Abstract
This study investigates the prevalence of Trypanosoma cruzi infection among solid organ transplant recipients in the United States from 2019 to 2023 before transplantation. Utilizing data from a large multicenter network, we identified a rising seroprevalence of 4.8% from 1523 solid organ transplant recipients at the time of the evaluation for transplantation, particularly among lung and heart transplant recipients. The findings highlight the need for improved screening protocols to address this neglected tropical disease in transplant populations.
Keywords: Chagas disease, reactivation, screening, solid organ transplant, Trypanosoma cruzi
Graphical Abstract
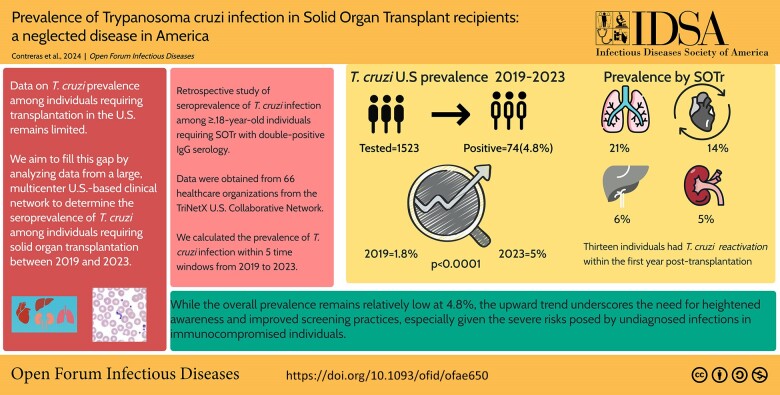
Graphical abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/prevalence-of-trypanosoma-cruzi-infection-in-solid-organ-transplant-recipients-a-neglected-disease-in-america-5effdb15-5cd2-4c40-8a17-8be39f09ff7d
This study reports a 4.8% prevalence of T. cruzi infection among 1,523 individuals who required solid organ transplantation from 2019 to 2023, with lung and heart transplant recipients showing the highest rates at the time of pre-transplant evaluation. These findings underscore the need for enhanced pre-transplant screening protocols.
Chagas disease, caused by the protozoan Trypanosoma cruzi, is a parasitic infection traditionally associated with rural areas of Latin America. However, due to increased migration from endemic regions, there is growing recognition of its presence in the United States [1, 2]. This migration has introduced new public health challenges, particularly for individuals undergoing solid organ transplantation, where undiagnosed T. cruzi infection can pose significant risks, including the potential for disease reactivation in an immunocompromised host [3–6].
Despite the increasing prevalence of Chagas disease in the United States, there remains a need for standardized pretransplant screening practices, especially in areas considered to have low endemicity. Previous studies have highlighted the importance of early detection and treatment to mitigate the risks of T. cruzi infection. Yet data on its prevalence among those requiring solid organ transplantation in the United States remain limited [7].
In this study, we aim to fill this gap by analyzing data from a large, multicenter, US-based clinical network to determine the seroprevalence of T. cruzi among individuals requiring solid organ transplantation between 2019 and 2023. We aim to underscore the need for more comprehensive screening protocols in individuals requiring solid organ transplantation by identifying the prevalence and associated demographic factors.
METHODS
We conducted a retrospective study to evaluate the prevalence of T. cruzi infection among solid organ transplant recipients (SOTrs) in the United States during pretransplant evaluation. Data were obtained from the TriNetX US Collaborative Network. This clinical database aggregates de-identified electronic medical records (EMRs) from >86 million patients across 66 health care organizations (HCOs); the study period spanned from 2019 to 2023.
Study Population
We included individuals aged 18 years or older who underwent heart, lung, kidney, or liver transplantation and had undergone double-positive immunoglobulin G (IgG) serology testing for T. cruzi during their pretransplant evaluation. The serological tests were conducted per World Health Organization (WHO) guidelines, with results reported as either positive or with an optical density (OD) of ≥1.20 [8]. The sensitivity and specificity of serological tests are typically around 85%–95% and 90%, respectively.
Data Collection
Patient data were collected from the TriNetX platform, which harmonizes data across participating HCOs using standardized health care information exchange codes such as codes from the International Classification of Diseases, Tenth Edition (ICD-10), Healthcare Common Procedure Coding System (HCPCS), Current Procedural Terminology (CPT), and Health Level Seven (HL7). The diagnosis, procedures, and medication codes used in this study are described in detail in Supplementary Tables 1–4. We defined T. cruzi reactivation as the presence of a diagnosis of Chagas disease within the first year after transplantation. The ICD-10 codes used to define Chagas disease reactivation are described in Supplementary Table 3. Information regarding microscopic, histological examination, or quantitative polymerase chain reaction for T. cruzi diagnosis is unavailable on the TriNext platform. Follow-up continued until each patient had completed 1 year since transplantation.
Due to the platform's data harmonization process, all data were de-identified, and no protected health information was accessible. This study was approved by the University of Texas Medical Branch Institutional Review Board (IRB #20–0085), with IRB exemption granted for using de-identified data [9].
Prevalence Calculation
We calculated the prevalence of T. cruzi infection among SOTrs within 5 discrete time windows from 2019 to 2023. For each time window, the prevalence was determined by identifying individuals with a positive serology result during the specified period. The lookback period was set to 1 day before the start of each time window to ensure comprehensive data capture.
Statistical Analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. Continuous variables were presented as medians with interquartile ranges (IQRs), while categorical variables were presented as frequencies and percentages. Comparisons between groups were performed using chi-square (χ2) or Fisher exact tests, as appropriate. Prevalence analyses were conducted using the TriNetX platform, which provides built-in statistical tools for data. We carried out the chi-square test to assess if the change in prevalence by year, ethnicity, and sex over time was significant in Python. A P value <.05 was considered significant.
RESULTS
We identified 1523 SOTrs tested for T. cruzi IgG serology during their pretransplant evaluation between 2019 and 2023. The cohort included recipients of kidney (n = 1102), liver (n = 165), heart (n = 69), lung (n = 49), and multiple organ transplants (n = 138). The population's median age (IQR) was 52 (26–74) years. Many patients were male (60%) and identified as non-Hispanic (49%) (Table 1). Among the 74 SOTrs who tested positive for T. cruzi before transplantation, 47% had a history of chronic ischemic heart disease, 26% had heart failure, 19% had a prior diagnosis of Chagas disease, and 18% had Chagas disease with heart involvement (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients With Double-Positive T. cruzi Serology Before Transplantation
| Demographicsa | No. (%) |
|---|---|
| Total tested positive | 74 (4.8) |
| Age, median (IQR), y | 52.6 (26–74) |
| Male | 50 (68.0) |
| Ethnicity | … |
| Hispanic | 36 (48.6) |
| Not Hispanic | 36 (48.6) |
| Race | … |
| White | 31 (42.0) |
| Asian | 14 (18.9) |
| Native Americanb | 10 (13.5) |
| Blackb | 10 (13.5) |
| Pacific Islanderb | 10 (13.5) |
| Comorbidities | … |
| Chronic kidney disease | 58 (78) |
| Hypertension | 56 (76) |
| Type 2 diabetes mellitus | 40 (54) |
| Chronic ischemic heart disease | 35 (47) |
| Chronic liver disease | 27 (36) |
| Obesity | 27 (36) |
| Heart failure | 19 (26) |
| Neoplasms | 17 (23) |
| Chagas disease | 14 (19) |
| Chagas disease with heart involvement | 13 (18) |
Abbreviation: IQR, interquartile range.
aUnless otherwise indicated, data are presented as the number/total number (%) of therapeutics.
bIf the patient count is ≤10, results show the count as 10.
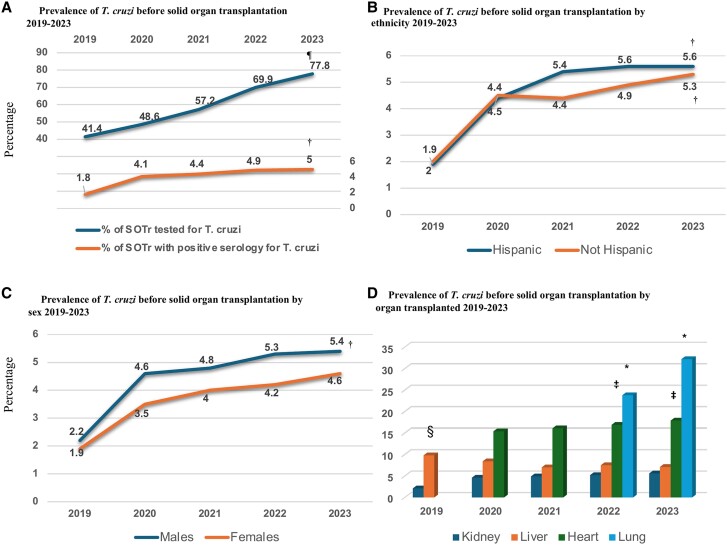
The overall prevalence of positive T. cruzi serology in this cohort was 4.8%, with a significant increase in prevalence from 2019 to 2023 (Figure 1A) (P < .0001). This progressive increase in prevalence was also driven by a significant progressive rise in infection screening (P = .043) (Figure 1A). Geographically, most positive cases were detected in the Western United States, with Hispanic individuals and males showing a significantly higher likelihood of having a positive T. cruzi serology throughout the observation period (Figure 1B and C). A breakdown by organ type revealed that lung (21%) and heart (14%) transplant recipients had the highest prevalence of positive serology compared with liver (6%) and kidney (5%) recipients (Figure 1D). Thirteen patients (17.6%) were diagnosed with T. cruzi reactivation within the first year after transplantation, with a median (IQR) of 8 (2–4) months. All reactivation cases were treated with benznidazole, and there were no deaths attributed to Chagas disease following treatment.
Figure 1.
Prevalence of T. cruzi serology in a cohort of solid organ transplant recipients from 2019 to 2023. (A–D) describe the prevalence of T. cruzi by year of screening, ethnicity, sex and transplanted organ. a2023 lung vs heart P = .004; lung vs liver P = .005; lung vs kidney P = .002; 2022 lung vs heart P = .004; lung vs liver P = .005; lung vs kidney P = .001. b2023 heart vs kidney P = .021; 2022 heart vs kidney P = .021; 2021 heart vs kidney P = .021 and 2019 heart vs kidney P = .003. c2019 liver vs kidney P = .003. dP < .0001. eP = .043.
DISCUSSION
Our study highlights the increasing prevalence of T. cruzi infection among solid organ transplant recipients in the United States from 2019 to 2023, with a notable rise observed, particularly among lung and heart transplant recipients. While the overall prevalence remains relatively low at 4.8%, the upward trend underscores the need for heightened awareness and improved screening practices, especially given the severe risks posed by undiagnosed infections in immunocompromised individuals [10–14].
The demographic trends observed in our study are consistent with prior research, showing a higher prevalence of positive T. cruzi serology among Hispanic individuals and males [10, 11]. This demographic association likely reflects the migration patterns from endemic regions, emphasizing the importance of considering patient origin and ethnicity in the risk assessment for Chagas disease. The higher prevalence rates in lung and heart transplant recipients could be attributed to existing comorbidities, such as chronic ischemic heart disease and heart failure, which are known to be associated with Chagas disease.
Our findings on T. cruzi reactivation rates post-transplantation provide essential insights into the clinical management of these patients. Although the reactivation rate was relatively low at 17.6%, it is critical to monitor these patients closely, as reactivation can lead to severe complications [3]. The successful treatment of all reactivation cases with benznidazole in our cohort suggests that early detection and timely intervention can effectively mitigate the risks associated with reactivation. However, the absence of deaths related to Chagas disease in this study does not diminish the potential severity of the disease if left untreated.
Despite these important findings, our study has several limitations that warrant cautious interpretation. The retrospective design and reliance on de-identified data from the TriNetX platform limit our ability to access detailed patient histories (ie, benznidazole prophylaxis) and specific serological test information. Trinext rounds to 10 if a patient or group has a number of events ≤10. The platform does this to safeguard protected health information, and because of this, we were able to determine the distribution of T cruzi reactivation by organ transplanted. The potential for selection bias exists due to the variable data update schedules across participating health care organizations. During this process, TriNetX carries out an extensive data quality assessment and harmonization. The platform rejects records that do not meet the TriNetX quality standards, thereby dropping data from transplant centers. Chagas disease reactivation might have been overestimated in our study by using ICD-10 codes instead of molecular or histopathological data that support the diagnosis of T. cruzi infection. Yet, our reactivation prevalence is similar to what is reported in the literature [3]. Given these limitations, future research should aim to include more granular data on patient ethnicity, migration history, and geographic origin to better understand the risk factors for T. cruzi infection and the rising prevalence among non-Hispanics observed in our cohort. Moreover, multicenter prospective studies with standardized screening protocols could provide a more comprehensive understanding of the disease burden and inform the development of targeted interventions.
In conclusion, our study underscores the need for expanded screening and awareness of Chagas disease among transplant candidates in the United States. As the prevalence of T. cruzi infection continues to rise, particularly in specific demographic groups, health care providers must remain vigilant in identifying and managing this neglected tropical disease. Prospective interventional studies are needed to assess the cost-effectiveness of screening all patients requiring transplantation, regardless of their ethnicity or geographic origin, to mitigate the risks associated with undiagnosed infection, given the growing prevalence in non-Hispanic individuals in our study. In the meantime, enhanced education and training for clinicians, coupled with the implementation of standardized screening protocols, will be crucial in ensuring the health and safety of transplant recipients.
Supplementary Material
Acknowledgments
Author contributions. German A. Contreras, MD, conceived the study, designed the research protocol, supervised the data collection, conducted the data analysis and interpretation, and wrote the initial draft of the manuscript. George Golovko, PhD, assisted in drafting and critically revising the manuscript for important intellectual content and provided expertise on the statistical methods and overall study design.
Financial support. This study was sponsored by the National Institutes of Health T32 Training Program in Center for Emerging Infectious Diseases (5T32AI155385-02) and by the Institute for Translational Health Sciences at the University of Texas Medical Branch at Galveston, supported in part by the Clinical & Translational Science Institute Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Data availability. TriNetX, as the primary data owner, will facilitate data sharing. To request access to the data, please get in touch with support@trinetx.com.
Patient consent. The UTMB IRB (IRB #20-0085) waived the patient's written consent because of the use of de-identified data. The study protocol was approved by the UTMB IRB (IRB #20-0085).
Potential conflicts of interest. The authors: no reported conflicts of interest.
Contributor Information
German A Contreras, Division of Infectious Diseases, Department of Internal Medicine, University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
George Golovko, Department of Pharmacology & Toxicology, University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Irish A, Whitman JD, Clark EH, Marcus R, Bern C. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg Infect Dis 2022; 28:1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark EH, Bern C. Chronic Chagas disease in the US. JAMA 2024; 331:2037–8. [DOI] [PubMed] [Google Scholar]
- 3. Jackson Y, Dang T, Schnetzler B, Pascual M, Meylan P. Trypanosoma cruzi fatal reactivation in a heart transplant recipient in Switzerland. J Heart Lung Transplant 2011; 30:484–5. [DOI] [PubMed] [Google Scholar]
- 4. Gray EB, La Hoz RM, Green JS, et al. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012–2016. Transpl Infect Dis 2018; 20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gómez-P CF, Mantilla-H JC, Rodriguez-Morales AJ. Fatal Chagas disease among solid-organ transplant recipients in Colombia. Open Forum Infect Dis 2014; 1:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallerano V, Consigli J, Pereyra S, et al. Chagas’ disease reactivation with skin symptoms in a patient with kidney transplant. Int J Dermatol 2007; 46:607–10. [DOI] [PubMed] [Google Scholar]
- 7. Clark EH, Messenger LA, Whitman JD, Bern C. Chagas disease in immunocompromised patients. Clin Microbiol Rev 2024; 37:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Guidelines for the diagnosis and treatment of Chagas disease. 2018. Available at: https://www.who.int/publications/i/item/9789275120439. Accessed July 19, 2023.
- 9. Palchuk MB, London JW, Perez-Rey D, et al. A global federated real-world data and analytics platform for research. JAMIA Open 2023; 6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meymandi SK, Forsyth CJ, Soverow J, et al. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin Infect Dis 2017; 64:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castro-Sesquen YE, Saldaña A, Patino Nava D, et al. Use of a latent class analysis in the diagnosis of chronic Chagas disease in the Washington metropolitan area. Clin Infect Dis 2021; 72:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henao-Martínez AF, Olivo-Freites C, Higuita NIA, et al. Clinical characteristics and outcomes of Chagas disease in the United States: a multicenter retrospective analysis. Am J Trop Med Hyg 2023; 109:1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salvador F, Sánchez-Montalvá A, Sulleiro E, et al. Prevalence of Chagas disease among solid organ-transplanted patients in a nonendemic country. Am J Trop Med Hyg 2018; 98:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis 2022; 225:1601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.