Abstract
Background
The benefit of antibiotic treatment of acute drops in FEV1 percent predicted (FEV1pp) has been clearly established, but data from the early 2000s showed inconsistent treatment. Further, there is no empirical evidence for what magnitude of drop is clinically significant.
Methods
We used data from the CF Foundation Patient Registry (CFFPR) from 2016 to 2019 to determine the association between treatment (any IV antibiotics, only oral or newly prescribed inhaled antibiotics, or no antibiotic therapy) following a decline of ≥5% from baseline FEV1pp and return to 100% baseline FEV1pp days using multivariable logistic regression including an interaction between the magnitude of decline and treatment category.
Results
Overall, 16,495 PWCF had a decline: 16.5% were treated with IV antibiotics, 25.0% non‐IV antibiotics, and 58.5% received no antibiotics. Antibiotic treatment was more likely for those with lower lung function, history of a positive PA culture, older age and larger FEV1 decline (p < 0.001). Treatment with IV antibiotics or oral/inhaled antibiotics was associated with a higher odds of recovery to baseline compared to no treatment across all levels of decline, including declines of 5%–10%.
Conclusions
A large proportion of acute drops in FEV1pp continue to be untreated, especially in younger patients and those with higher baseline lung function. Acute drops as small as 5% predicted are less likely to be recovered if antibiotic treatment is not prescribed. These findings suggest the need for more aggressive antimicrobial treatment of acute drops in FEV1, including those of a magnitude previously believed to be associated with self‐recovery.
Keywords: antibiotics, cystic fibrosis, lung function, pulmonary exacerbation
1. INTRODUCTION
CF lung disease is chronic and progressive and characterized by intermittent episodes of worsening signs and symptoms that clinicians identify as acute pulmonary exacerbations (PEx). While considerable disagreement and controversy exist around the pathophysiology of these events, as well as their definition and diagnostic criteria, the association of PEx with long term lung decline in people with CF is well accepted, and surveillance for and the periodic treatment of discrete PEx with antibiotics and augmentation of chronic airway clearance therapies (ACT) has become the hallmark of CF treatment around the world. 1 , 2 , 3 , 4 , 5 , 6 , 7
The most easily identifiable objective finding of a PEx, both in the clinical setting and databases such as CF patient registries, is an acute drop in FEV1 percent predicted (FEV1pp) from baseline. While this finding is present in no more than 80% of clinician‐diagnosed PEx, 5 and there is no existing research that has determined how much of an acute drop is clinically meaningful, previous studies have indicated that antibiotic treatment of FEV1 declines from baseline of 10% or more is associated with better likelihood of acute recovery in FEV1 and that more consistent antibiotic treatment of such drops may be associated with better long‐term outcomes. 8 The source of much of these data was the Epidemiological Study of CF (ESCF), which collected data for over a decade, ending in 2005. 9 ESCF analyses also showed that over 1/3 of acute drops in FEV1pp of 10% or more were not treated with antibiotics, particularly in children and patients with higher baseline FEV1pp. 10 The absence of treatment among patients with higher baseline FEV1pp perhaps accounts for why high FEV1pp is associated with a higher risk of accelerated decline. 11
The first aim of this study was to determine whether the use of antibiotic treatment for acute lung function declines in patients attending CFF‐accredited care centers has changed. The second aim was to determine empirically the magnitude of acute drop in FEV1pp that is clinically significant by comparing the impact of treatment on the probability of recovery to 100% of baseline FEV1pp following different degrees of decline.
2. METHODS
The study population included individuals with a CF diagnosis who attended a CFF‐accredited care center and participated in the CF Foundation Patient Registry (CFFPR) between 2016 and 2019. The CFFPR maintains longitudinal data reported over the course of routine CF clinical care from 1986 to the present. 12 To be considered for inclusion in the study, patients must have been aged 6 to ≤40 years of age with ≥3 FEV1 measures reported within a 1.5‐year period. In the event an individual reported a lung transplant or pregnancy, their data were censored at the date of transplant or on January 1 in the first calendar year a pregnancy was reported. FEV1 measures reported after the date of elexacaftor/tezacaftor/ivacaftor (ETI) initiation were not included in the analysis.
We reviewed all FEV1pp measures reported from 2016 to 2018 to establish baseline lung function, defined as the highest FEV1pp in a 365‐day period from the first FEV1 measure reported. If a patient had an IV‐treated exacerbation in the first possible 365‐day baseline period, then baseline lung function was defined by the highest FEV1pp reported within 365 days from the exacerbation start date (defined as the start date for a hospitalization or home IV care episode). Once baseline FEV1pp was established, a decline event was identified as the first relative decline of ≥5% from baseline that occurred within 365 days after the baseline period. Relative decline was calculated as [(baseline FEV1pp—visit FEV1pp)/baseline FEV1pp]. Only one decline event was ascertained for each individual. Decline events that occurred within 1 year from the baseline FEV1 measurement were then categorized by the magnitude of relative decline: 5– < 7.5%, 7.5– < 10%, 10– < 15%, 15%+. Individuals who did not experience ≥5% relative decline over the study period were excluded from the study as well as individuals who experienced a relative decline >50%. All FEV1pp values were calculated using Global Lung Initiative reference equations. 13 Follow‐up FEV1pp was determined as the best value within 28 and 180 days following the decline event. Individuals missing follow‐up FEV1 data were excluded from analysis. Treatment of the decline event was ascertained by reviewing the medication and hospitalization data reported to the CFFPR and categorizing any treatment within 14 days before the decline and 28 days after the decline event hierarchically as either any IV antibiotics, only oral or newly prescribed inhaled antibiotics, or no antibiotic therapy.
We summarized patient characteristics as proportions (for categorical variables) or by median and interquartile range (for continuous variables). To estimate the effect of treatment on the probability a person would return to 100% of their baseline ppFEV1, we employed multivariable logistic regression controlling for the following sources of confounding bias: baseline FEV1pp, magnitude of relative FEV1pp decline, age at relative FEV1pp decline event, reported asthma diagnosis, positive Pseudomonas aeruginosa (PA) culture in the year before decline. We also considered the impact of CFRD status and any modulator use but they were excluded from the final model for the sake of parsimony as their inclusion did not alter the point estimates or 95% confidence intervals (CIs). We did not include mycobacterial culture positivity in our model because 40% of the total study population did not have a mycobacterial culture at the time of the decline event and would have been excluded from the study, limiting its generalizability. We tested an interaction term between magnitude of acute FEV1pp decline and antibiotic treatment (Any IV, only oral/inhaled, no antibiotics) to determine if odds of recovery was different across levels of decline. SAS 9.4 was used for all data analyses (SAS Institute, Inc.).
This research was classified as exempt by Advarra (IRB#37633).
3. RESULTS
The study cohort included 16,495 PWCF who met inclusion criteria to establish baseline FEV1pp and who had at least one decline in FEV1pp between 5% and <50% (Figure 1). Overall, the cohort included 8,035 (49%) who were female, 12,658 (76.7%) individuals who were classified as having a class 1 to 3 CFTR variant, and the mean age at the decline event was 17.7 years (95% CI: 17.5, 17.8) (Table 1). Overall, the mean relative drop in FEV1 at decline event was 13.3% (95% CI: 13.2%, 13.4%) and the mean FEV1pp at decline event was 78.8 (95% CI: 78.5, 79.1). Overall, treatment distribution included 2722 (16.5%) events that were treated with IV antibiotics, 4,121 (25.0%) with oral or new inhaled antibiotics, and 9652 (58.5%) with no antibiotics reported in CFFPR. Among the 2722 individuals that were prescribed any IV antibiotics, 90% were hospitalized for their decline event compared to 10% receiving home IV therapy with no hospitalization. Among those who were hospitalized, 30% received home IV antibiotics in conjunction with that episode. Mean age in years at decline by treatment was 19.7 (95% CI: 19.4, 20.0) for IV antibiotics, 17.7 (95% CI: 17.5, 18.0) for non‐IV antibiotics, and 17.1 (95% CI: 16.9, 17,2) for no antibiotics reported (p < 0.001). Mean FEV1pp at decline by treatment was 64.6 (95% CI: 63.9, 65.3) for IV antibiotics, 75.6 (95% CI: 75.0, 76.1) for non‐IV antibiotics, and 84.2 (95% CI: 83.8, 84.5) no antibiotics reported (p < 0.001). The mean relative decline was 19.3 (95% CI: 18.9, 19.7) for those treated with IV antibiotics, 15.0 (95% CI: 14.7, 15.2) for non‐IV antibiotics, and 10.9 (95% CI: 10.7, 11.0) for no antibiotics reported (p < 0.001). Prescribed antibiotic treatment was more likely for those with lower lung function, asthma, those who had a positive PA culture during year before decline event, older age and larger FEV1 decline (p < 0.001). The proportion of people treated with antibiotics increased with the magnitude of relative decline (Table 1): among those experiencing a 5%‐7.5% decline, 21.7% were prescribed an antibiotic compared to 64.5% of those with >15% decline. Antibiotic prescription rates varied with age, peaking at age 18‐26, and increased with magnitude of decline and lower baseline FEV1pp (Figure 2). Notably, among those with ≥100% FEV1pp at their decline event, over 80% were not prescribed antibiotics.
Figure 1.

Cohort flow diagram.
Table 1.
Descriptive statistics by treatment.
| IV Abx | Oral or inhaled Abx only | No Abx Treatment | Total | p‐value | |
|---|---|---|---|---|---|
| N | 2722 (16.5%) | 4121 (25.0%) | 9652 (58.5%) | 16495 (100%) | |
| Female Sex | 1492 (54.8%) | 2084 (50.6%) | 4459 (46.2%) | 8035 (48.7%) | <0.0001 |
| Mean Age at Decline (95% CI) | 19.7 (19.4, 20.0) | 17.7 (17.5, 18.0) | 17.1 (16.9, 17,2) | 17.7 (17.5, 17.8) | |
| Age at Decline Event | <0.0001 | ||||
| 6 to <10 Years | 408 (15.0%) | 914 (22.2%) | 2626 (27.2%) | 3948 (23.9%) | |
| 10 to <14 years | 348 (12.8%) | 681 (16.5%) | 1796 (18.6%) | 2825 (17.1%) | |
| 14– <18 years | 515 (18.9%) | 755 (18.3%) | 1551 (16.1%) | 2821 (17.1%) | |
| 18– <26 | 808 (29.7%) | 1040 (25.2%) | 1927 (20.0%) | 3775 (22.9%) | |
| 26– <41 | 643 (23.6%) | 731 (17.7%) | 1752 (18.2%) | 3126 (19.0%) | |
| Mean FEV1pp at decline (95% CI) | 64.6 (63.9, 65.3) | 75.6 (75.0, 76.1) | 84.2 (83.8, 84.5) | 78.8 (78.5, 79.1) | |
| FEV1pp at decline | <0.0001 | ||||
| FEV1pp < 60% | 1235 (45.4%) | 889 (21.6%) | 1186 (12.3%) | 3310 (20.1%) | |
| FEV1pp 60– <75% | 671 (24.7%) | 1049 (25.5%) | 1639 (17.0%) | 3359 (20.4%) | |
| FEV1pp 75– <90% | 535 (19.7%) | 1264 (30.7%) | 2792 (28.9%) | 4591 (27.8%) | |
| FEV1pp 90– <100% | 180 (6.6%) | 570 (13.8%) | 2158 (22.4%) | 2908 (17.6%) | |
| FEV1pp 100%+ | 101 (3.7%) | 349 (8.5%) | 1877 (19.4%) | 2327 (14.1%) | |
| Mean relative FEV 1 pp decline (95% CI) | 19.3 (18.9, 19.7) | 15.0 (14.7, 15.2) | 10.9 (10.8, 11.0) | 13.3 (13.2, 13.4) | |
| Relative decline groups | <0.0001 | ||||
| 5–<7.5% | 301 (11.1%) | 695 (16.9%) | 3411 (35.3%) | 4407 (26.7%) | |
| 7.5– <10% | 295 (10.8%) | 700 (17.0%) | 2267 (23.5%) | 3262 (19.8%) | |
| 10– <15% | 609 (22.4%) | 1123 (27.3%) | 2255 (23.4%) | 3987 (24.2%) | |
| 15 + % | 1517 (55.7%) | 1603 (38.9%) | 1719 (17.8%) | 4839 (29.3%) | |
| Nonwhite Race a | 183 (6.7%) | 267 (6.5%) | 602 (6.2%) | 1052 (6.4%) | 0.6 |
| Hispanic Ethnicity | 300 (11.0%) | 358 (8.7%) | 874 (9.1%) | 1532 (9.3%) | 0.002 |
| Ever Medicaid (%) b | 1293 (47.5%) | 1661 (40.3%) | 3606 (37.4%) | 6560 (39.8%) | <0.0001 |
| Mutation Class | <0.0001 | ||||
| Class 1 to 3 | 2210 (81.2%) | 3213 (78.0%) | 7235 (75.0%) | 12658 (76.7%) | |
| Class 4 and 5 | 144 (5.3%) | 338 (8.2%) | 1040 (10.8%) | 1522 (9.2%) | |
| Other/Unknown | 368 (13.5%) | 570 (13.8%) | 1377 (14.3%) | 2315 (14.0%) | |
| Allergic Bronchial Pulmonary Aspergillosis (ABPA) | 236 (8.7%) | 256 (6.2%) | 485 (5.0%) | 977 (5.9%) | <0.0001 |
| Asthma | 1144 (42.0%) | 1547 (37.5%) | 3389 (35.1%) | 6080 (36.9%) | <0.0001 |
| Microbiology in year prior | |||||
| Any PA | 1637 (60.1%) | 1950 (47.3%) | 4120 (42.7%) | 7707 (46.7%) | <0.0001 |
| Any MRSA | 1002 (20.2%) | 1322 (26.6%) | 2638 (53.2%) | 4962 | <0.0001 |
| Mean days from Baseline PFT to Decline (95% CI) | 150.1 (146.6, 153.6) | 167.5 (164.7, 170.5) | 175.2 (173.3, 177.1) | 169.2 (167.7, 170.6) | <0.0001 |
CFFPR race fields are not mutually exclusive and individuals can be identified as having more than one race. In the event that any race category other than (or in addition to) White was reported, individuals were classified as “nonwhite” for this analysis. CF Patients are asked to report their race by their CF Care team.
Ever Medicaid reported in any calendar year ‐between baseline and decline event.
Figure 2.
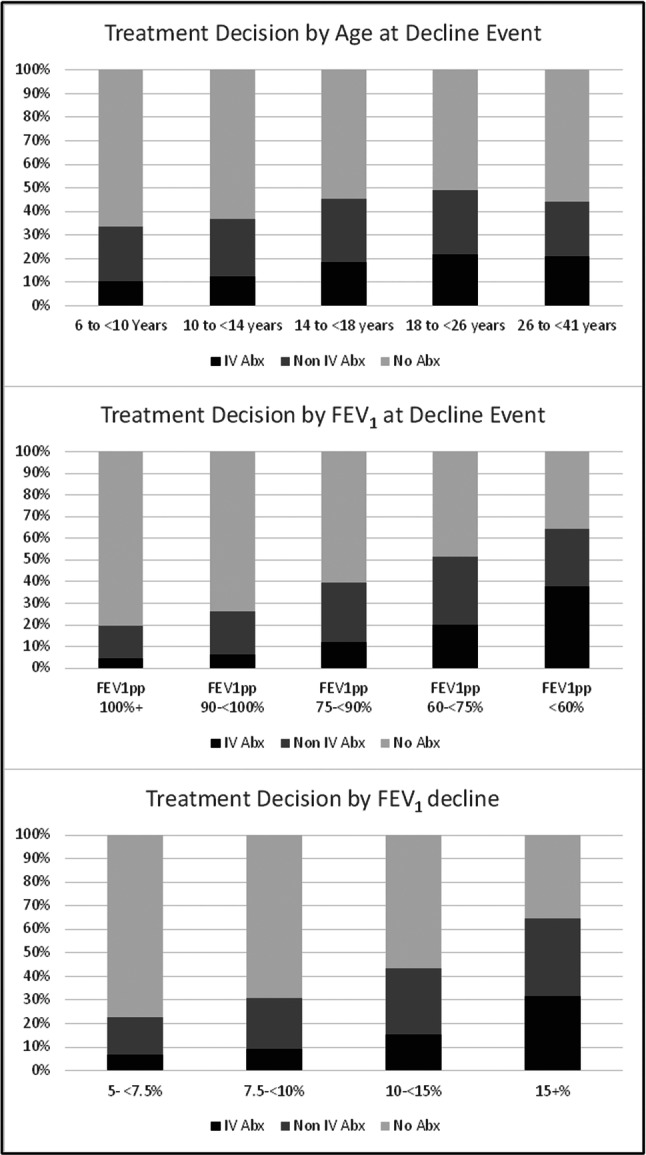
Clinical criteria associated with Treatment Decision.
Overall, a return to 100% of baseline FEV1pp was seen in 46.7% of patients prescribed IV antibiotics, 31.1% of those prescribed non‐IV antibiotics, and 20.6% of those prescribed no antibiotics (Table 2). Of the 2722 individuals treated with IV antibiotics, 293 were treated exclusively with Home IV antibiotics, of which 37% recovered to 100% of baseline. Of the 1690 patients who received IV antibiotics exclusively in the hospital, 840 recovered to 100% of baseline (49.7%). In the multivariable logistic regression model, we found that treatment with IV antibiotics or oral/inhaled antibiotics was associated with a higher odds of recovery to baseline compared to no antibiotic treatment across all levels of decline (Table 3). Interaction between treatment and magnitude of decline was statistically significant for decline levels treated with IV antibiotics compared to no antibiotic treatment but not significant for oral/inhaled antibiotics v. no treatment (see Table S1 for parameter estimates). This indicates that the odds of recovery of treatment of smaller acute drops in FEV1 are greater with IV antibiotic treatment, but not significantly different across magnitude of drop when treated with oral or new inhaled antibiotics. Odds of recovery to baseline are illustrated by magnitude of decline and treatment category in Figure 3.
Table 2.
Number (%) of people who return to 100% of baseline by treatment category and magnitude of decline.
| Magnitude of decline | Persons treated with IV Abx | Persons treated with only Oral/inhaled antibiotics | Persons with no report of antibiotics | All persons | P value (chisquare) |
|---|---|---|---|---|---|
| 5– <7.5 (N = 4407) | 176/301 (58.5%) | 261/695 (37.6%) | 765/3411 (22.4%) | 1202/4407 (27.3%) | <0.0001 |
| 7.5– <10 (N = 3262) | 162/295 (54.9%) | 211/700 (30.1%) | 463/2267 (20.4%) | 836/3267 (25.6%) | <0.0001 |
| 10– <15 (N = 3987) | 298/609 (48.9%) | 344/1123 (30.6%) | 439/2255 (19.5%) | 1081/3987 (27.1%) | <0.0001 |
| 15+ (N = 4839) | 634/1517 (41.8%) | 464/1603 (28.9%) | 324/1719 (18.8%) | 14224839 (29.4%) | 0.002 |
| Overall (16,495) | 1270/2722 (46.7%) | 1280/4121 (31.1%) | 1991/9652 (20.6%) | 4541/16495 (27.5%) | <0.0001 |
Table 3.
Logistic regression of 100% recovery to baseline by treatment vs FEV1 Decline: Odds Ratios.
| Parameter | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|
| FEV 1 pp relative decline 5– <7.5% | ||
| IV Antibiotic vs No Antibiotic Treatment | 4.9 (3.8, 6.2) | 4.0 (3.1, 5.1) |
| Non‐IV Antibiotic vs No Antibiotic Treatment | 2.1 (1.7, 2.5) | 1.9 (1.6, 2.3) |
| FEV 1 pp Relative Decline 7.5– <10% | ||
| IV Antibiotic vs No Antibiotic Treatment | 4.7 (3.7, 6.1) | 4.1 (3.2, 5.4) |
| Non‐IV Antibiotic vs No Antibiotic Treatment | 1.7 (1.4, 2.0) | 1.6 (1.3, 1.9) |
| FEV 1 pp relative decline 10– <15% | ||
| IV Antibiotic vs No Antibiotic Treatment | 4.0 (3.3, 4.8) | 3.4 (2.8, 4.2) |
| Non‐IV Antibiotic vs No Antibiotic Treatment | 1.8 (1.6, 2.2) | 1.7 (1.5, 2.0) |
| FEV 1 pp relative decline ≥ 15% | ||
| IV Antibiotic vs No Antibiotic Treatment | 3.1 (2.6, 3.6) | 2.8 (2.3, 3.2) |
| Non‐IV Antibiotic vs No Antibiotic Treatment | 1.8 (1.5, 2.1) | 1.6 (1.4, 1.9) |
adjusted for baseline FEV1pp, magnitude of relative FEV1pp decline, age at relative FEV1pp decline event, reported asthma diagnosis, positive Pseudomonas aeruginosa (PA) culture in the year before decline.
Figure 3.
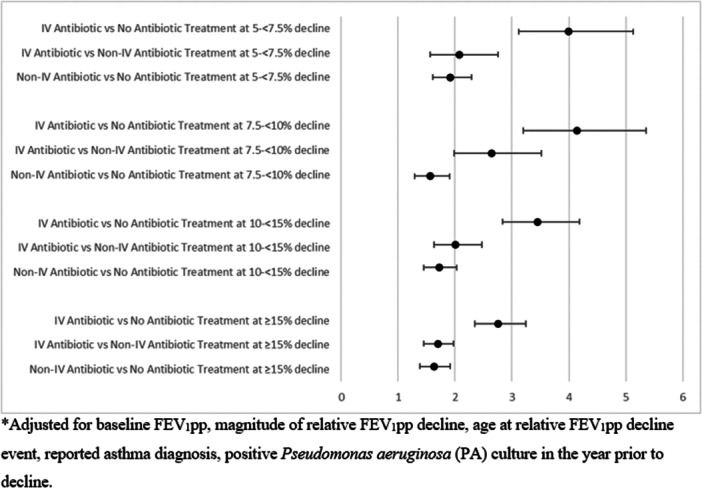
Logistic Regression of 100% Recovery to Baseline by treatment vs FEV1 Decline: Adjusted Odds Ratios*.
4. CONCLUSIONS AND DISCUSSION
The findings of this study indicate that a surprisingly high percentage (56.6%) of acute FEV1pp decline events of 10% or more in PWCF were still not treated with antibiotics in the years immediately preceding the widespread adoption of elexacaftor/tezacaftor/ivacaftor (ETI). Remarkably, this is a higher percentage than what was found in ESCF reports going back nearly 2 decades (although our methods were slightly different from that used in the ESCF analyses and we used a different database). 10 It also continues to be true that younger patients, and those with a high baseline FEV1pp, are least likely to be treated with antibiotics. This may be due to doubts about the validity of effort in younger children and/or acceptance of drops that still leave the patient with a very high FEV1, leading to recommendations for alternative actions such as an increase in airway clearance therapy. These considerations may certainly be valid in individual cases, but the overall outcome is that this is exactly the population that has previously been shown to exhibit the greatest overall annual rate of FEV1 decline. 11 , 14
Our findings regarding the odds of recovery of acute drops in FEV1pp associated with antibiotic usage are also comparable to previous reports. 8 IV antibiotic use is most likely to be associated with recovery of acute declines in FEV1pp, but oral antibiotic use is also associated with a higher likelihood of FEV1 recovery than no antibiotic use. Previous reports showing that CF care programs with the highest average FEV1pp are more likely to treat acute drops in FEV1pp with antibiotics; this may suggest that a more consistent approach to antibiotic treatment of these acute drops leads to better long‐term pulmonary outcomes. 15 Further evidence to support this approach was provided by a recent Quality Improvement report of substantial, relatively rapid improvements in the mean FEV1pp of a clinic population that was obtained by designing and adhering to processes intended to ensure that acute FEV1pp drops were noticed and followed up on carefully, with antibiotics prescribed as default treatment. 16
An important additional finding of our report is that the increased odds of recovery associated with antibiotic treatment of relatively small declines (as low as 5% predicted) in FEV1pp are comparable to those achieved by antibiotic treatment of much greater declines. The idea that a drop of 10% FEV1pp is the appropriate threshold of concern (as used by Fuchs and others 17 ) is primarily based upon analyses of FEV1 variability in the normal healthy population, and fails to take into account the need to consider the relative importance of Type 1 and Type 2 statistical errors in clinical decision making. 18 Specifically, if preservation of lung function is a priority in CF patients, the goal should be to minimize the likelihood of overlooking a true drop in FEV1, because this may lead to a permanent and life‐shortening loss of lung function, even if the tradeoff is a possibly unnecessary course of antibiotic therapy. In our analytic group, only 22.4% of events characterized by an acute drop of 5– < 7% recovered to baseline without antibiotic therapy, no greater than the proportion that spontaneously recovered from greater drops, and the increased odds of recovery with antibiotic treatment was also comparable to what was found after treatment of greater declines. Studies of lung function decline before the availability of highly effective CFTR modulator therapy (HEMT) show a mean annual decline in FEV1 of just under 1.5%, 19 so a loss of 5%–7% represents about 3–5 years of lung function decline. For the majority of untreated patients who do not recover, this represents a substantial loss of lung function that could be prevented by antibiotic treatment.
Our findings are aligned with those of two recent papers that have evaluated variations in FEV1pp in people with CF. Stanojevic et al. 20 examined longitudinal spirometry measurements in healthy White children and found a mean coefficient of variation of 5.2%, with 1 standard deviation from the mean equal to 8.6% (which, if used as a cutoff, would lead to a 10% false positive rate). Notably, they pointed out that the degree of FEV1 variation was greater the longer the time interval between measurements, and the average time interval between repeated measurements in their test population was 0.9 years. They found that the variation among the CF population at their local clinic to be comparable to the health population. Heltshe et al. 21 compared recovery to drops in FEV1 found in the context of physician‐diagnosed (and treated) PEx to those found followed patient birthdays (presumably untreated). They reported that FEV1 recovered to 100% baseline in about half (49.6%) of PEx events, compared to only 36.6% of birthday events, even though the average drop in the PEx group was higher. The difference they found in recovery between PEx events and birthday events is similar to the difference we report here between decline events treated with IV antibiotics and those treated without antibiotics. They also note the importance of adjusting for baseline FEV1pp and FEV1pp drops in analyses comparing treatment options, which we have done here.
It should be pointed out that our finding that IV antibiotic treatment is more likely than non‐IV antibiotic treatment to be associated with a recovery of acute drops in FEV1pp do not necessarily imply that all drops in FEV1pp should be immediately treated with IV antibiotics. In the clinical setting, a multi‐staged approach to responding to acute declines in FEV1 may be feasible, starting with a lower intensity treatment (eg, an increase in airway clearance therapy and/or oral antibiotics) with follow‐up to ensure recovery and then prescription of a higher intensity treatment such as IV antibiotics for those who do not respond. There is some evidence for the effectiveness of such an algorithm. 16 On the other hand, it is important to consider previous research that suggests that failure to recover FEV1pp is more likely when time to initiation of IV antibiotics is more prolonged. 22
We have purposely focused on acute drops in FEV1 as our events of interest, and do not call these events PEx because PEx as a clinical entity take physical signs and symptoms into consideration and in fact are not always associated with any drop in FEV1. 23 The CFFPR does not record patient signs and symptoms so in our analysis we are not able to determine which patients with acute drops in FEV1 were symptomatic; we suspect that patients with symptoms along with an acute drop in FEV1 are more likely to be diagnosed with PEx and treated with antibiotic than those who have a drop in FEv1 but no signs or symptoms. 24
The lack of antibiotic treatment of acute drops in FEV1 does not imply that these drops are disregarded; there may have been other responses, such as a recommendation to improve adherence to and/or increase airway clearance; we adjusted for the diagnosis of asthma primarily to account for possible stepping up of asthma therapy as an alternative.
Potential weaknesses of this analysis include the threats to validity of any observational data analysis. If patients who were untreated, as reported in the CFFPR, did not receive treatment because they did not return for follow‐up, and were poorly adherent in other ways, this might have biased our findings towards seeing an advantageous association with antibiotic treatment. As a significant portion of our patients receiving IV antibiotic treatment were hospitalized, the impact that we interpret as being associated with IV antibiotic treatment might have been associated with hospitalization. In fact, given the overarching problem of confounding by indication that plagues observational studies comparing effectiveness of different treatments, it is more likely that indication bias has decreased our measurement of the magnitude of effect of antibiotic treatment. In other words, patients who are perceived by their care providers as higher risk in ways that are not well documented in the database may be more likely to receive higher intensity treatment but less likely to respond to that treatment. 25 Finally, this analysis was done before the widespread use of ETI, and we do not know if our findings would be different in those patients who are on this treatment. However, it is important to point out that a significant proportion of the population of PWCF does not currently use ETI. In the U.S., the CF Foundation estimates that this comprises about 20% of the CF population and outside of North America, Western Europe, Israel, Australia and New Zealand, use of ETI is quite limited. Thus, the findings of this study are relevant to a significant proportion of the PWCF in the world. Nonetheless, a parallel analysis focused on those who are currently using ETI is planned.
In summary, we found that a substantial proportion of acute drops in FEV1pp in PWCF are not treated with antibiotics, especially if they are young and/or have relatively high baseline FEV1. We found that the likelihood of recovery of FEV1 is substantially increased with IV antibiotic therapy, and to a lesser extent, non‐IV antibiotic therapy, even when the relative drop is as low as 5% predicted. Further investigations are planned looking into the long term impact of repeated antibiotic treatment and nontreatment, and further analyses should be done looking at the impact of HEMT on antibiotic response to acute drops in FEV1.
AUTHOR CONTRIBUTIONS
Michael S. Schechter: Conceptualization; methodology; investigation; formal analysis; writing—original draft; writing—review and editing. Joshua S. Ostrenga: Investigation; methodology; formal analysis; writing—review and editing. Elizabeth A. Cromwell: Methodology; data curation; investigation; validation; formal analysis; supervision; project administration; resources; writing—review and editing. Clement L. Ren: Conceptualization; methodology; investigation; writing—review and editing. Aliza K. Fink: Methodology; data curation; investigation; formal analysis; supervision; project administration; resources; writing—review and editing. D.B. Sanders: Conceptualization; methodology; investigation; writing—review & editing. Wayne J. Morgan: Conceptualization; methodology; investigation; formal analysis; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Schechter MS, Ostrenga JS, Cromwell EA, et al. Treatment of small as well as large declines in lung function enhances recovery to baseline in people with CF. Pediatr Pulmonol. 2024;59:3212‐3220. 10.1002/ppul.27176
This research was classified as exempt by Advarra (IRB#37633).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Cystic Fibrosis Foundation. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the author(s) with the permission of Cystic Fibrosis Foundation.
REFERENCES
- 1. Schechter MS. Reevaluating approaches to cystic fibrosis pulmonary exacerbations. Pediatr Pulmonol. 2018;53(S3):S51‐s63. [DOI] [PubMed] [Google Scholar]
- 2. Sanders DB, Zhao Q, Li Z, Farrell PM. Poor recovery from cystic fibrosis pulmonary exacerbations is associated with poor long‐term outcomes. Pediatr Pulmonol. 2017;52(10):1268‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boucher RC. On the pathogenesis of acute exacerbations of mucoobstructive lung diseases. Ann Am Thorac Soc. 2015;12(2):S160‐S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heltshe SL, Goss CH. Optimising treatment of CF pulmonary exacerbation: a tough nut to crack. Thorax. 2016;71(2):101‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders DB, Solomon GM, Beckett VV, et al. Standardized treatment of pulmonary exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCourt F, O'Neill B, Logan I, et al. Indicators of pulmonary exacerbation in cystic fibrosis: a Delphi survey of patients and health professionals. J Cyst Fibros. 2015;14(1):90‐96. [DOI] [PubMed] [Google Scholar]
- 7. Flume PA, Mogayzel PJ Jr., Robinson KA, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802‐808. [DOI] [PubMed] [Google Scholar]
- 8. Morgan WJ, Wagener JS, Pasta DJ, Millar SJ, VanDevanter DR, Konstan MW. Relationship of antibiotic treatment to recovery after acute FEV1 decline in children with cystic fibrosis. Ann Am Thorac Soc. 2017;14(6):937‐942. [DOI] [PubMed] [Google Scholar]
- 9. Konstan MW, Pasta DJ, VanDevanter DR, Wagener JS, Morgan WJ. Epidemiologic study of cystic fibrosis: 25 years of observational research. Pediatr Pulmonol. 2021;56(5):823‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan WJ, Wagener JS, Yegin A, Pasta DJ, Millar SJ, Konstan MW. Probability of treatment following acute decline in lung function in children with cystic fibrosis is related to baseline pulmonary function. J Pediatr. 2013;163(4):1152‐1157.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134‐139.e1. [DOI] [PubMed] [Google Scholar]
- 12. Knapp EA, Fink AK, Goss CH, et al. The cystic fibrosis foundation patient registry. design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173‐1179. [DOI] [PubMed] [Google Scholar]
- 13. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cogen J, Emerson J, Sanders DB, et al. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol. 2015;50(8):763‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson C, Butler SM, Konstan MW, Morgan W, Wohl MEB. Factors influencing outcomes in cystic fibrosis. Chest. 2003;123(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 16. Schechter MS, Schmidt HJ, Williams R, Norton R, Taylor D, Molzhon A. Impact of a program ensuring consistent response to acute drops in lung function in children with cystic fibrosis. J Cyst Fibros. 2018;17(6):769‐778. [DOI] [PubMed] [Google Scholar]
- 17. Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331(10):637‐642. [DOI] [PubMed] [Google Scholar]
- 18. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. [DOI] [PubMed] [Google Scholar]
- 19. Szczesniak R, Andrinopoulou ER, Su W, et al. Lung function decline in cystic fibrosis: impact of data availability and modeling strategies on clinical interpretations. Ann Am Thorac Soc. 2023;20(7):958‐968. [DOI] [PubMed] [Google Scholar]
- 20. Stanojevic S, Filipow N, Ratjen F. Paediatric reproducibility limits for the forced expiratory volume in 1 s. Thorax. 2020;75(10):891‐896. [DOI] [PubMed] [Google Scholar]
- 21. Heltshe SL, Russell R, VanDevanter DR, Sanders DB. Re‐examining baseline lung function recovery following IV‐treated pulmonary exacerbations. J Cyst Fibros. 2023;22(5):864‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders DB, Solomon GM, Beckett VV, et al. Standardized treatment of pulmonary exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bouzek DC, Ren CL, Thompson M, Slaven JE, Sanders DB. Evaluating FEV1 decline in diagnosis and management of pulmonary exacerbations in children with cystic fibrosis. Pediatr Pulmonol. 2022;57(7):1709‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosco JLF, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from Cystic Fibrosis Foundation. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the author(s) with the permission of Cystic Fibrosis Foundation.


