Abstract
Background
Immune‐based therapy targeting immunoglobulin E (IgE), anti‐IgE treatment, has emerged as an adjunct treatment for children with severe allergic asthma. After start of anti‐IgE treatment, an effect of the treatment cannot be monitored by Total‐IgE, because current methods measure both bound and free IgE molecules. Basophil activation test may be very useful for monitoring anti‐IgE treatment efficacy. The objective of this paper is to evaluate if basophil activation test is applicable in regulating the anti‐IgE treatment.
Methods
A case series of 20 children with IgE‐mediated severe allergic asthma were treated according to guidelines with anti‐IgE (Omalizumab). Blood samples were drawn for total IgE, specific IgE, number of IgE receptors (FcεRI) and basophil sensitivity were measured at baseline before anti‐IgE treatment and 4 months after initiation of anti‐IgE treatment.
Results
A total of 19 out of 20 children had statistically significant and clinically relevant effects of anti‐IgE treatment on symptom score, lung function and medication. All 20 children had a significant reduction in basophil allergen sensitivity and the number of IgE receptors (FcεRI) on blood basophils. Anti‐IgE treatment was found to be well controlled by measuring basophil allergen sensitivity and FceRI density on blood basophils.
Conclusion
This cohort study demonstrates a promising method, measuring basophil allergen sensitivity and in particular blood basophil FceRI density, concerning the monitoring of anti‐IgE treatment in different clinical situations. There are no randomized controlled trials evaluating this method in clinical settings.
Keywords: anti IgE treatment, asthma control, basophil FceRI expression, basophile activation test, children, severe allergic asthma
1. INTRODUCTION
Immune‐based therapy targeting immunoglobulin E (IgE), anti‐IgE treatment, is well documented in the treatment of a variety of allergic diseases, in children specifically for severe allergic asthma. 1 , 2 Anti‐IgE treatment consists of monoclonal humanized IgG antibodies capable of binding circulating IgE at the constant region on the tail of the IgE molecule. This prevents the binding of IgE to cognate high‐affinity receptors (FcεRI receptors) located on effector cells such as blood basophils and mast cells, ultimately inhibiting the release of inflammatory mediators 3 , 4
Omalizumab (Xolair©) is an anti‐IgE therapeutic drug. It is approved as an add‐on drug in the treatment of severe allergic asthma in patients above 6 years of age where (1) high dose anti‐asthmatic treatment is not sufficient in achieving satisfying asthma control. (2) Presence of all‐year sensibilization and allergy towards one or more airborne allergens. (3) Frequent exacerbations or multiple asthma symptoms during day and/or night. The initial dose and frequency of administration are decided based on the weight and total IgE‐level of the patient. 3 , 5 Besides severe allergic asthma, Oamlizumab can also be considered in cases with severe urticaria, Chronic rhinosinuitis with nasal polyps, and severe food anaphylaxis. There are no well‐documented methods to regulate dosage or monitor treatment efficacy of Omalizumab. Omalizumab and IgE molecules gather in complexes and the standard methods used to quantify IgE do not differentiate between bound and unbound IgE making it impossible to monitor total IgE after treatment with Omalizumab has started. Nopp et al. 6 demonstrated that “free IgE” is reduced by up to 99% within 3 days of the first administered Omalizumab dose. 7 Detection of “free IgE” in a clinical setting is, however, difficult. A study by Korn et al. 8 has found that detection of free IgE by enzyme‐linked immunosorbent assay (ELISA) is possible, but there was no correlation between the level of “free IgE” and clinical response. 8 The present monitoring of treatment with Omalizumab is, therefore, based solemnly on clinical presentation—e.g., lung function (forced expiratory volume in 1 s [FEV1]) and asthma control score (ACT). However, it has been demonstrated that despite no improvement in FEV1, patients still have clinical improvement in ACT and other clinical parameters as need for systemic steroids. 9
Basophil activation test (BAT) measures the degree of IgE‐mediated response towards a defined allergen. With BAT an objective value for the basophile sensitivity determined as the half‐maximal concentration of basophils reactivity (EC50) is obtained and that makes it a promising player in monitoring anti‐IgE treatment efficacy. 6 , 7 , 10 , 11 , 12 CD123 and CD193 are used for basophil identification, and CD63 is the most commonly used marker for basophil activity. 12 CD‐sens is defined as the inverted value of the eliciting concentration at which 50% of basophils respond (EC50) multiplied by 100. 13 Several studies have shown that CD‐sens is reduced with anti‐IgE treatment 14 , 15 , 16 and other studies have evaluated if CD‐sens can be used in monitoring the treatment of chronic urticaria 17 and food allergy. 18 Nopp et al. 19 showed that patients 3 years after discontinuation of 6 years of treatment with Omalizumab had mild and stable asthma along with a considerable, downregulation of CD‐sens. 19 In addition to assessing basophil sensitivity as EC50 (the inverse of CD‐sens) by BAT, we measured the density of FceRI on blood basophils as a clinical outcome. In a previous study, we found that allergen immunotherapy dramatically decreases the density of FceRI on blood basophils during allergen immunotherapy, 20 and we explored whether this readily available biomarker also is useful here. We hypothesize monitoring of treatment for allergic asthma is possible.
With this cohort study, we aim to evaluate if BAT can be used in monitoring anti‐IgE treatment of children with IgE‐mediated severe allergic asthma. We found measurement of FceRI on blood basophils to be a more useful metric of Omalizumab response than basophil sensitivity.
2. METHODS
2.1. Study group and measurements
The study group consisted of 20 patients treated with Omalizumab at the Center of Pediatric Pulmonology and Allergology, Department of Child and Adolescent Health, Aarhus University Hospital, Denmark, from 2010 to 2022. All 20 children met the standard criteria for Omalizumab treatment in children. At baseline and after 16 weeks all children performed (1) lung function test (including reversibility) (2) bronchial provocation with mannitol and (3) BAT and determination of FceRI density on blood basophils. In addition, (4) medication and (5) status of asthma control (GINA/BTS) were registered. Systemic oral prednisolone treatment dose was calculated and registrered as accumulated annual dose. The research project is reported to the Data Protection Agency through the joint notification in the Central Jutland Region and approved by the legal office and Scientifical Ethical Committee in the Central Jutland Region (j.nr. 631742) with conclusion of no need for informed consent from patients due to data collected from patient records without need of additional sampling to the study. The study follows the standards of the Declaration of Vancouver and European Medicines Agency Guidelines for Good Clinical Practice.
2.2. Method of intervention—measurements of basophile activation test
Blood samples were drawn for total IgE, specific IgE, number of IgE receptors (FcεRI), and sensitivity to allergen by basophil activation test, essentially as described. 19 Heparinised blood was analyzed in 100 µL aliquots stimulated with 9 log10 dilutions of relevant allergen for 30 min at 37°C. 12 , 13 FceRI density was determined with a QiFiKit (DAKO, DK) with antibodies to FecRI and to CD3 on blood T cells as a control, according to manufacturer instructions.
2.3. Statistical method
The fraction of activated basophils was plotted against allergen concentration to establish an EC50 with GraphPad Prism. Two‐tailed nonparametric statistics were used on paired samples, and p < .05 was considered statistically significant. Receiver operating characteristic (ROC) analysis was performed with GraphPad Prism.
3. RESULTS
3.1. Study population
Twenty children aged 6–18 years (mean: 12.65 years) were treated with Omalizumab for severe perennial allergic asthma (Table 1). Treatment was monitored at baseline and after 16 weeks.
Table 1.
Case summary: anti‐IgE treatment within standard indication in severe allergic asthma.
| Prestart anti‐IgE treatment | Post 16‐week anti‐IgE treatment | p‐value | |
|---|---|---|---|
| Age | 6–18 years | 6–18 years | NA |
| Allergies, sensitizations | HDM*, Grass, Birch, Aspergillus | HDM*, Grass, Birch, Aspergillus | NA |
| Dose Omalizumab treatment |
Range: 150 mg per 4 weeks to 600 mg per 2 weeks |
Range: 150 mg per 4 weeks to 600 mg per 2 weeks | NA |
| Total IgE | Range: 44–1945 | NA | |
| FceRI* | Range: 32.457–1.768.208 | Range: 297–3.334 | p < .0001 |
| EC50* | Range: −8.0 to 4.8 × 10−7 | Range: −3.3 to 1.8 × 10−6 | p = .0002 |
| Symptom score (GINA/BTS*) | Not‐controlled asthma/ARC | Controlled asthma/controlled ARC | p < .0001 |
| Lung function (FEV1*) | Range: 55%–109% | Range: 72%–127% | p < .0001 |
| Lung function (Reversibility test) | Positive reversibility test | Negative reversibility test | p < .0001 |
| Lung function (Mannitol test) | Positive mannitol test at 40 mg | Negative mannitol test at 635 mg | p < .0001 |
Abbreviations: FEV1, forced expiratory volume in 1 s; IgE, immunoglobulin E.
There was a statistically and clinically significant (p = .014) change in asthma status from uncontrolled to controlled in 19 out of 20 children (95%) (Table 2, Figure 1A). Mannitol test was significantly (p < .001) changed to a negative (normal) test and lung function (FEV1) was significantly increased in the accumulated study population (Figure 1A,B). Six of 19 (31%) children with improved asthma control had only marginal improvements in FEV1, and one had a slight decrease in FEV1 (Figure 1A). Regular anti‐asthmatic treatment was reduced especially for inhaled corticosteroids where 17 out of 20 (85%) were reduced from high‐dose treatment (step 4 GINA) to medium or low dose treatment (steps 2–3, GINA) (Table 3). The number of accumulated annual systemic treatments with corticosteroid decreased from 84 before Omalizumab treatment to four systemic corticosteroid treatments after Omalizumab treatment (Table 3).
Table 2.
Case overview—effect of anti‐IgE treatment within standard indication in severe allergic asthma.
| Case | Age/years | Allergy/total‐IgE |
Prestart FceRI*/EC50* |
Post 16 weeks FceRI*/EC50* |
Dose Omalizumab |
Pretreatment symptom scoreGINA/BTS* |
Posttreatment symptom scoreGINA/BTS* |
Pretreatment lung function (FEV1*) |
Posttreatment lung function (FEV1*) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 |
Grass IgE 1243 |
150.000/−5.4 | 715/−0.7 | 600 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 109 Pos. reversibility Pos. mannitol, 315 mg |
FEV1% 126 Neg. reversibility Neg. mannitol, 635 mg |
| 2 | 9 |
HDM* IgE 413 |
171.241/−0.92 | 297/0.77 | 300 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 105 Pos. reversibility Pos. mannitol, 40 mg |
FEV1% 107 Neg. reversibility Pos. mannitol, 475 mg |
| 3 | 11 |
Grass IgE 682 |
55.535/−1.97 | 729/0.22 | 375 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 101 Neg. reversibility Pos. mannitol, 155 mg |
FEV1% 106 Neg. reversibility Neg. mannitol, 635 mg |
| 4 | 13 |
HDM* IgE 1890 |
Nd*/−7.3 | 1.000/−3.3 | 600 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 101 Pos. reversibility Pos. mannitol, 315 mg |
FEV1% 108 Neg. reversibility Neg. mannitol, 635 mg |
| 5 | 14 |
Grass IgE 806 |
110.470/−0.84 | 991/−1.77 | 375 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 72 Pos. reversibility Pos. mannitol, 475 mg |
FEV1% 96 Neg. reversibility Neg. mannitol, 635 mg |
| 6 | 11 |
Aspergillus IgE 443 |
168.335/−2.92 | 3.334/−2.01 | 300 mg/4 weeks | Not‐controlled asthma/CF* | Controlled Asthma/CF |
FEV1% 55 Pos. reversibility Pos. mannitol, 155 mg |
FEV1% 72 Neg. reversibility Neg. mannitol, 635 mg |
| 7 | 17 |
Grass IgE 164 |
1.768.208/−2.81 | 1.315/−1.67 | 300 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 95 Pos. reversibility Pos. mannitol, 635 mg |
FEV1% 109 Neg. reversibility Neg. mannitol, 635 mg |
| 8 | 12 |
HDM* IgE 362 |
102.515/−5.48 | 1.087/−2.08 | 300 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 83 Pos. reversibility Pos. mannitol, 315 mg |
FEV1% 95 Neg. reversibility Pos. mannitol, 315 mg |
| 9 | 14 |
HDM* IgE 292 |
123.000/−4.93 | 645/−2.94 | 450 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 102 Neg. reversibility Pos. mannitol, 40 mg |
FEV1% 109 Neg. reversibility Neg. mannitol, 635 mg |
| 10 | 12 |
Grass IgE 1945 |
145.164/−3.14 | 1.110/0.27 | 600 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 95 Neg. reversibility Pos. mannitol, 635 mg |
FEV1% 113 Neg. reversibility Neg. mannitol, 635 mg |
| 11 | 12 |
Grass HDM* IgE 453 |
83.700/−2.6 | 592/0.12 | 600 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 93 Pos. reversibility Pos. mannitol, 75 mg |
FEV1% 104 Neg. reversibility Neg. mannitol, 635 mg |
| 12 | 14 |
HDM* IgE 44 |
32.457/−8.0 | 571/−2.04 | 150 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 75 Pos. reversibility Pos. mannitol, 75 mg |
FEV1% 115 Neg. reversibility Neg. mannitol, 635 mg |
| 13 | 18 |
Aspergillus IgE 502 |
92.797/0.077 | 777/423 | 600 mg/4 weeks | Not‐controlled asthma/cystic fibrosis | Controlled asthma/cystic fibrosis |
FEV1% 93 Neg. reversibility Pos. mannitol, 315 mg |
FEV1% 87 Neg. reversibility Neg. mannitol, 635 mg |
| 14 | 12 |
HDM* IgE 391 |
156.468/4.8 × 10−7 | 961/1.8 × 10−6 | 600 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 109 Pos. reversibility Pos. mannitol, 635 mg |
FEV1% 127 Neg. reversibility Neg. mannitol, 635 mg |
| 15 | 9 |
Grass HDM* IgE 124 |
83.739/0.029 | 1.000/0.49 | 150 mg/4 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 71 Pos. reversibility Pos. mannitol, 75 mg |
FEV1% 86 Neg. reversibility Neg. mannitol, 635 mg |
| 16 | 15 |
Grass HDM* IgE 823 |
150.000/0.84 | 724/0.0 | 450 mg/2 weeks | Not‐controlled asthma | Controlled asthma |
FEV1% 74 Pos. reversibility Pos. mannitol, 75 mg |
FEV1% 107 Neg. reversibility Neg. mannitol, 635 mg |
| 17 | 14 |
Grass Birch IgE 646 |
90.515/0.012 | 1.910/0.184 | 450 mg/2 weeks | Not‐controlled asthma/ARC | Not‐controlled asthma/ARC |
FEV1% 65 Neg. reversibility Mannitol negative |
FEV1% 69 Neg. reversibility Neg. mannitol, 635 mg |
| 18 | 11 |
Grass Birch IgE 704 |
158.000/0.0017 | 1.300/1.9 | 600 mg/4 weeks | Not‐controlled ARC | Controlled ARC |
FEV1% 97 Neg reversibility Pos. mannitol v. 475 mg |
FEV1% 113 Neg reversibility Mannitol. negative |
| 19 | 6 |
HDM* Grass Birch IgE 375 |
134.750/0.024 | 2.713/0.1 | 225 mg/4 weeks | Not controlled asthma | Controlled asthma |
FEV1% 108 Neg. reversibility Mannitol. Negative |
FEV1% 129 Neg. reversibility |
| 20 | 16 |
HDM* IgE 111 |
97.236/0.011 | 2.169/0.993 | 300 mg/4 weeks | Controlled asthma/not controlled ARC | Controlled asthma/controlled ARC |
FEV1% 73 Neg. reversibility Pos. mannitol, 155 mg |
FEV1% 75 Neg. reversibility Neg. mannitol, 635 mg |
Note: *EC50: The half‐maximal concentration of basophils reactivity (EC50, CD‐sens, basophil sensitivity). *FEV1: forced expiratory volume in 1 s. *FceRI: Fc epsilon RI, is the high‐affinity receptor for the Fc region of immunoglobulin E (IgE). *HDM: House Dust Mite. *GINA/BTS: Global Initiative for Asthma/British Thoracic Society, PRN: Pro Re Nata (as needed), Nd: No data, CF: cystic fibrosis.
Abbreviation: IgE, immunoglobulin E.
Figure 1.
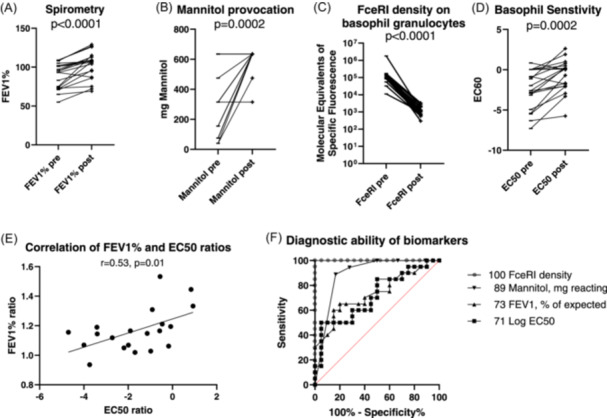
(A–F) Treatment effects on clinical outcome, basophil sensitivity, and FcεRI receptors and their diagnostic ability at baseline and after 16 weeks.
Table 3.
Case overview—effect of anti‐IgE treatment on asthma and allergy medication.
| Case |
Pre‐anti‐IgE treatment asthma medication |
Post‐anti‐IgE treatment asthma medication | Pre‐anti‐IgE treatment allergy medication | Post‐anti‐IgE treatment allergy medication |
|---|---|---|---|---|
| 1 |
Singulair* 10 mg 2 Prednisolon OR min ×10 pr. year |
Singulair 10 mg ×1 Prednisolon OR max ×2 |
Avamys* ×4 Opatanol* ×4 Aerius* 10 mg ×3 Tobradex* |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 2 |
Singulair 5 mg ×2 Seretide* 50/250 µg ×2 Prednisolon OR min ×10 pr. year |
Singulair 5 mg ×1 Seretide 50/100 µg ×1 |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×3 Tobradex* |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 3 |
Singulair 10 mg ×2 Spirocort* 200 µg ×4 Prednisolon OR min ×10 pr. year |
Singulair 10 mg ×1 Spirocort 200 µg ×1 |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×2 Tobradex |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 4 |
Singulair 10 mg x3 Symbicort* 9/320 µg ×2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Aerobec* 100 µg ×1 |
Allergodil* ×2 Flixonase* ×2 Opatanol ×4 Aerius 5 mg ×2 Tobradex |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 5 |
Singulair 10 mg ×2 Symbicort 9/320 µg ×2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Symbicort 4.5/160 µg ×1 Prednisolon OR ×1 pr year |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×2 |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 6 |
Singulair 10 mg ×2 Symbicort 9/320 µg ×2 Theo‐dur* 100 mg ×2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Symbicort 4.5/160 µg ×1 Theo‐dur 100 mg ×2 Prednisolon OR ×1 pr year |
Avamys ×2 | Avamys ×1 |
| 7 |
Singulair 10 mg ×1 Symbicort 320/9 µg 2 × 2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Symbicort 160/4.5 µg ×1 |
Avamys ×2 Zaditen* ×4 Cetirizin 10 mg ×1 |
Avamys PRN Zaditen PRN Cetirizin 10 mg PRN |
| 8 |
Singulair 10 mg ×1 Symbicort 9/320 µg ×2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Relvar Ellipta*184/22 µg ×1 |
Aerius 5 mg ×2 | Aerius 5 mg PRN |
| 9 |
Singulair 10 mg ×2 Symbicort 9/320 µg ×2 Aerobec 100 µg ×2 Spiriva* 18 µg ×2 Prednisolon OR min ×5 pr. Year |
Singulair 10 mg ×1 Symbicort 4.5/160 µg ×2 |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×2 |
Avamys PRN Opatanol PRN Aerius 5 mg PRN |
| 10 |
Singulair 10 mg ×1 Oxis* 4.5 µg ×2 Spirocort 200 µg ×2 Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×4 |
Avamys x×1 Opatanol PRN Aerius 5 mg PRN |
| 11 |
Singulair 10 mg ×1 Symbicort 9/320 µg ×2 Bricanyl 0.5 mg ×2 + PRN Prednisolon OR min ×5 pr. year |
Singulair 10 mg ×1 Symbicort 4.5/160 µg ×2 |
Avamys ×2 Opatanol ×4 Xyzal* 10 mg ×4 |
Avamys x×1 Opatanol PRN Aerius 5 mg PRN |
| 12 |
Singulair 10 mg ×1 Formo* 12 µg ×2 Giona* 200 µg 2 × 2 Theo‐dur 200 mg ×2 Prednisolon OR min ×3 pr. year |
Singulair 10 mg ×1 Formo 12 µg ×1 Giona 200 µg ×2 |
Avamys ×2 Opatanol ×4 Alnok* 10 mg ×4 |
Opatanol PRN Aerius 5 mg PRN |
| 13 |
Symbicort 9/320 µg ×2 Prednisolon OR min ×5 pr. year |
No preventer treatment | Aerius 5 mg ×2 | Aerius 5 mg PRN |
| 14 |
Bricanyl 0,5 mg ×2 + PRN Singulair 10 mg ×1 Symbicort 9/320 µg ×2 Spirocort 200 µg ×2 Spiriva* 18 µg ×2 Prednisolon OR min ×5 pr. year |
Bricanyl 0.5 mg ×2 + PRN Symbicort 4.5/160 µg ×2 Spirocort 200 µg ×2 |
Aerius 5 mg ×4 | Aerius 5 mg PRN |
| 15 |
Bricanyl 0.5 mg ×2 + PRN Singulair 5 mg ×1 Symbicort 4.5/160 µg ×2 Spirocort 200 µg ×2 Theo‐dur 100 mg ×2 Prednisolon OR min ×3 pr. year |
Singulair 5 mg x1 Buventol 0.1 mg PRN Bufomix* 4.5/160 µg ×2 |
Avamys ×2 Opatanol ×4 Aerius 5 mg ×4 |
Avamys ×1 Opatanol PRN Aerius 5 mg PRN |
| 16 |
Bricanyl 0.5 mg ×2 + PRN Singulair 5 mg ×1 Symbicort 4.5/160 µg ×2 Aerobec 200 µg ×2 Prednisolon OR min ×3 pr. year |
Bricanyl 0.5 mg PRN Singulair 5 mg ×1 Symbicort 4.5/160 µg ×2 Aerobec 100 µg ×2 |
Opatanol ×4 Aerius 5 mg ×4 |
Opatanol PRN Aerius 5 mg PRN |
| 17 |
Innovair* 200/6 µg ×2 Buventol PRN |
Innovair 200/6 µg ×2 Buventol PRN |
Dymista* 1 × 2 Alnok 20 mg ×2 Opatanol PRN |
Dymista 1 × 2 Aerius 5 mg PRN |
| 18 |
Giona 200 µg ×2 Prednisolon OR ×1 pr. year |
Airomir* PRN |
Dymista 1 × 2 day Telfast* 120 mg ×2 Opatanol ×3 Steroid OC drops PRN |
Avamys ×1 Telfast 120 mg PRN Opatanol PRN |
| 19 |
Innovair 100/6 µg 2 × 2 Singulair 5 mg ×1 Ventoline* PRN Atrovent* PRN |
Innovair 200/6 µg ×2 Singulair 5 mg ×1 Ventoline PRN Atrovent PRN |
||
| 20 |
Innovair 200/6 µg ×2 Buventol PRN |
Innovair 200/6 µg ×2 Buventol PRN |
Avamys 2 × 1 | Avamys 1 × 1 |
Note: Inhaled Corticosteroids: Aerobec: Beclometasondipropionat, Giona: Budesonide, Spirocort: Budesonid. Inhaled Beta2agonists: Buventol + Ventoline: Salbutamol, Bricanyl: Terbutalin, Formo: Formoterol, Oxis: Formoterol. Inhaled Beta2agonists/ICS: Symbicort + Bufomix: Budesonid/Formoterol, Seretide: Fluticasonpropionat/Salmeterol, Relvar Ellipta: Fluticasonfuroat/Vilanterol, Innovair: Beclometasondipropionat/Formoterolfumaratdihydrat. Inhaled others: Atrovent: Ipratropium, Tobramycin: Aminoglykoside, Spiriva: Tiotropium. Antiasthmatic tablet: Theo‐dur: Theophyllin/Methylxanthinderivat, Singulair: Leukotrien D4‐receptorantagonist. Antihistamin tablet: Alnok: Cetirizin, Aerius: Desloratadin, Telfast: Fexofenadinhydrochlorid. Intranasal medicine: Avamys: Fluticasonfuroat, Allergodil: Azelastin, Flixonase: Fluticasonpropionat, Dymista: Azelastin/Fluticasonpropionat. Eyedrops: Tobradex: Dexamethason, Zaditen: Ketotifen, Xyzal: Levocetirizin, Opatanol: Olopatadin.
Abbreviation: IgE, immunoglobulin E.
All 20 children had a significant improvement in basophil sensitivity (EC50) toward relevant allergens (p = .0001) as well as a significant decrease in the number of FcεRI receptors per basophil (p = .002) (Figure 1C,D). There was no difference with regard to sensitization and outcome. Improvement in basophil sensitivity correlated well with improvement in FEV1% of predicted (Figure 1E). We assessed the performance of these biomarkers in a ROC curve (Figure 1F). The FceRI density appears to be the best diagnostic marker for effect of anti‐IgE treatment, followed by threshold of Mannitol response, FEV1, % of expected and finally EC50.
4. DISCUSSION
In this descriptive study of 20 Danish children treated with Omalizumab for IgE‐mediated perennial severe allergic asthma it was shown that there was a statistically significant and clinically relevant improvement on asthma control score (defined by GINA guideline level of control), bronchial provocation with mannitol, lung function, and medication, especially systemic prednisolone treatment was reduced. Basophil sensitivity improved and the number of FcεRI‐receptors on basophils was significantly reduced to below detection limits in all 20 children with no difference regarding type of sensitization. This suggests that Omalizumab controls basophil sensitivity. It also suggests that the effects of Omalizumab on basophil FcεRI‐receptors are indicative of the individual's condition.
The unambiguous reduction of FceRI density on blood basophils is a novel consequence of treatment. Scmid et al. 21 have previously found that subcutaneous immunotherapy of adult grass pollen allergic patients reduced FceRI density on basophils by 66%. 21 FecRI density on blood basophils may be a quick and effective biomarker of allergic activity. A surprising finding was that this biomarker performed best in this small cohort of patients.
Previous studies have shown that the inverse of basophil sensitivity, CD‐sens, correlates significantly with nasal airway challenge 7 and bronchial allergen threshold sensitivity 16 providing evidence that these measures can be used as a biomarker of allergen sensitivity in allergic asthma. Pereira‐Santos et al. 9 support this theory and describe two such cases. Konradsen et al. 14 found that cat‐allergic children with severe uncontrolled allergic asthma had higher allergen sensitivity than cat‐allergic children with controlled allergic asthma. This suggests that the severity of asthma is related to the level of allergen sensitivity. This suggests that the level of allergen exposure is a key factor in asthma development. Basophil sensitivity measurement is a complex procedure and may require more optimization.
The results presented here suggest that monitoring anti‐IgE treatment in IgE‐mediated asthma by measuring FceRI density on basophil and measuring basophil sensitivity to allergen is a useful supplement. This study does not only demonstrate a promising method to assess the efficacy of anti‐IgE treatment. It may also serve as an explanation method when experiencing a lack of effect on on‐going treatment as has been shown previously. 10 It raises the question of whether basophil sensitivity or its inverse, CD‐sens, may be used quantitatively in the attempt to optimize the dosage and administration frequency to an appropriate level where maximum effect is obtained. The method may also be useful when desiring to interrupt treatment i.e., when treatment has reached a period with controlled asthma for 5 years and deciding whether to continue or terminate treatment. In this situation having a method to evaluate whether there is a risk of potentially severe asthma symptoms evolving immediately after treatment termination is highly relevant. It could also be applied when a seasonal airborne allergy is treated with anti‐IgE, to determine when the treatment should be terminated and re‐managed.
This case series demonstrates a promising method of using FceRI density or basophil allergen sensitivity in monitoring anti‐IgE treatment in different clinical situations of IgE‐mediated disease. The validity of this study is limited according to small number of patients. However, it seems highly relevant to investigate the findings of this study further with randomized clinical trials evaluating the specific use of this method in a clinical setting.
AUTHOR CONTRIBUTIONS
Britta Eilertsen Hjerrild, Hans Jürgen Hoffmann, and Sune Leisgaard Mørck Rubak conceived and designed the study, thus responsible for methodology. Hans Jürgen Hoffmann and Sune Leisgaard Mørck Rubak were responsible for formal analysis, software. Sune Leisgaard Mørck Rubak and Britta Eilertsen Hjerrild were responsible for projekt administration, resources. All authors played a role in the intellectual content, conceptualization, data curation, investigation, supervision, validation, visualization, and writing of the manuscript (original draft, review, and editing). All authors critically revised the manuscript for important intellectual content and approved the final version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Aspergillus extract was supplied by Maiken Cavling Ahrendrup, State Serum Institute, Denmark.
Rubak SLM, Bonne NL, Hjerrild BE, Hoffmann HJ. Basophil FceRI expression—A management tool in anti‐IgE treatment of allergic asthma. Pediatr Pulmonol. 2024;59:3355‐3363. 10.1002/ppul.27206
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data available on request due to privacy/ethical restrictions: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti‐IgE) for asthma in inner‐city children. N Engl J Med. 2011;364(11):1005‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;Dec 136(6):1476‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicinrådet, Omalizumab. https://medicinraadet.dk/media/crhplg4b/medicinrådets‐lægemiddelrek‐og‐beh‐vejl‐vedr‐svær‐astma‐vers‐2‐1.pdf, 2022.
- 4. Saini SS, MacGlashan DW. Assessing basophil functional measures during monoclonal anti‐IgE therapy. J Immunol Methods. 2012;383:60‐64. 10.1016/j.jim.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omalizumab, produktresume.
- 6. Nopp A, Johansson SGO, Ankerst J, Palmqvist M, Öman H. CD‐sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy. 2007;62:1175‐1181. 10.1111/j.1398-9995.2007.01476.x [DOI] [PubMed] [Google Scholar]
- 7. Nopp A, Johansson SGO, Ankerst J, et al. Basophil allergen threshold sensitivity: a useful approach to anti‐IgE treatment efficacy evaluation. Allergy. 2006;61:298‐302. 10.1111/j.1398-9995.2006.00987.x [DOI] [PubMed] [Google Scholar]
- 8. Korn S, Haasler I, Fliedner F, et al. Monitoring free serum IgE in severe asthma patients treated with Omalizumab. Respir Med. 2012;106:1494‐1500. 10.1016/j.rmed.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 9. Pereira Santos MC, Campos Melo A, Caetano A, et al. Longitudinal study of the expression of FcεRI and IgE on basophils and dendritic cells in association with basophil function in two patients with severe allergic asthma treated with Omalizumab. Eur Ann Allergy Clin Immunol. 2015;47:38‐40. [PubMed] [Google Scholar]
- 10. Johansson SGO, Lilja G, Hallberg J, Nopp A. A clinical follow‐up of Omalizumab in routine treatment of allergic asthma monitored by CD‐sens. Immun Inflamm Dis. 2018;6:382‐391. 10.1002/iid3.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann HJ, Knol EF, Ferrer M, et al. Pros and cons of clinical basophil testing (BAT). Curr Allergy Asthma Rep. 2016;16:56. 10.1007/s11882-016-0633-6 [DOI] [PubMed] [Google Scholar]
- 12. Mayorga C, Çelik GE, Pascal M, et al. Flow‐based basophil activation test in immediate drug hypersensitivity. An EAACI task force position paper. J Allergy Clin Immunol. 2024;79(3):580‐600. 10.1111/all.15957 [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70:1393‐1405. 10.1111/all.12698 [DOI] [PubMed] [Google Scholar]
- 14. Konradsen JR, Nordlund B, Nilsson OB, et al. High basophil allergen sensitivity (CD‐sens) is associated with severe allergic asthma in children. Pediatr Allergy Immunol. 2012;23:376‐384. 10.1111/j.1399-3038.2011.01260.x [DOI] [PubMed] [Google Scholar]
- 15. Chanez P, Contin‐Bordes C, Garcia G, et al. Omalizumab‐induced decrease of FcξRI expression in patients with severe allergic asthma. Respir Med. 2010;104:1608‐1617. 10.1016/j.rmed.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 16. Dahlén B, Nopp A, Johansson SGO, Eduards M, Skedinger M, Adédoyin J. Basophil allergen threshold sensitivity, CD‐sens, is a measure of allergen sensitivity in asthma. Clinical & Experimental Allergy. 2011;41:1091‐1097. 10.1111/j.1365-2222.2011.03763.x [DOI] [PubMed] [Google Scholar]
- 17. Poddighe D, Vangelista L. Effects of Omalizumab on basophils: potential biomarkers in asthma and chronic spontaneous urticaria. Cell Immunol. 2020;358:104215. 10.1016/j.cellimm.2020.104215 [DOI] [PubMed] [Google Scholar]
- 18. Brandström J, Vetander M, Lilja G, et al. Individually dosed Omalizumab: an effective treatment for severe peanut allergy. Clinical & Experimental Allergy. 2017;47:540‐550. 10.1111/cea.12862 [DOI] [PubMed] [Google Scholar]
- 19. Nopp A, Johansson SGO, Adédoyin J, Ankerst J, Palmqvist M, Öman H. After 6 years with Xolair; a 3‐year withdrawal follow‐up. Allergy. 2010;65:56‐60. 10.1111/j.1398-9995.2009.02144.x [DOI] [PubMed] [Google Scholar]
- 20. Pascal M, Edelman SM, Nopp A, et al. Task force report: a consensus protocol for the basophil activation test for collaboration and external quality assurance. Allergy. 2024;79(2):290‐293. 10.1111/all.15907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmid JM. Changes in Allergen Specific Immunoglobulins and Basophil Sensitivity during Subcutaneous Immunotherapy in Grasspollen Allergic Subjects: PhD Dissertation. Health, Aarhus University, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data available on request due to privacy/ethical restrictions: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


