Abstract
Pregnant BALB/c mice were inoculated intravaginally on day 5 of gestation with the Chlamydia trachomatis mouse pneumonitis biovar. Animals that received 105, 106, or 107 inclusion-forming units (IFU) of C. trachomatis delivered prematurely on days 15 to 16 of gestation. A focal inflammatory infiltrate was observed in the wall of the uterus on the day 14 of gestation in animals inoculated with 105 IFU. In this group of mice, immunohistochemical analysis showed chlamydial inclusions in the endometrium and fetal membranes.
Infant mortality rates in the United States continue to be higher than those of most industrialized countries and have recently increased (9, 13, 18). These high infant mortality rates are mainly due to high rates of premature birth and associated low birth weight. The magnitude of this problem is such that recently, Hillier et al. (13) concluded that preterm delivery, low birth weight, and neonatal mortality are the most important problems in obstetrics. Determinants that affect low birth weight include genetic, social, environmental, and behavioral factors. Among these, infections of the genital tract are considered to account for up to 40% of preterm births and thus are probably the most significant contributors to high infant mortality rates (20). Organisms that have been associated with this problem include, among others, Chlamydia trachomatis, Gardnerella vaginalis, Mycoplasma hominis, Neisseria gonorrhoeae, Streptococcus agalactiae, Trichomonas vaginalis, Ureaplasma urealyticum, and other pathogens involved in bacterial vaginosis (9, 13).
C. trachomatis is one of the most common sexually transmitted pathogens in the Western world (4, 10, 24). Several studies over the last two decades have attempted to determine the impact that a C. trachomatis genital infection has on pregnancy outcome. Some of these studies found maternal and fetal morbidity and mortality associated with both acute and past chlamydial infections, while others did not confirm these data (2, 5, 6, 8, 12, 14, 16, 19). These contradictory results are not surprising considering the problems encountered in conducting these types of studies in humans, where assessment of a chlamydial infection is very difficult. Thus, only in an animal model can we start to characterize the role that a C. trachomatis infection may play in the outcome of pregnancy and on the mechanisms that may be involved in the pathogenesis of the disease. In this study, we describe a new murine model in which we determined the effect that an acute chlamydial genital infection during gestation has on pregnancy outcome.
C. trachomatis mouse pneumonitis (MoPn) biovar (strain Nigg II; American Type Culture Collection, Rockville, Md.) was grown in HeLa 229 cells (American Type Culture Collection), and elementary bodies (EB) were purified and stored in 0.2 M sucrose–20 mM sodium phosphate (pH 7.2)–5 mM glutamic acid (SPG) as previously described (3, 17). Eight- to 9-week-old female and proven breeder male BALB/c (H-2d) mice were purchased from Charles River (Wilmington, Mass.). Mice received normal diet and water ad libitum and were kept in isolation cubicles at a constant temperature of 24°C, with a cycle of 12 h of fluorescence light and 12 h of darkness. Groups of four female mice were housed with one male mouse and examined every morning for the presence of a vaginal plug as an indication of successful mating. When a vaginal plug was seen, the mouse was marked, weighed, and placed in a separate cage. The day the vaginal plug was observed was considered day 0 of gestation. Mice were inoculated intravaginally with C. trachomatis MoPn in 20 μl of SPG on day 5 of gestation with doses ranging from 101 to 107 inclusion-forming units (IFU) (7, 17). Three control groups were included in this study. The first control group received mock-infected HeLa 229 cell extracts in 20 μl of SPG processed in the same way as purified EB. The second group was inoculated with 105 C. trachomatis IFU that had been heat killed (HK) in 20 μl of SPG. A third control group was inoculated with 20 μl of SPG. Mice were examined and weighed daily to ascertain the progress of the pregnancy starting on day 10 of gestation. Within 24 h after birth, pups were weighed and body lengths were recorded. For histopathological studies, 15 fetuses from animals inoculated with 105 IFU of C. trachomatis MoPn and 12 controls from mice injected with HeLa 229 cell extracts were examined on day 14 of gestation. The uterine horns were fixed with the fetuses in situ, and tissue sections stained with hematoxylin and eosin (H&E). For immunohistological (IHC) analysis, staining with a rabbit anti-C. trachomatis MoPn serum followed by a biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, Calif.) was used to detect chlamydial inclusions, and the sections were counterstained with hematoxylin (17). To confirm that the staining was specific for C. trachomatis MoPn, normal rabbit serum was used as a control. For statistical analyses, differences between the control and infected animals in the occurrence of prematurity and birth rates were determined by Fisher’s exact test. Differences between groups in body weight and body length were compared by unpaired Student’s t test. The protocol was approved by the University of California, Irvine, Institutional Animal Care and Use Committee.
Mice infected with 105, 106, and 107 IFU of C. trachomatis showed signs of lethargy, hunched posture, and ruffled hair starting day 14 of pregnancy. Animals inoculated with 101, 103, or 104 IFU and the controls inoculated with HK C. trachomatis, HeLa cell extracts, and SPG showed no clinical abnormalities. All mice inoculated with 106 or 107 IFU of C. trachomatis delivered prematurely (Table 1). The mean gestation times at which delivery occurred for these groups were days 16.3 and 16.4, respectively. Of the 13 mice inoculated with 105 IFU, 12 (92.3%) delivered prematurely and only 1 delivered on day 19 of pregnancy. The mean gestation time for delivery to occur in this group was day 15.8. In contrast, the mean delivery time for the mice receiving 104, 103 and 101 IFU was day 19.6 or 19.5. All control mice inoculated with HK C. trachomatis MoPn, HeLa 229 cell extracts, or SPG delivered normal pups. The mean delivery date for the three control groups was day 19.3. Animals born prematurely were cannibalized by their mothers and so were not available for measurement. The mean numbers of babies born per pregnant mouse were similar for all groups. No significant differences were observed in the mean body weight or length between the control animals and those inoculated with C. trachomatis MoPn that were born on day 19 or 20 of gestation.
TABLE 1.
Effects of different C. trachomatis MoPn inocula on pregnancy outcome
| Inoculum dose/mouse | No. of pregnant mice that delivered
|
Mean no. of babies born/pregnant mouse ± 1 SD | Mean no. of gestation days at time of delivery
|
Characteristic of conceptus at birtha
|
|||
|---|---|---|---|---|---|---|---|
| Prematurely/total (%) | At term/total (%) | Premature | Normal | Mean wt (g) ± 1 SD | Mean length (cm) ± 1 SD | ||
| C. trachomatis MoPn | |||||||
| 107 IFU | 7/7 (100)b | 0/7 (0)b | NAc | 16.4 | NA | NAMd | NAM |
| 106 IFU | 4/4 (100)b | 0/4 (0)b | NA | 16.3 | NA | NAM | NAM |
| 105 IFU | 12/13 (92.3)b | 1/13 (7.7)b | 5f | 15.8 | 19.0 | 1.43 ± 0.08 | 2.70 ± 0.07 |
| 104 IFU | 0/7 (0) | 6/7 (85.7)e | 4.1 ± 2.4 | NA | 19.6 | 1.48 ± 0.19 | 2.82 ± 0.12 |
| 103 IFU | 0/4 (0) | 4/4 (100) | 6.3 ± 1.7 | NA | 19.5 | 1.44 ± 0.17 | 2.75 ± 0.11 |
| 101 IFU | 0/3 (0) | 3/3 (100) | 5.3 ± 1.2 | NA | 19.6 | 1.62 ± 0.16 | 2.87 ± 0.15 |
| 105 HK C. trachomatis MoPn | 0/6 (0) | 6/6 (100) | 6.3 ± 1.9 | NA | 19.3 | 1.33 ± 0.23 | 2.65 ± 0.16 |
| HeLa cell extract | 0/22 (0) | 22/22 (100) | 5.3 ± 1.3 | NA | 19.3 | 1.55 ± 0.21 | 2.80 ± 0.25 |
| SPG | 0/9 (0) | 9/9 (100) | 5.4 ± 1.5 | NA | 19.3 | 1.39 ± 0.19 | 2.76 ± 0.18 |
Body length and weight were measured within 24 h after birth.
P < 0.05 by Fisher’s exact test compared with the HeLa cells extract or HK C. trachomatis MoPn-inoculated group.
NA, not applicable.
NAM, not available for measurement.
One mouse died before delivery.
Babies delivered from one mother.
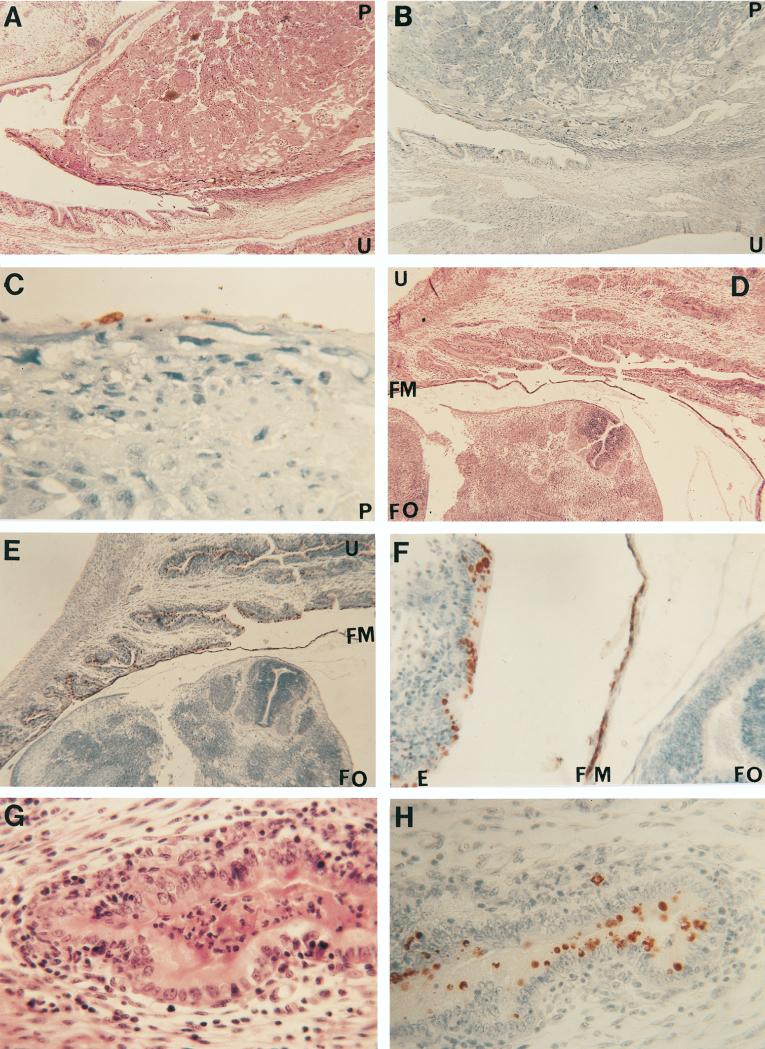
In a majority (12 of 15) of the fetuses processed for histopathology from the pregnant mice inoculated with C. trachomatis MoPn, a mild focal acute inflammatory reaction consisting mainly of polymorphonuclear leukocytes with scattered mononuclear inflammatory cells, including some plasma cells, was observed in the maternal uterine wall (Fig. 1). In 3 of the 15 specimens studied, the inflammatory response was more severe. No inflammatory reaction was detected in the fetal tissues. IHC staining with a specific chlamydial rabbit polyclonal antibody revealed C. trachomatis inclusions in the maternal endometrium, in the splanchnopleure of the yolk sac, and in the periplacental bilaminar omphalopleure (15). Chlamydial inclusions were more numerous in the tissues in which there was a severe inflammatory reaction. No chlamydial inclusions were found in the zone of giant cells, trophospongium, the labyrinth, or the chorionic plate of the chorioallantoic placenta. Similarly, no chlamydial inclusions were detected in the amnion or in the fetal organs. Fetal and maternal tissues from 12 control fetuses had no inflammatory response, and no chlamydial inclusion were detected.
FIG. 1.
(A to C) Histological section at the placental site of insertion stained with H&E (A [magnification, ×30]) and an IHC stain for C. trachomatis (B [×30] and C [×250]). The overall architecture of the placenta and the uterine wall is well preserved (A). Chlamydial inclusions can be detected in the endometrium (B) and in the periplacental bilaminar omphalopleure (B and C). (D to F) Section of the fetus and uterine wall stained with H&E (D [×30]) and an IHC stain for C. trachomatis (E [×30] and F [×160]). Fetal tissues appear normal and at a developmental stage corresponding to 14 to 15 days of gestation (D). Chlamydial inclusions can be observed in the endometrium and in splanchnopleure of the yolk sac (E and F). (G and H) Uterus stained with H&E (G [×250]) and the IHC stain for C. trachomatis (H [×250]). A moderate acute and chronic inflammatory reaction (G) and multiple chlamydial inclusions (H) can be observed in the endometrium. Abbreviations: E, endometrium; FM, fetal membranes; FO, fetal organs; P, placenta; U, uterus.
The effects of a C. trachomatis infection on pregnancy remain controversial (2, 5, 6, 8, 12, 14, 16, 19). Most likely, depending on the infecting inoculum, time of gestation, and susceptibility of the host, a wide variety of clinical manifestations ranging from asymptomatic infection to termination of pregnancy may occur. In a preliminary report, Spiliopoulou et al. (21) indicated that intravenous inoculation of Swiss mice on day 11 of gestation with doses ranging from 105 to 107 IFU of the C. trachomatis serovars E and L1 resulted in a reduced number of infant mice. A strong colonization of the placenta was observed, whereas colonization of the fetus was less extensive. Tuffrey et al. (23) inoculated intraperitoneally, or intravenously and intravaginally, TO mice with C. trachomatis serovar E either 14 days before detection of a vaginal plug or from 1 to 9 days thereafter. C. trachomatis was isolated from the placental disk in approximately 25% of the mice but not from fetal tissue or from maternal spleens. However, litter size and percentage of fetuses dying were not significantly different between the test and control animals. Thus, the conclusion from these experiments was that C. trachomatis did not affect pregnancy outcome and did not cross the placenta. As indicated by Tuffrey et al. (23), the main weakness of the model is that there is no evidence that intravaginal inoculation with the human C. trachomatis serovars in mice nonpretreated with progesterone results in infection of the upper genital tract. In fact, even in mice pretreated with progesterone, the ability of the human serovars of C. trachomatis to cause significant upper genital infection has been questioned (22). With the C. trachomatis MoPn biovar, on the other hand, we have shown that intravaginal inoculation, without pretreatment with progesterone, can result in salpingitis and infertility (7). Thus, the mouse serovar in this respect more closely parallels a human genital infection, and as a result, we should be able to better assess the effects of a C. trachomatis infection on pregnancy outcome. Here, using this model, we have shown that C. trachomatis MoPn inoculated intravaginally on day 5 of gestation infects the endometrium and the membranes of the yolk sac, resulting in early termination of pregnancy. This is not surprising since chlamydial endometritis commonly occurs during a genital infection and the ability of C. trachomatis to infect amniotic cells has been demonstrated in vitro (11). Most likely, the fetal membranes were affected following infection of the endometrium. It is possible that the direct damage to the fetal membranes resulting from the infection, in combination with the endotoxin activity of the chlamydial lipopolysaccharide, is a significant factor in the premature termination of pregnancy.
In conclusion, we have shown that a genital infection early in gestation with a high chlamydial inoculum can result in premature termination of pregnancy, while a low inoculum does not appear to affect the course of gestation. We realize that due to the anatomical and physiological differences between a human and a murine pregnancy, there are limitations in this model. However, mice have been successfully used to characterize some of the effects of bacteria on pregnancy outcome (1). Thus, we think that this model could be very helpful for gaining an understanding of the possible effects and pathogenesis of chlamydial infections during gestation and for assessing the possibilities for developing preventive measures.
Acknowledgments
This work was supported by Public Health Service grants AI-32248 and AI-30499 from the National Institute of Allergy and Infectious Diseases.
We thank Gerald Spear (University of California, Irvine) and Kurt Benirschke (University of California, San Diego) for help in assessing the fetal tissues.
REFERENCES
- 1.Baumgartner W, Bachmann S. Histological and immunocytochemical characterization of Coxiella burnetii-associated lesions in the murine uterus and placenta. Infect Immun. 1992;60:5232–5241. doi: 10.1128/iai.60.12.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman S M, Harrison H R, Boyce W T, Haffner W J J, Lewis M, Arthur J B. Low birth weight, prematurity and postpartum endometritis. Association with prenatal cervical Mycoplasma hominis and Chlamydia trachomatis infections. JAMA. 1987;257:1189–1194. [PubMed] [Google Scholar]
- 3.Caldwell H D, Kromhout J, Schachter J. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cates W, Rolfs R T, Aral S O. Sexually transmitted diseases, pelvic inflammatory disease and infertility: an epidemiologic update. Epidemiol Rev. 1990;12:199–220. doi: 10.1093/oxfordjournals.epirev.a036054. [DOI] [PubMed] [Google Scholar]
- 5.Claman P, Toye B, Peeling R W, Jessamine P, Belcher J. Serologic evidence of Chlamydia trachomatis infection and risk of preterm birth. Can Med Assoc J. 1995;153:259–262. [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen I, Veille J C, Calkins B M. Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA. 1990;263:3160–3163. [PubMed] [Google Scholar]
- 7.de la Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gencay M, Puolakkainen M, Wahlstrom T, Ammala P, Mannonen L, Vaheri A, Koskiniemi M L. Chlamydia trachomatis detected in human placenta. J Clin Pathol. 1997;50:852–855. doi: 10.1136/jcp.50.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg R L, Rouse D J. Prevention of premature birth. N Engl J Med. 1998;339:313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 10.Grayston J T, Wang S P. New knowledge of Chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Harrison H R, Riggin R T. Infection of untreated primary human amnion monolayers with Chlamydia trachomatis. J Infect Dis. 1979;140:968–971. doi: 10.1093/infdis/140.6.968. [DOI] [PubMed] [Google Scholar]
- 12.Harrison H R, Alexander E R, Weinstein L, Lewis M, Nash N, Sim D A. Cervical Chlamydia trachomatis and mycoplasmal infections in pregnancy. Epidemiology and outcomes. JAMA. 1983;250:1721–1727. [PubMed] [Google Scholar]
- 13.Hillier S L, Nugent R P, Eschebach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek J G, Rao A V, McNellis D, Regan J A, Carey J C, Klebanoff M A for the Vaginal Infections and Prematurity Study Group. Association between bacterial vaginosis and preterm delivery of a low birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 14.Martin D H, Koutsky, Eschebach D A, Daling J R, Alexander E R, Benedetti J K, Holmes K K. Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA. 1982;247:1585–1599. [PubMed] [Google Scholar]
- 15.Mossman H W. Vertebrate fetal membranes. New Brunwsick, N.J: Rutgers University Press; 1987. [Google Scholar]
- 16.Osser S, Persson K. Chlamydial antibodies in women who suffer miscarriage. Br J Obstet Gynaecol. 1996;103:137–141. doi: 10.1111/j.1471-0528.1996.tb09665.x. [DOI] [PubMed] [Google Scholar]
- 17.Pal S, Fielder T J, Peterson E M, de la Maza L M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philip A G S. Neonatal mortality rate: is further improvement possible? J Pediatr. 1995;126:427–433. doi: 10.1016/s0022-3476(95)70463-9. [DOI] [PubMed] [Google Scholar]
- 19.Rae R, Smith I W, Liston W A, Kilpatrick D C. Chlamydial serologic studies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;170:782–785. doi: 10.1016/s0002-9378(94)70282-9. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Spiliopoulou D, Psarrou E, Rodolakis A, Vretou E. Protection of mice infected with C. trachomatis from abortion by passive immunization. In: Mårdh P A, La Placa M, Ward M, editors. Proceedings of the European Society for Chlamydia Research. Uppsala, Sweden: Uppsala University Center for STD Research; 1992. p. 103. [Google Scholar]
- 22.Su H, Parnell M, Caldwell H D. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital infection. Vaccine. 1995;13:1023–1031. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 23.Tuffrey M, Falder P, Taylor-Robinson D. Failure of Chlamydia trachomatis to pass transplacentally to fetuses of TO mice infected during pregnancy. J Med Microbiol. 1987;25:1–5. doi: 10.1099/00222615-25-1-1. [DOI] [PubMed] [Google Scholar]
- 24.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson S E. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]