected using RT-qPCR. Malondialdehyde (MDA) levels, which indicate the degree of lipid peroxidation and cellular damage, were increased in the AD group, and the AD + Dex T1 and AD + Dex T2 groups showed decreased MDA levels compared to the AD group (Figure 3J). The expression of CXCL2, IL-1β, iNOS, and SPHK1 was significantly upregulated in the Aβ-induced AD model, and the DX treatment dramatically decreased CXCL2, iNOS, and SPHK1 expression, but not IL-1β, in AD mice. The mRNA level of Bcl2 was decreased in AD mice compared with the control mice and increased again in AD mice after treatment with Dex T2. No significant changes were observed between the mRNA levels of IFN-γ, TNF-α, and Caspase 1 among all groups (Figure 3K–R).
Dex Improved Cognitive Decline and Anxiety-Like Behavior in AD Mice by Regulating CXCL2
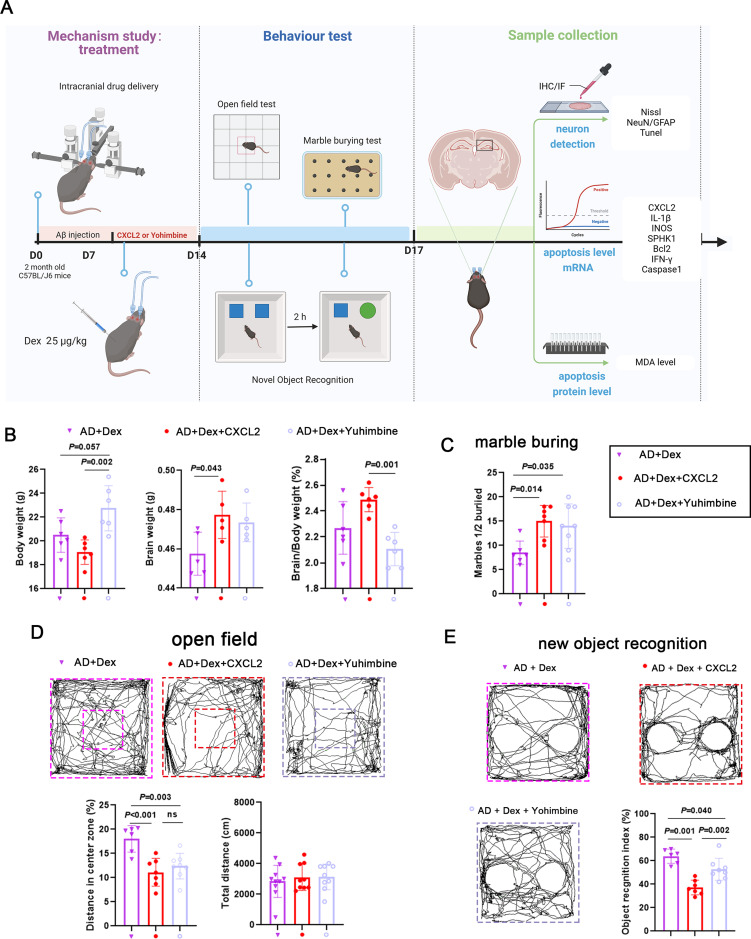
To explore the potential mechanism by which Dex alleviates AD development, the role of CXCL2 was investigated. The AD mouse model was treated with Dex (i.p)., Dex (i.p). + CXCL2 monomer (intracerebral injection), or Dex (i.p). + Yohimbine (i.p). for 7 days. The experimental procedure is illustrated in Figure 4A. The body weight of mice in the AD + Dex + CXCL2 group was significantly decreased compared with that of the AD + Dex group, and body weight was increased in the AD + Dex + Yohimbine group. In addition, the brain weight proportion of mice in the AD + Dex + CXCL2 group was higher than that in the AD + Dex group and decreased further in the AD + Dex + Yohimbine group (Figure 4B). Animal behavior test results indicated that the injection of CXCL2 monomer and Yohimbine reversed the inhibitory effect of Dex on the marble-burying ability of AD mice (Figure 4C). The results of the open-field test showed that the total traveling distance of AD mice receiving the Dex treatment was not affected by CXCL2 monomer and Yohimbine treatment, whereas the traveling distance in the center zone was shortened by CXCL2 monomer and Yohimbine treatments (Figure 4D). In addition, mice in the AD + Dex + CXCL2 group spent less time exploring new objects compared with mice in the AD + Dex and AD + Dex + Yohimbine groups (Figure 4E). These results indicate that reloading CXCL2 and Dex inhibitors reversed the protective effect of Dex against AD.
Figure 4.
Dex improves the behavior of AD mice by downregulating CXCL2. (A) Schematic of the experimental design for studying the role of Dex/CXCL2 in the neuron loss of AD mice. Mice were divided into 3 groups: AD + Dex, AD + Dex + CXCL2, and AD + Dex + Yohimbine. Aβ was injected into mouse brains on day 0 (D0); 7 days after Aβ injection, the AD mice model was treated with Dex (0.5 μg/kg, ip), CXCL2 (intracerebral injection), or Yohimbine (ip) for 7 days, respectively. (B) The body weight and brain weight of the mice were monitored (n = 5 in each group). The body weight and brain weight of each mouse at D20 were measured and are shown in the bar graph. (C) Bar graph comparing number of marbles buried by each group (n = 7 in AD + Dex + CXCL2 group and n = 8 in AD + Dex + Yohimbine group). (D) Bar graphs showing the total distance and percentage of distance in the open zone in the open-field test (n = 6 in AD + Dex group, n = 7 in AD + Dex + CXCL2 group, and n = 8 in AD + Dex + Yohimbine group). (E) The percentage of time mice spent exploring new objects was calculated and is shown in the bar graph (n = 7 in AD + Dex + CXCL2 group and n = 8 in AD +Dex + Yohimbine group).
CXCL2 Injection Reversed Dex-Depressed Neuron Damage in the CA1 Region of AD Mice
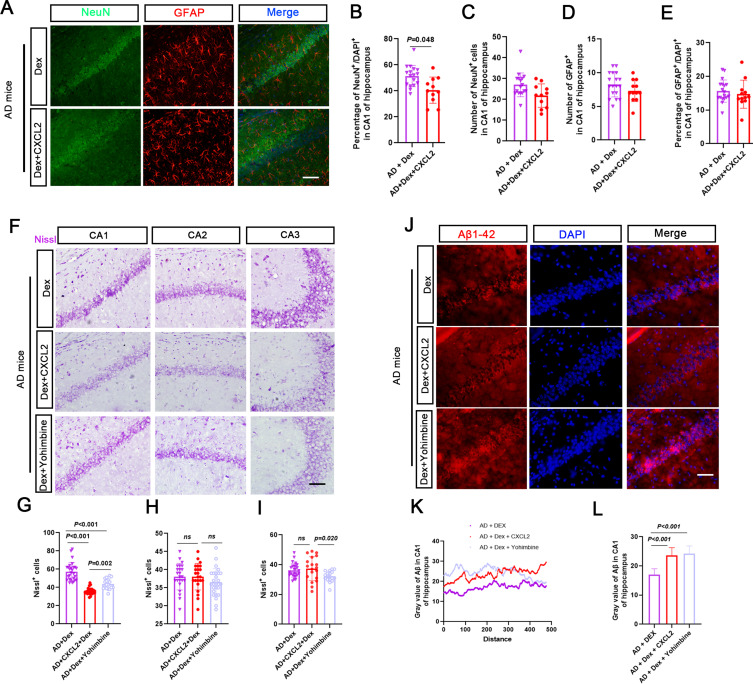
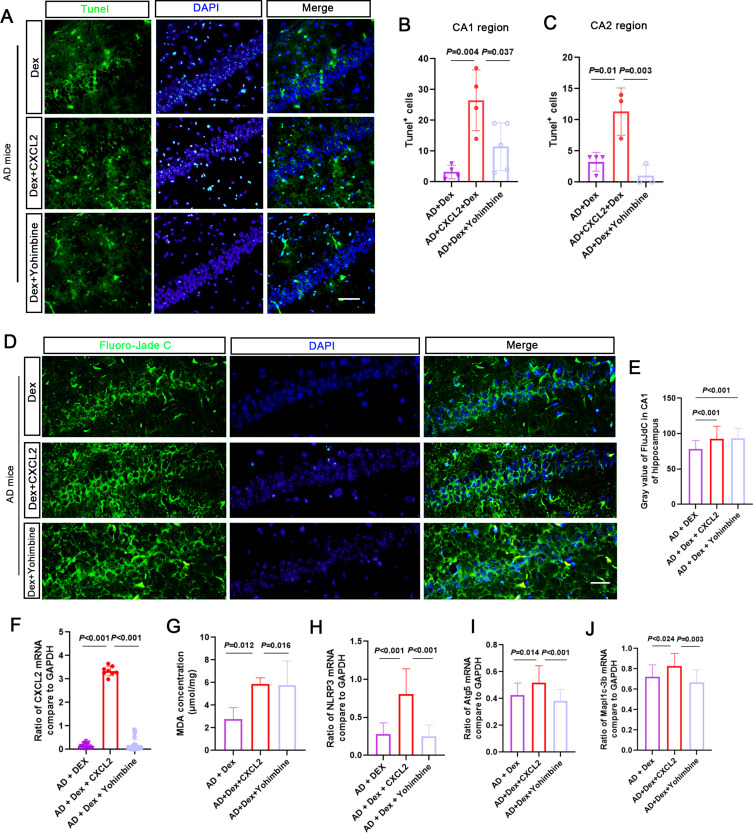
The results showed that the number of NeuN-positive cells decreased in the AD + Dex + CXCL2 group, and the number of GFAP-positive cells showed no apparent change (Figure 5A–E). Nissl-positive cell numbers in the hippocampal CA1 region of the AD + Dex + CXCL2 and AD + Dex + Yohimbine groups were significantly decreased compared with the AD + Dex group, while CXCL2 monomer and Yohimbine treatments had no significant effects on Nissl-positive cells in the CA2 and CA3 regions, except that a slight decrease was found in the Nissl-positive cell number in the CA3 region in the AD + Dex + Yohimbine group (Figure 5F–I). Moreover, the number of Aβ-positive particles in the CA1 region in the AD + Dex + CXCL2 and AD + Dex + Yohimbine groups was higher than in the AD + Dex group (Figure 5J–L). As shown in Figure 6A–C, the AD + Dex + CXCL2 and AD + Dex + Yohimbine groups presented more TUNEL-positive cells in the hippocampal CA1 region than the AD + Dex group. Although the CXCL2 monomer dramatically increased TUNEL-positive cells in the CA2 region in Dex-treated AD mice, Yohimbine showed an inhibitory effect. The results of FJC staining and MDA levels showed the same phenomenon (Figure 6D and E). Moreover, the mRNA levels of CXCL2, NLPR3, Atg5, and Mapl1c-3b were significantly upregulated in the AD + Dex + CXCL2 group and decreased in Yohimbine-treated AD + Dex mice (Figure 6F–J).
Figure 5.
Role of CXCL2 and Yohimbine in regulating neuron damage in Dex-treated AD mice. (A) Representative immunofluorescence images of dual NeuN (green) or GFAP (red) and DAPI (blue) from the hippocampal CA1 region. (B–E) The bar chart shows the number of NeuN or GFAP-positive cells in each brain slide from 3 mice, and each dot in the bar chart represents the NeuN or GFAP-positive cell number in the CA1 region from one brain slide. (F) Nissl staining was used to observe the neuron numbers in hippocampal CA1, CA2, and CA3. (G–I) The bar chart shows the Nissl body positive cell numbers in the hippocampal regions in the AD + Dex, AD + Dex + CXCL2, and AD + Dex + Yohimbine groups. (J) Aβ1-42 was used to stain the Aβ-42-positive particles (red) and DAPI to stain the nucleus (blue). (K and L) The fluorescence intensity was measured using ImageJ. The x axis of the line chart represents the distance (arrowhead line). The y-axis represents the fluorescence intensity of Aβ-42-positive particles. The fluorescence intensity is shown in the bar chart.
Figure 6.
Increasing CXCL2 reverses the protective effect of Dex in AD mice. (A–C) Representative immunofluorescence images of TUNEL (green) and DAPI (blue) staining from hippocampal CA1. The bar chart shows the number of TUNEL-positive cells in each brain slide from 3 mice. (D and E) Fluoro-Jade C staining was used to observe the FJC level in the hippocampus. The bar chart shows the intensity of FJC levels in the hippocampus in the AD + Dex, AD + Dex + CXCL2, and AD + Dex + Yohimbine groups. (F–J) Samples were collected from the hippocampus of mouse brains in each group. qRT-PCR was performed to detect the mRNA expression of CXCL2, NLRP3, Atg5, and Mapl1c-3b. GAPDH was used as a control. Concentration of MDA in the hippocampus was measured using an ELISA kit (n = 4 in each group).
Discussion
Patients with AD suffer from progressive cognitive decline, memory loss, anxiety, and depression, which have also been observed in Aβ-stimulated AD mice. Aβ is one of the main neuropathological hallmarks of AD.2 Aβ plaques can be recognized by the brain as foreign bodies, leading to activated microglia and triggering inflammation and immune responses, ultimately causing neuronal death and degeneration.20 Research on drug therapy for AD is continuously developing, mainly focusing on anti-amyloid and anti-tau therapeutics, cholinergic neurotransmission, symptom relief, and immunotherapy.21 However, due to the multifactorial pathogenesis of AD, finding an effective anti-AD drug is still complex. Dex is a lipophilic compound that can pass through the blood–brain barrier and penetrate brain tissue, reduces the inflammation in some disease model.4
Dex is being used during anesthesia, mostly because it reduces the incidence of postoperative cognitive dysfunction and postoperative delirium.22 But its role in the AD mice model still needs further study. In the present study, we showed that DEX ameliorated cognitive and anxiety abnormalities in AD mouse model, with a remarkably drop of the body weight.
On one hand, we find that Dex (25μg and 50μg/kg) decreased the body weight of AD and wild type C57Bl/J6 male mice. Body weight dropped remarkably in mice treated with Dex. It was reported that Dex using at high dose (20mg/kg body weight for 10 days, i.p). could cause skeletal muscle atrophy, with loss of body weight and impaired quality of life.23 Chronic Dex treatment is also associated with increased fat mass and weight gain. After intraperitoneally injection of Dex (5 mg/kg every other day for 6 weeks), the adipose tissue of C57BL/6J mice expanded and the body weight decreased.24 Previous study revealed Dex were used at mg/kg body weight,25 here we illustrate that Dex causes the loss of body weight even in a low dose range.
On the other hand, we find that 25μg and 50μg/kg Dex attenuates the cognitive decline and anxiety in AD mice model. Dex was used to alleviates the cognitive decline in different kind of disease models in mice and rat. The dose ranged from 20 μg/kg −100 μg/kg. Our result illustrated that the performance of mice in Dex 25 μg/kg and 50 μg/kg group were the same in behavior test. About 25 μg/kg of Dex treatment was proved to attenuate the cognitive decline in previous studies. Previous results from the Morris water maze illustrated 20 μg/kg Dex decreased the post-operative cognitive dysfunction in rat.26 Pretreatment with 25 µg/kg Dex prior to ischemic manipulation reduced neuro behavioral scores and cerebral infarct volume in mice at 1 hour of ischemia and 24 hours of reperfusion injury.11 Administration of 25µg/kg Dex once daily for one week to mice with chronic pain-induced depression resulted in an increase in realistic sucrose preference and a shortening of immobility time in the forced swimming test, indicating that Dex ameliorates depression induced by chronic pain in mice.12 Therefore, our result is in line with the previous studies in other animal models, which is Dex attenuates the cognitive and anxiety in neurovegetative disease models.
Our findings illustrate the crucial role of the protective effect of Dex on neuronal loss. Dex protected neurons against apoptosis and neurological deficits through several ways,6 anti-inflammation, reducing Aβ degradation and other neuro-protective mechanisms.
Firstly, Dex attenuates anxiety and cognitive decline in AD mice partly through its protective effect on CA1 neurons. We find that Dex reduces the neuron loss in the CA1 region. The dorsal CA1 subregion of the hippocampus is enriched in place cells, ventral CA1 is enriched in anxiety cells. These anxiety cells were enriched in the vCA1 and projected to the lateral hypothalamic.27 Which means other than contributing to working memory, CA1 region of the hippocampus is also important for the memory of anxiety. Dex may contributes to the cognitive decline and anxiety through protecting the neurons in the CA1 region of the hippocampus in AD mice.
Secondly, the neuron protective function of Dex on CA1 neurons may due to the decrease of Aβ level in CA1 region. This study reveals that Dex dramatically decreased Aβ levels in the CA1 region and protects neuron degeneration in this region. Dex injection decreased Fe2+ levels and alleviated cognitive decline and neuronal loss in Aβ-induced AD mice.28 Dex increased the expression of LC3-LAMP2 and LC3-cathepsin D, which are one of the main factors for Aβ degradation.29 Deng et al indicated that Dex plays a role in LAMP5-mediated hyperexcitation and Dex is a potent regulator of AD therapy due to its beneficial effect on neuronal activation, which promotes Aβ and tau clearance to weaken AD neuronal function deficits.30
Thirdly, the protective effect of Dex on AD mice may due to its anti-inflammation function. Neuro inflammation caused by Aβ is a major cause for the development of AD, and is a critical contributor for neuron damage in the hippocampus of AD mice model. Dex administered at 0, 2 and 4 h after systemic lipopolysaccharide injection can attenuate the LPS administration-induced elevation of IL-1β and TNF-α, and alleviate neuronal turnover.31 Recent research has demonstrated the effectiveness of Dex in attenuating hippocampal neuronal apoptosis induced by LPS; meanwhile, Dex also suppresses IL-1β, IL-6, IL-18, and TNF-α levels in the hippocampus, thereby inhibiting LPS-induced inflammation.32
More importantly, other than the previous studies. In the present study, Dex suppressed CXCL2 expression and ameliorated neuronal inflammatory injury and cognitive decline. CXCL2 is a pro-inflammatory cytokine with chemotactic activity toward neutrophils.33 A previous clinical study based on blood expression levels of cytokines revealed that CXCL2 was significantly upregulated in AD patients, contributing to cognitive impairment and brain amyloidosis.34 In another study, gene expression from the intact micro vessels of the dorsolateral prefrontal cortex derived from 16 advanced AD and control subject groups was compared, and the results indicated that SERPINE1, IL8, CXCL1, ICAM-2, TIE1, and CXCL2 were promoted in the late stage of cognitive impairment.35 Moreover, CXCL2 is a crucial factor regulating neuronal dysfunction, and the suppression of CXCL2 may be an effective way to resolve inflammatory injury in the nervous system. Zhao et al found that the overproduction of CXCL2 in neurons induced by Cathepsin C aggravated cryogenic brain lesions.36 Another study used MIP-2, an agonist of CXCL2, to stimulate rat primary motor neurons, and discovered dose-dependent neurotoxicity in the CNS.37 Although these findings support our conclusion about the important role of CXCL2 in nervous system regulation, few studies have reported the regulatory mechanism of CXCL2 in AD. Our study proves that CXCL2 is a key influence factor at Dex treated AD mice which may become a future therapy target for reducing the cognitive decline in Alzheimer’s disease.
Conclusion
DEX can produce neuroprotective effects through various mechanisms, such as antioxidation, inhibition of apoptosis, inhibition of neuron inflammation, promotion of neurogenesis, and through the influence of various cell signaling pathways. Our research concluded that Dex attenuated abnormal behavior and pathological changes in AD mice by mediating CXCL2 and attenuating Aβ degradation and neuronal degeneration in the hippocampal CA1 region. Our study demonstrated the effectiveness of Dex in AD therapy and primarily investigated its mechanism of action, presenting a promising therapeutic target for AD treatment. However, there are also some limitations in this study which provokes further study. For instance, whether other inflammation-related cytokines are related with Dex still needed further exploration. The mechanism by which Dex affects CXCL2 expression by interacting with post-transcriptional modifications requires further investigation. Moreover, since Dex could promote neurogenesis, it is interesting to find out whether any neurogenesis was activated through Dex. More importantly, astrocyte and microglia were acknowledged to be crucial for neuron inflammation, CXCL2 is closely related to microglial function, whether Dex exerts effects on microglia to attenuate AD progression might be an interesting aspect to focus on in the future. The role of these two types of cells in Dex treated AD mice should be addressed further.
Acknowledgments
Mice were kept in an environmentally enriched cage. Additional toys, nesting material and tubes were used as a standard housing condition, with these animal toys kindly provided by Golden Horse Arts & Crafts Co., LTD.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 82001493, from Ma KG; No. 82171308 from Chen XL), China Postdoctoral Science Foundation (No.2019M653662, from Ma KG). Fundamental Research Funds for the Central Universities (No. xzy012022098, from Zhu K); Natural Science Basic Research Plan in Shaanxi Province of China (No. 2023-JC-QN-0862, from Zhu K). Science and Technology Talent Support Program of Shaanxi Provincial People’s Hospital (No. 2023BJ-02, from Zhang YM), Research Incubation Fund of Shaanxi Provincial People’s Hospital (No. 2023YJY-01, from Zhang YM).
Data Sharing Statement
All data are available on reasonable request from the corresponding author.
Ethics Statement
All efforts were made to minimize the number of animals used and their suffering. All experimental animal procedures were rigorously reviewed and approved by the Animal Committee of Xi’an Jiaotong University Health Science Center (No: XJTUAE2024-28).
Author Contributions
The problem can be resolved by instead using the following sentence (if it is accurate): All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
- 1.Rostagno AA. Pathogenesis of Alzheimer’s Disease. International Journal of Molecular Sciences. 2022;24(1):107. doi: 10.3390/ijms24010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. European Journal of Neurology. 2018;25(1):59–70. doi: 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- 3.Jucker M, Walker LC. Alzheimer’s disease: from immunotherapy to immunoprevention. Cell. 2023;186(20):4260–4270. doi: 10.1016/j.cell.2023.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clinical Pharmacokinetics. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian XL, Zhang W, Liu M-Z, et al. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur J Pharmacol. 2015;746:206–212. doi: 10.1016/j.ejphar.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Chen D, Chen P, et al. Dexmedetomidine Attenuates Apoptosis and Neurological Deficits by Modulating Neuronal NADPH Oxidase 2-Derived Oxidative Stress in Neonates Following Hypoxic Brain Injury. Antioxidants. 2022;11(11):2199. doi: 10.3390/antiox11112199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng K, Chen W-R, Xia F, et al. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1α signalling. Journal of Cellular and Molecular Medicine. 2020;24(1):850–861. doi: 10.1111/jcmm.14795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Y, Li Y-P, Yin Y-Q, et al. Dexmedetomidine inhibits pyroptosis by down-regulating miR-29b in myocardial ischemia reperfusion injury in rats. International Immunopharmacology. 2020;86:106768. doi: 10.1016/j.intimp.2020.106768 [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y, Li J, Qiu L, et al. Dexmedetomidine Protects Human Cardiomyocytes Against Ischemia-Reperfusion Injury Through α2-Adrenergic Receptor/AMPK-Dependent Autophagy. Frontiers in Pharmacology. 2021;12:615424. doi: 10.3389/fphar.2021.615424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glumac S, Kardum G, Sodic L, et al. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34(11):776–784. doi: 10.1097/EJA.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 11.Hu M, Men Y, Chen L, et al. Dexmedetomidine exerts its protective effect on cerebral ischemia reperfusion injury in mice by inhibiting ferroptosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47(5):600–609. doi: 10.11817/j.issn.1672-7347.2022.210443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Zhao X, Zhu Z, et al. A New Potential Antidepressant: dexmedetomidine Alleviates Neuropathic Pain-Induced Depression by Increasing Neurogenesis in the Hippocampus. Pharmacology. 2022;107(5–6):317–329. doi: 10.1159/000521737 [DOI] [PubMed] [Google Scholar]
- 13.Kirkley KS, Popichak KA, Afzali MF, et al. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. Journal of Neuroinflammation. 2017;14(1):99. doi: 10.1186/s12974-017-0871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antiorio AT, Alemán-Laporte J, Zanatto DA, et al. Mouse Behavior in the Open-field Test after Meloxicam Administration. Journal of the American Association for Laboratory Animal Science: JAALAS. 2022;61(3):270–274. doi: 10.30802/AALAS-JAALAS-21-000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraeuter A-K, Guest PC, Sarnyai Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods in Molecular Biology. 2019;1916:1. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar D. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clinical Psychopharmacology and Neuroscience: the Official Scientific Journal of the Korean College of Neuropsychopharmacology. 2020;18(3):341–351. doi: 10.9758/cpn.2020.18.3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prajapati RP, Kalaria MV, Karkare VP, et al. Effect of methanolic extract of Lagenaria siceraria (Molina) Standley fruits on marble-burying behavior in mice: implications for obsessive-compulsive disorder. Pharmacognosy Research. 2011;3(1):62–66. doi: 10.4103/0974-8490.79118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28(5):497–505. doi: 10.1016/j.neubiorev.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 19.Takao K, Kobayashi K, Hagihara H, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38(8):1409–1425. doi: 10.1038/npp.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S, Barve KH, Kumar MS. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr Neuropharmacol. 2020;18(11):1106–1125. doi: 10.2174/1570159X18666200528142429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep. 2021;48(7):5629–5645. doi: 10.1007/s11033-021-06512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin X, Chen J, Hua W, et al. Intraoperative dexmedetomidine for prevention of postoperative delirium in elderly patients with mild cognitive impairment. International Journal of Geriatric Psychiatry. 2021;36(1):143–151. doi: 10.1002/gps.5406 [DOI] [PubMed] [Google Scholar]
- 23.Wang BY-H, Hsiao AW-T, Shiu HT, et al. Mesenchymal stem cells alleviate dexamethasone-induced muscle atrophy in mice and the involvement of ERK1/2 signalling pathway. Stem Cell Research & Therapy. 2023;14(1):195. doi: 10.1186/s13287-023-03418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Du C, Wang W, et al. Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages. Nature Communications. 2024;15(1):1190. doi: 10.1038/s41467-024-45477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong J, Tran LT, Lynch KA, et al. Dexamethasone exacerbates cytotoxic chemotherapy induced lethargy and weight loss in female tumor free mice. Cancer Biology & Therapy. 2018;19(1):87–96. doi: 10.1080/15384047.2017.1394549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H, Zhu M, Lyu Y, et al. Effects and possible mechanisms of dexmedetomidine on post-operative cognitive dysfunction. Chin Med J (Engl). 2023;136(19):2392–2394. doi: 10.1097/CM9.0000000000002372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez JC, Su K, Goldberg AR, et al. Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron. 2018;97(3):670–683.e6. doi: 10.1016/j.neuron.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao L, Li G, Yuan H-X. Dexmedetomidine mediates the mechanism of action of ferroptosis in mice with Alzheimer’s disease by regulating the mTOR-TFR1 pathway. World Journal of Psychiatry. 2023;13(8):511–523. doi: 10.5498/wjp.v13.i8.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YY, Han JI, Lee KE, et al. Neuroprotective effect of dexmedetomidine on autophagy in mice administered intracerebroventricular injections of Aβ25-35. Frontiers in Pharmacology. 2023;14:1184776. doi: 10.3389/fphar.2023.1184776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Bi M, Delerue F, et al. Loss of LAMP5 interneurons drives neuronal network dysfunction in Alzheimer’s disease. Acta Neuropathol. 2022;144(4):637–650. doi: 10.1007/s00401-022-02457-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning Q, Liu Z, Wang X, et al. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol Res. 2017;39(4):357–366. doi: 10.1080/01616412.2017.1281197 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Li L, Zhang J, et al. Dexmedetomidine Alleviates Lipopolysaccharide-Induced Hippocampal Neuronal Apoptosis via Inhibiting the p38 MAPK/c-Myc/CLIC4 Signaling Pathway in Rats. Mol Neurobiol. 2021;58(11):5533–5547. doi: 10.1007/s12035-021-02512-9 [DOI] [PubMed] [Google Scholar]
- 33.Iwasa T, Afroz S, Inoue M, et al. IL-10 and CXCL2 in trigeminal ganglia in neuropathic pain. Neurosci Lett. 2019;703:132–138. doi: 10.1016/j.neulet.2019.03.031 [DOI] [PubMed] [Google Scholar]
- 34.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 35.Bryant AG, Hu M, Carlyle BC, et al. Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer’s Disease. Frontiers in Neurology. 2020;11:575953. doi: 10.3389/fneur.2020.575953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Liu S, Yang X, et al. Cathepsin C aggravates neuroinflammation via promoting production of CCL2 and CXCL2 in glial cells and neurons in a cryogenic brain lesion. Neurochem Int. 2021;148:105107. doi: 10.1016/j.neuint.2021.105107 [DOI] [PubMed] [Google Scholar]
- 37.De Paola M, Buanne P, Biordi L, et al. Chemokine MIP-2/CXCL2, acting on CXCR2, induces motor neuron death in primary cultures. Neuroimmunomodulation. 2007;14(6):310–316. doi: 10.1159/000123834 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on reasonable request from the corresponding author.