Abstract
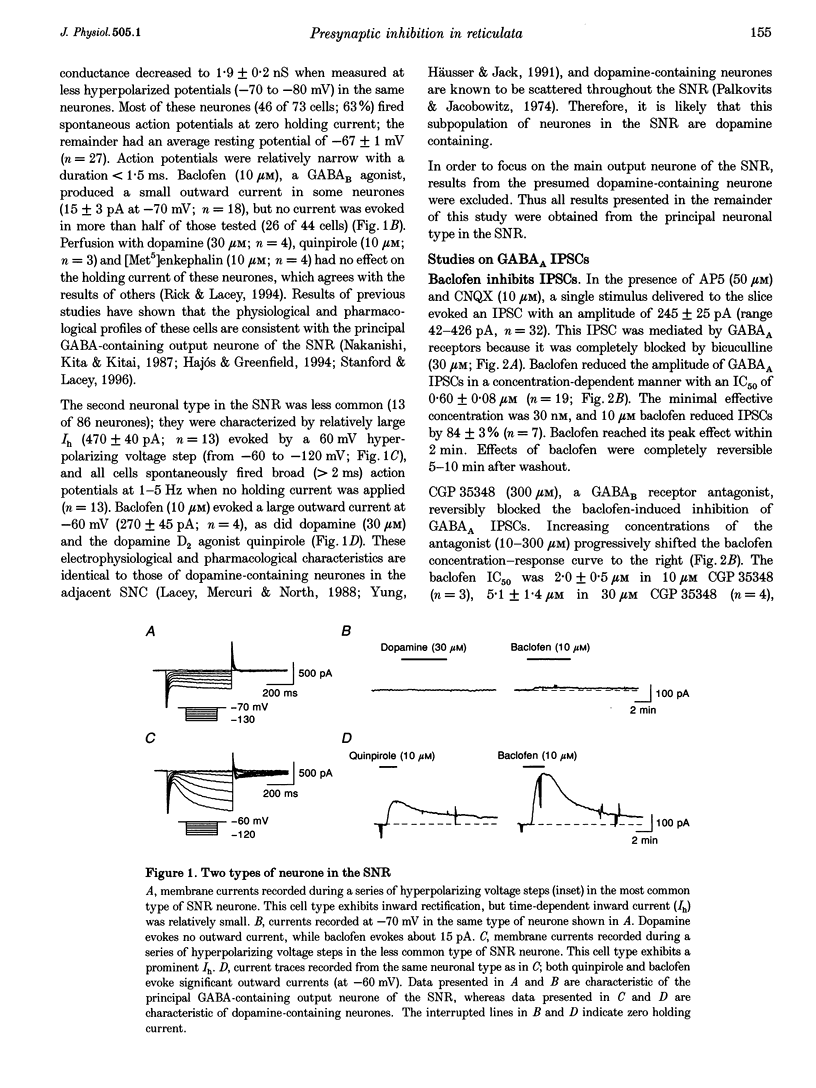
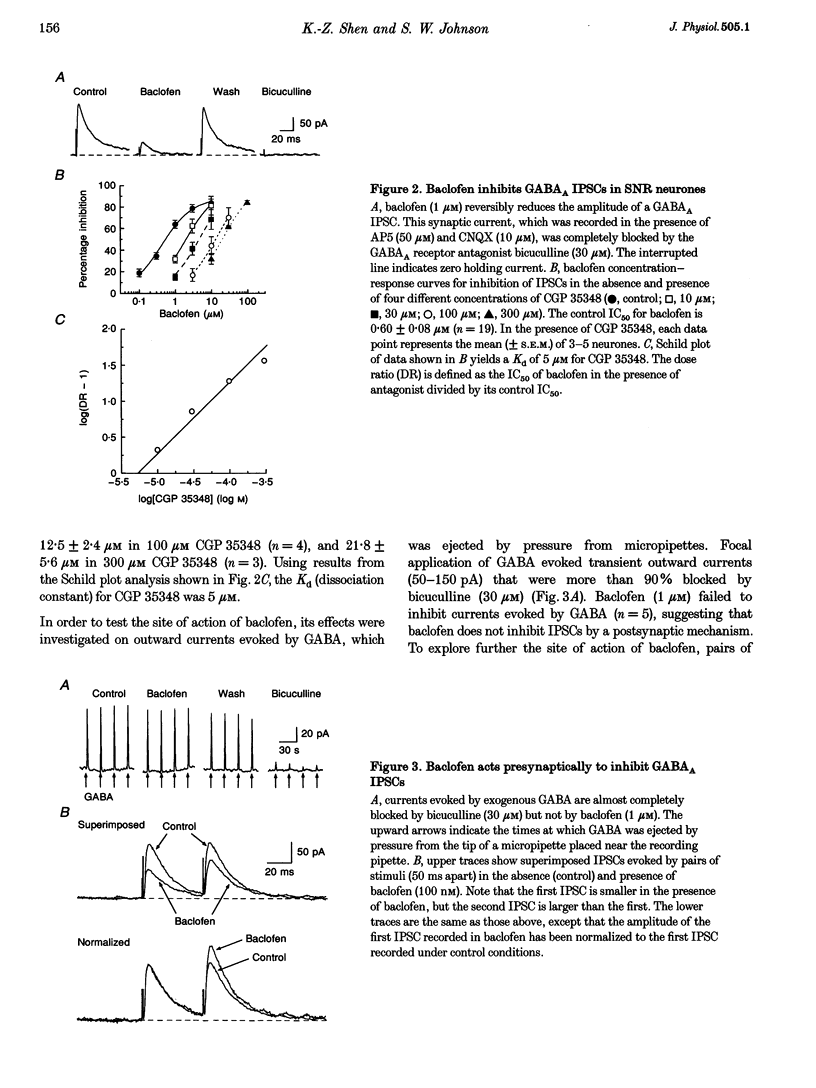
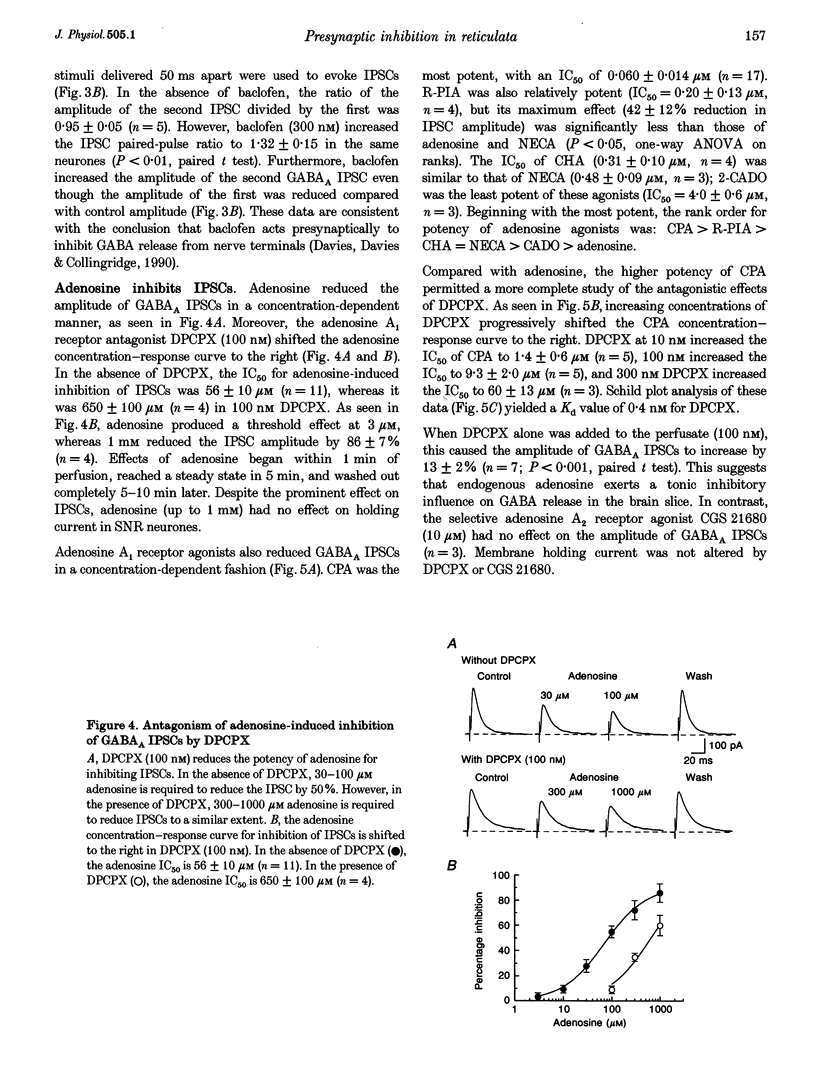
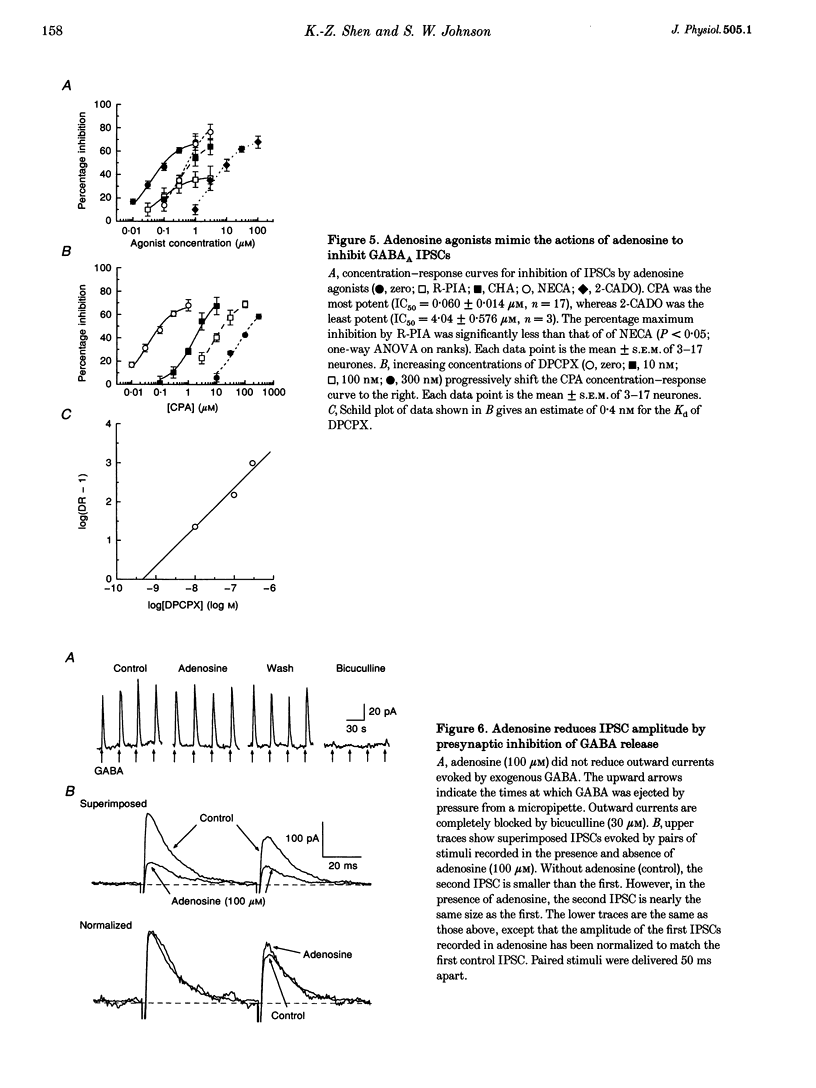
1. Patch pipettes were used to record whole-cell currents under voltage clamp in substantia nigra zona reticulata (SNR) neurones in the rat midbrain slice. Bipolar electrodes evoked synaptic currents mediated by glutamate (EPSCs) and GABAA receptors (IPSCs). 2. Baclofen reduced the amplitude of IPSCs by 48% at its IC50 value of 0.60 microM. The GABAB antagonist CGP 35348 blocked this effect with a Kd value estimated by Schild analysis of 5 microM. 3. Adenosine reduced IPSCs by 48% at its IC50 value of 56 microM. Adenosine agonists reduced IPSCs with the following rank order of potency: CPA (N6-cyclopentyladenosine) > R-PIA (R(-)N6-(2-phenylisopropyl)adenosine) > CHA (N6-cyclohexyladenosine) = NECA (5'-N-ethylcarboxamidoadenosine) > 2-CADO (2-chloroadenosine) > adenosine. Schild analysis yielded a Kd value of 0.4 nM for antagonism of CPA by the adenosine A1 receptor antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine). 4. Both baclofen and adenosine reduced the magnitude of paired-pulse depression of IPSCs, and neither blocked currents evoked by GABA, which was pressure-ejected from micropipettes. 5. Glutamate EPSCs were reduced by baclofen (IC50 = 0.78 microM) and adenosine (IC50 = 57 microM). Schild analysis yielded a Kd value of 11 microM for antagonism of baclofen-induced inhibition of EPSCs by CGP 35348. DPCPX (1 microM) completely blocked the inhibitory effects of adenosine (100 microM) and CPA (100 nM) on EPSCs. Neither adenosine nor baclofen reduced inward currents evoked by glutamate which was pressure-ejected from micropipettes. 6. These results show that presynaptic GABAB and A1 receptors reduce glutamate and GABA release from nerve terminals in the SNR.
Full text
PDF

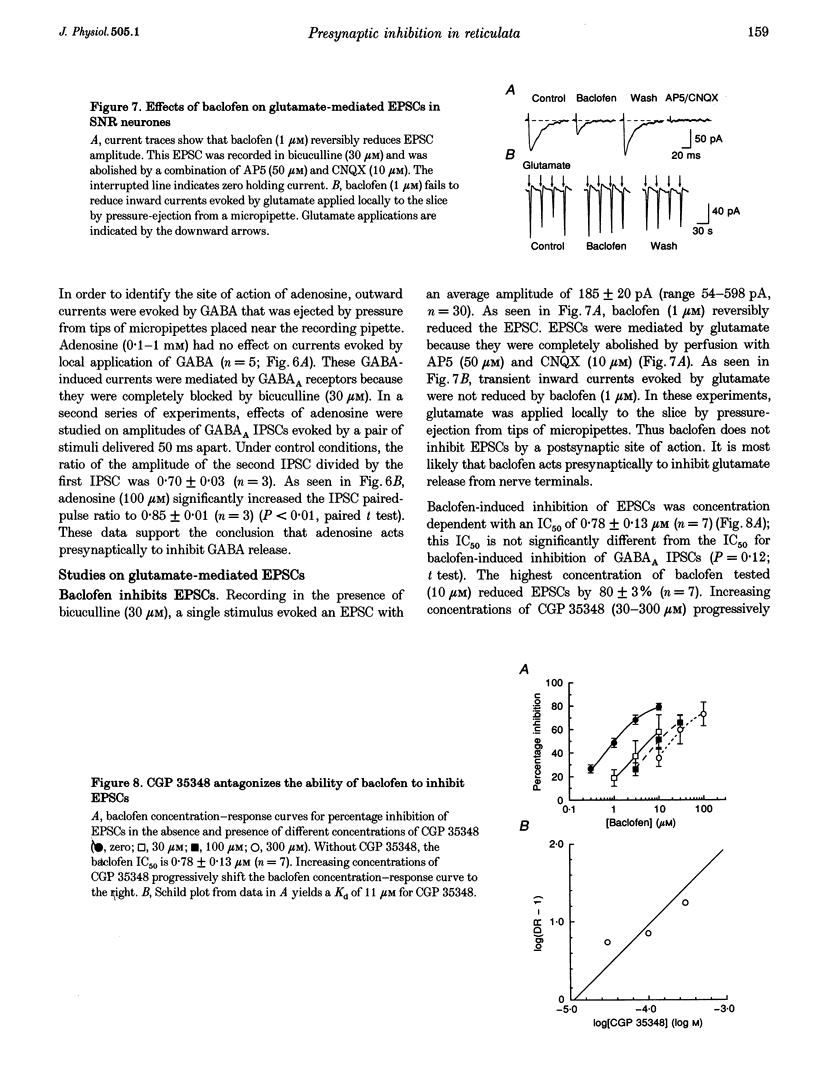



Selected References
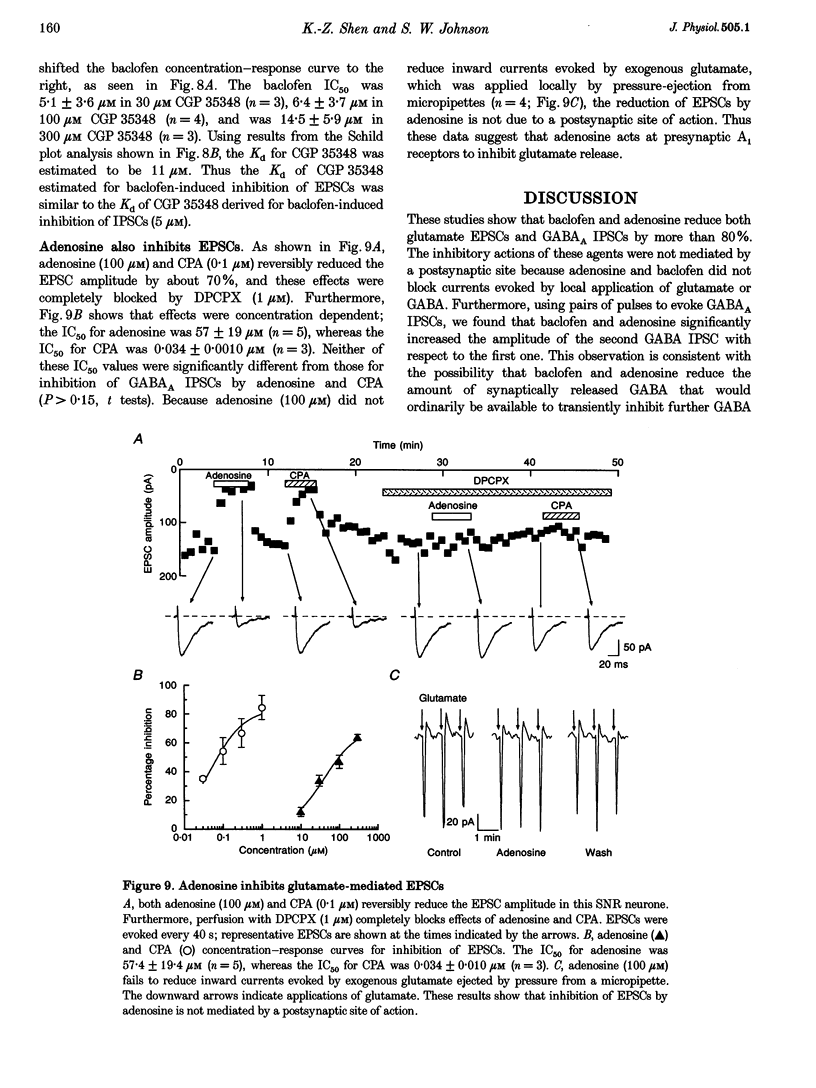
These references are in PubMed. This may not be the complete list of references from this article.
- Bon C., Galvan M. Electrophysiological actions of GABAB agonists and antagonists in rat dorso-lateral septal neurones in vitro. Br J Pharmacol. 1996 Jun;118(4):961–967. doi: 10.1111/j.1476-5381.1996.tb15493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G., Fassio A., Schmid G., Severi P., Sala R., Raiteri M. Pharmacologically distinct GABAB receptors that mediate inhibition of GABA and glutamate release in human neocortex. Br J Pharmacol. 1997 Jan;120(1):60–64. doi: 10.1038/sj.bjp.0700852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993 Jul;14(7):259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. gamma-Aminobutyric acid (GABA) autoreceptors in rat cerebral cortex and spinal cord represent pharmacologically distinct subtypes of the GABAB receptor. J Pharmacol Exp Ther. 1993 May;265(2):765–770. [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F., Delfs J. M. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996 Oct;19(10):417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience. 1984 Jun;12(2):347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990 Jul;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Double K. L., Crocker A. D. Dopamine receptors in the substantia nigra are involved in the regulation of muscle tone. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1669–1673. doi: 10.1073/pnas.92.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassio A., Bonanno G., Cavazzani P., Raiteri M. Characterization of the GABA autoreceptor in human neocortex as a pharmacological subtype of the GABAB receptor. Eur J Pharmacol. 1994 Oct 3;263(3):311–314. doi: 10.1016/0014-2999(94)90727-7. [DOI] [PubMed] [Google Scholar]
- Fastbom J., Pazos A., Palacios J. M. The distribution of adenosine A1 receptors and 5'-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987 Sep;22(3):813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- Gale K. Mechanisms of seizure control mediated by gamma-aminobutyric acid: role of the substantia nigra. Fed Proc. 1985 May;44(8):2414–2424. [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol. 1979 Nov 16;59(3-4):211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Hajós M., Greenfield S. A. Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 1994 Oct 17;660(2):216–224. doi: 10.1016/0006-8993(94)91292-0. [DOI] [PubMed] [Google Scholar]
- Holstein G. R., Pasik P., Hámori J. Synapses between GABA-immunoreactive axonal and dendritic elements in monkey substantia nigra. Neurosci Lett. 1986 May 23;66(3):316–322. doi: 10.1016/0304-3940(86)90038-8. [DOI] [PubMed] [Google Scholar]
- Johnson S. W., North R. A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992 Feb;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K., Huggel K., Heid J., Flor P. J., Bischoff S., Mickel S. J., McMaster G., Angst C., Bittiger H., Froestl W. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997 Mar 20;386(6622):239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989 Apr;9(4):1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M., Fassio A., Gemignani A., Bonanno G., Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993 Jun 24;237(2-3):191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Mitchell I. J., Crossman A. R., Liminga U., Andren P., Gunne L. M. Regional changes in 2-deoxyglucose uptake associated with neuroleptic-induced tardive dyskinesia in the Cebus monkey. Mov Disord. 1992;7(1):32–37. doi: 10.1002/mds.870070106. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Lupica C. R., Dunwiddie T. V. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993 Aug;13(8):3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Kita H., Kitai S. T. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987 Dec 22;437(1):45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Parent A., Côté P. Y., Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995 Jun;46(2-3):131–197. [PubMed] [Google Scholar]
- Rick C. E., Lacey M. G. Rat substantia nigra pars reticulata neurones are tonically inhibited via GABAA, but not GABAB, receptors in vitro. Brain Res. 1994 Oct 3;659(1-2):133–137. doi: 10.1016/0006-8993(94)90872-9. [DOI] [PubMed] [Google Scholar]
- Rick C. E., Stanford I. M., Lacey M. G. Excitation of rat substantia nigra pars reticulata neurons by 5-hydroxytryptamine in vitro: evidence for a direct action mediated by 5-hydroxytryptamine2C receptors. Neuroscience. 1995 Dec;69(3):903–913. doi: 10.1016/0306-4522(95)00283-o. [DOI] [PubMed] [Google Scholar]
- Robertson H. A. Dopamine receptor interactions: some implications for the treatment of Parkinson's disease. Trends Neurosci. 1992 Jun;15(6):201–206. doi: 10.1016/0166-2236(92)90034-6. [DOI] [PubMed] [Google Scholar]
- SCHILD H. O. Drug antagonism and pAx. Pharmacol Rev. 1957 Jun;9(2):242–246. [PubMed] [Google Scholar]
- Shirakawa O., Tamminga C. A. Basal ganglia GABAA and dopamine D1 binding site correlates of haloperidol-induced oral dyskinesias in rat. Exp Neurol. 1994 May;127(1):62–69. doi: 10.1006/exnr.1994.1080. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Bolam J. P. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990 Jul;13(7):259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Solís J. M., Nicoll R. A. Pharmacological characterization of GABAB-mediated responses in the CA1 region of the rat hippocampal slice. J Neurosci. 1992 Sep;12(9):3466–3472. doi: 10.1523/JNEUROSCI.12-09-03466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford I. M., Lacey M. G. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996 Dec 1;16(23):7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper J. M., Martin L. P., Anderson D. R. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995 Apr;15(4):3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier P. C., Wicki P., Feldtrauer J. J., Mickel S. J., Bittiger H., Baumann P. A. GABA and glutamate release affected by GABAB receptor antagonists with similar potency: no evidence for pharmacologically different presynaptic receptors. Br J Pharmacol. 1994 Dec;113(4):1515–1521. doi: 10.1111/j.1476-5381.1994.tb17168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. N., Mercuri N. B., Johnson S. W. Presynaptic inhibition of gamma-aminobutyric acidB-mediated synaptic current by adenosine recorded in vitro in midbrain dopamine neurons. J Pharmacol Exp Ther. 1995 May;273(2):576–581. [PubMed] [Google Scholar]
- Yamamoto T., Staines W. A., Dewar K., Geiger J. D., Daddona P. E., Nagy J. I. Distinct adenosine deaminase-containing inputs to the substantia nigra from the striatum and tuberomammillary nucleus. Brain Res. 1988 Nov 22;474(1):112–124. doi: 10.1016/0006-8993(88)90674-9. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Tsumori T., Ando A., Domoto T. Demonstration of axon collateral projections from the substantia nigra pars reticulata to the superior colliculus and the parvicellular reticular formation in the rat. Brain Res. 1995 Mar 13;674(1):122–126. doi: 10.1016/0006-8993(94)01459-u. [DOI] [PubMed] [Google Scholar]
- Yung W. H., Häusser M. A., Jack J. J. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991 May;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]



