Abstract
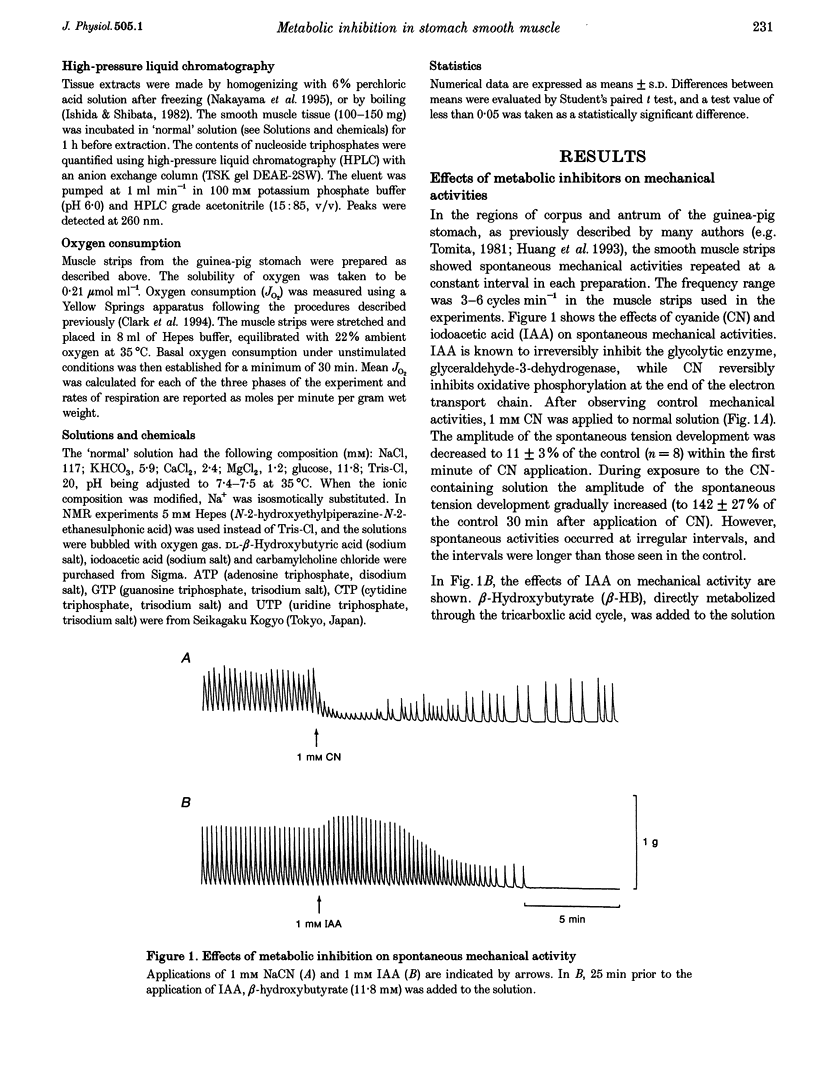
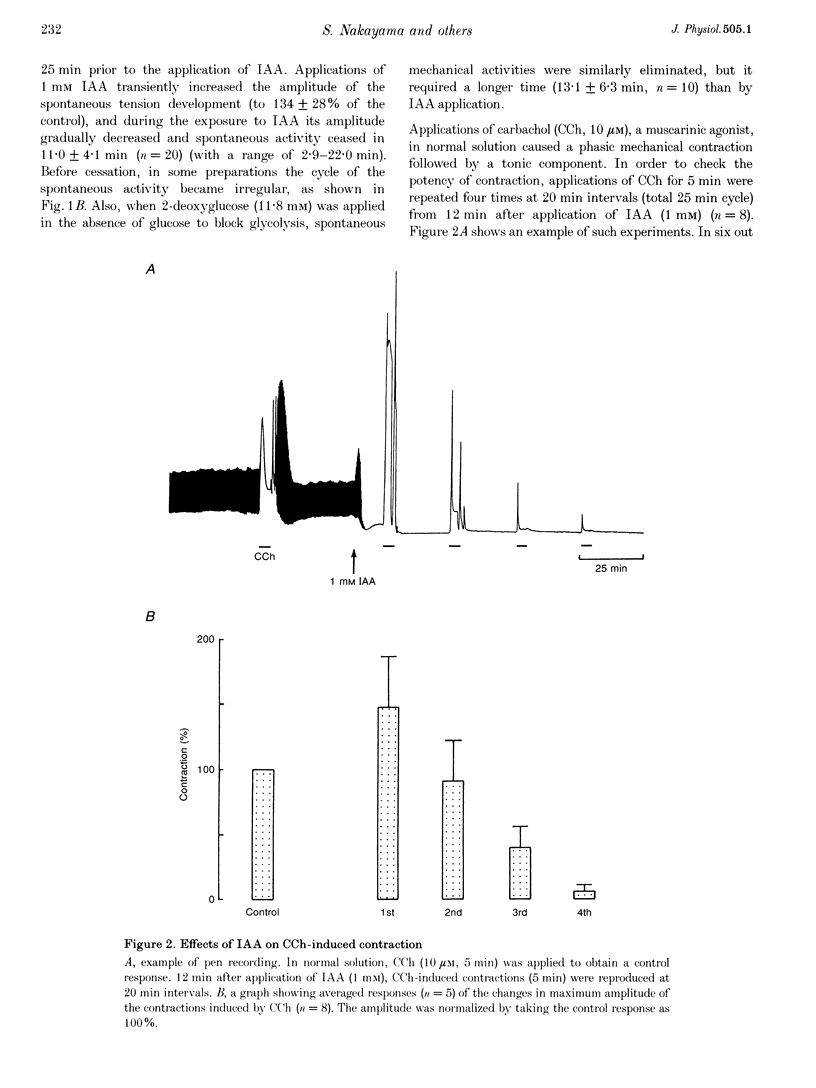
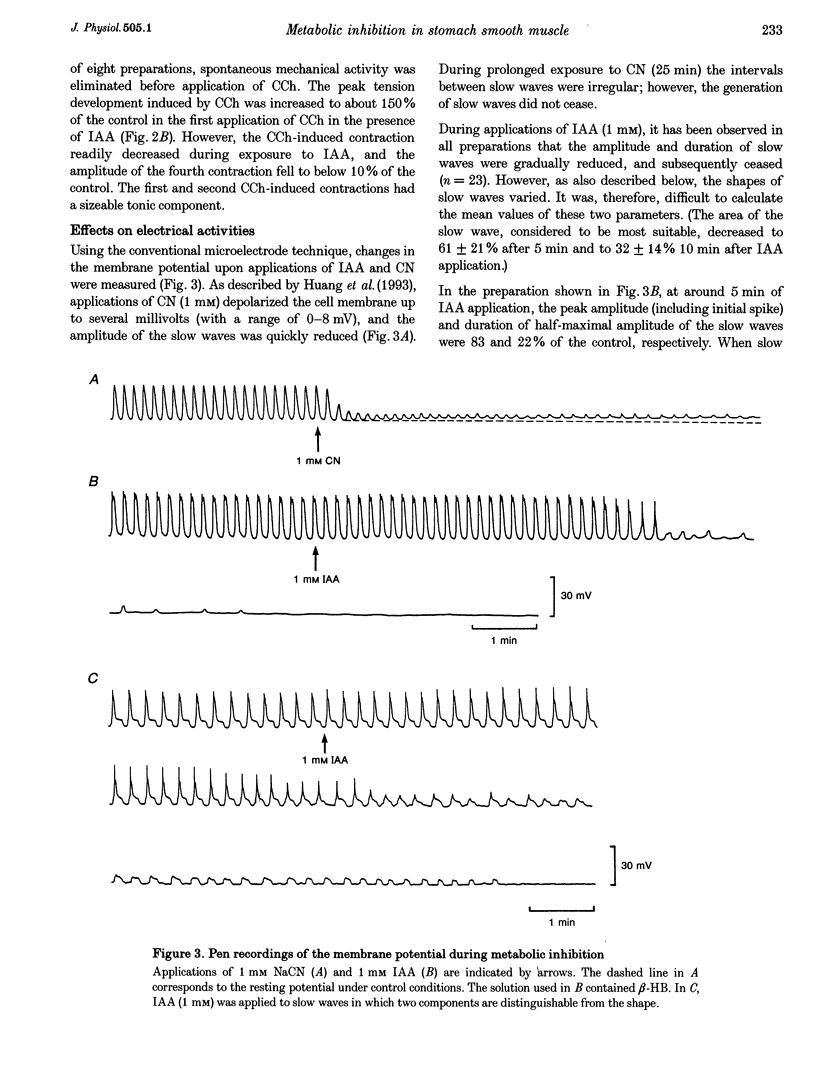
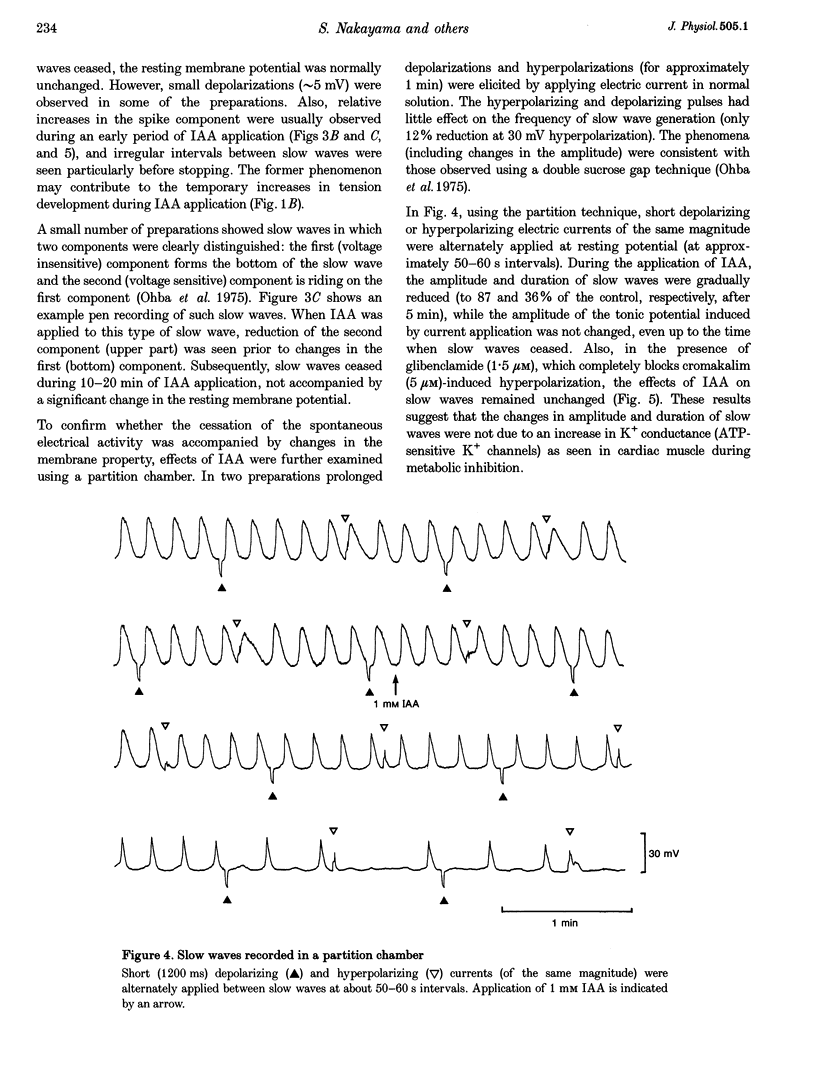
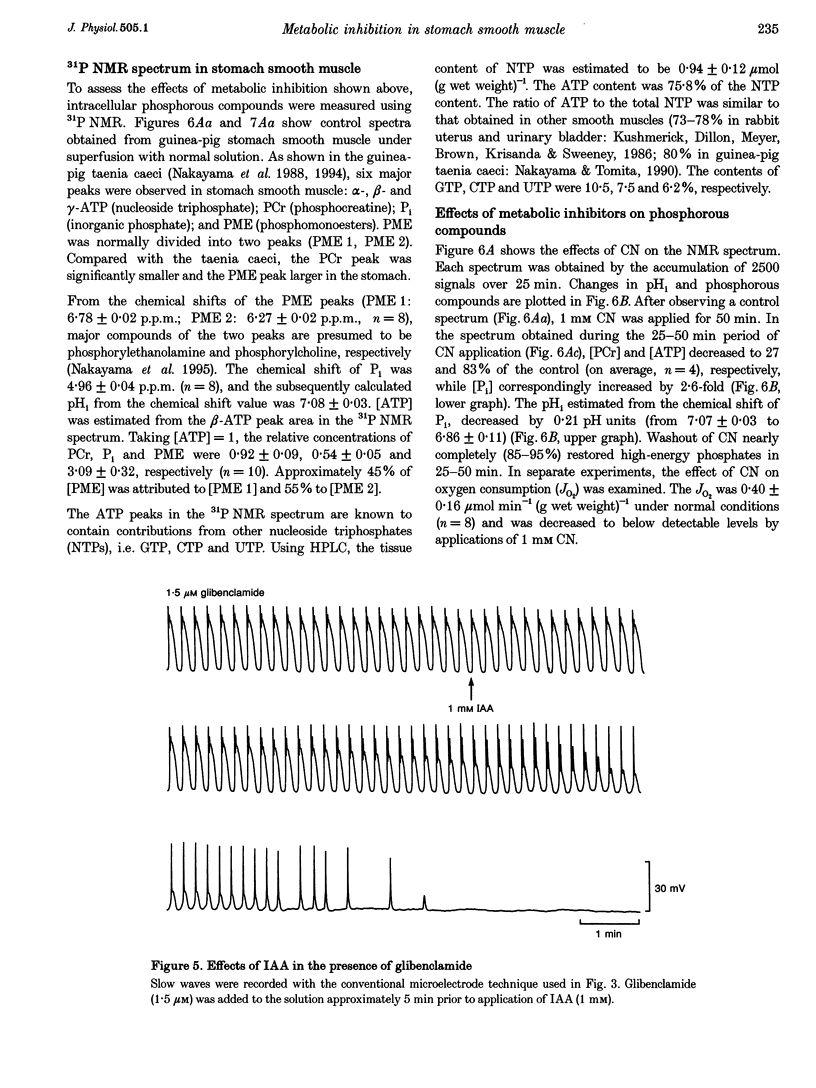
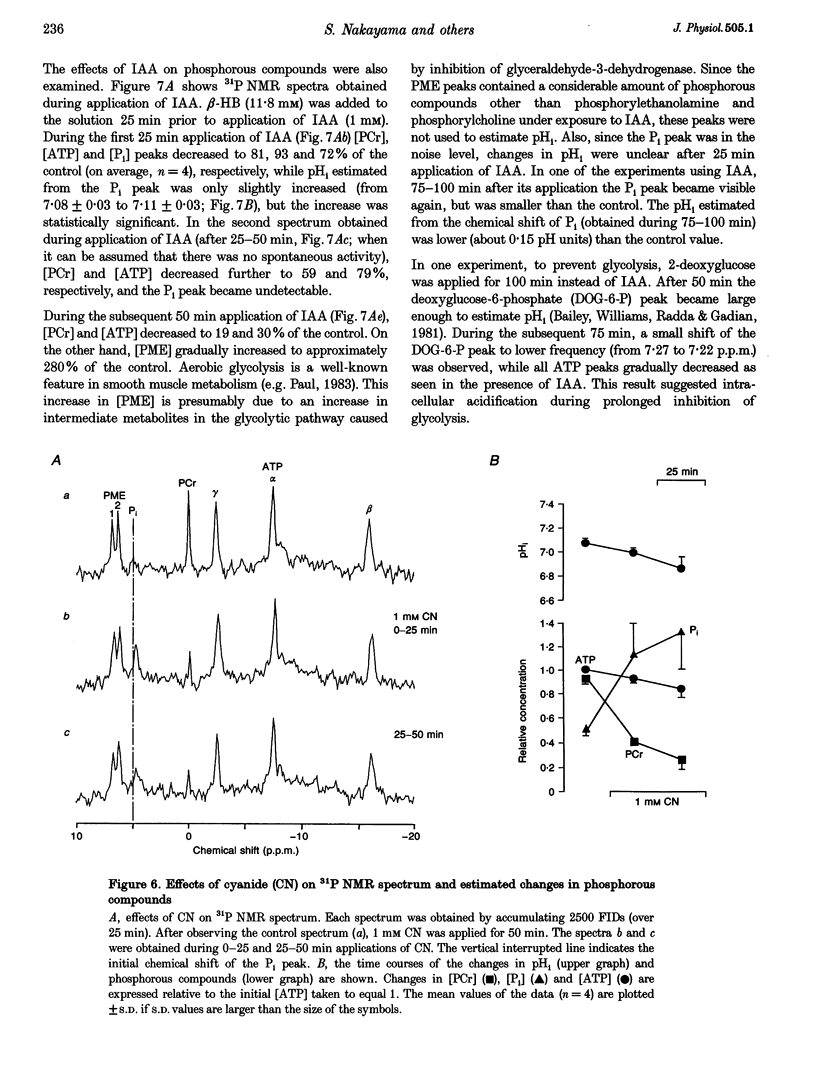
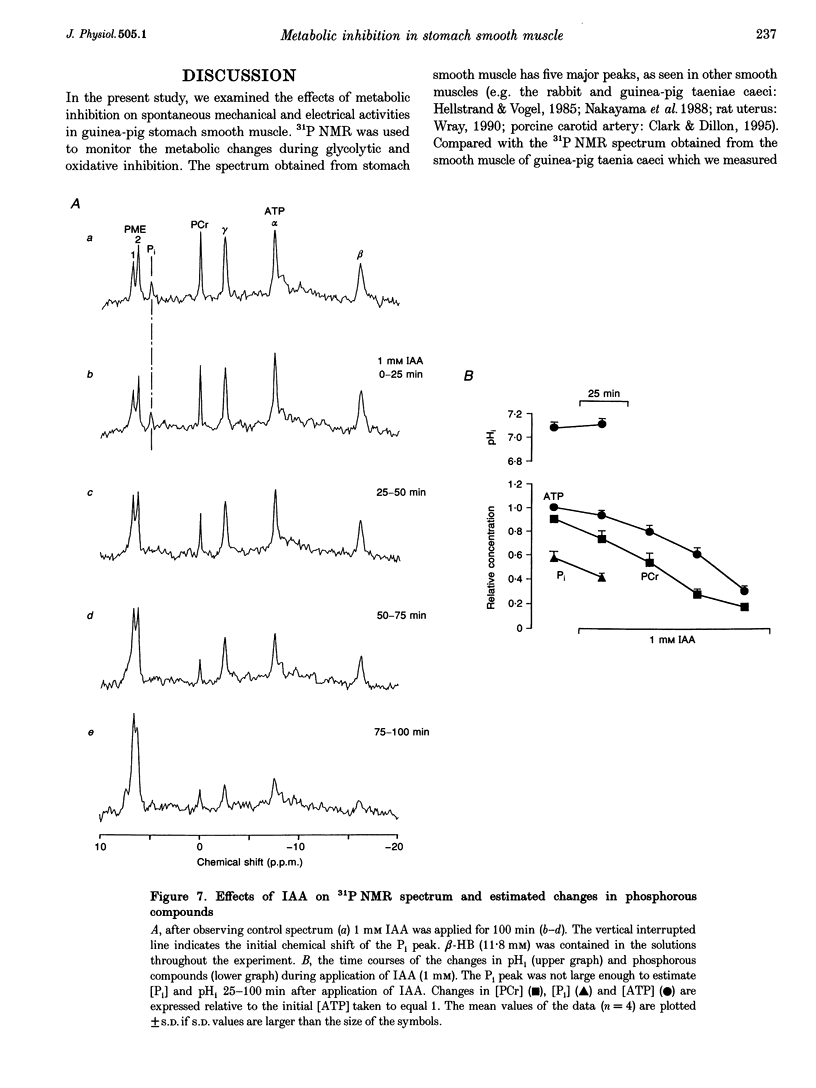
1. In smooth muscle isolated from the guinea-pig stomach, cyanide (CN) and iodoacetic acid (IAA) were applied to block oxidative phosphorylation and glycolysis, respectively. Effects of IAA on generation of spontaneous mechanical and electrical activities were systematically investigated by comparing those of CN. Spontaneous activity ceased in 10-20 min during applications of 1 mM IAA. On the other hand, application of 1 mM CN also reduced the spontaneous activity, but never terminated it. In the presence of CN the negativity of the resting membrane potential was slightly reduced. 2. When spontaneous activity ceased with IAA, the resting membrane potential was not significantly affected. Also, before ceasing, the amplitude and duration of the spontaneous electrical activity were significantly reduced. The amplitude of the electrotonic potential was, however, not changed by IAA. Further, glibenclamide did not prevent the effects of IAA. These results suggest that, unlike cardiac muscle, activation of metabolism-dependent K+ channels in stomach smooth muscle does not seem to play a major role in reducing and terminating spontaneous activity during metabolic inhibition. 3. Carbachol-induced contraction transiently increased, and subsequently decreased gradually during application of IAA. 4. After 50 min application of IAA, when there was no spontaneous activity, the concentrations of phosphocreatine (PCr) and ATP measured with 31P nuclear magnetic resonance decreased to 60 and 80% of the control, respectively, while inorganic phosphate (Pi) concentration paradoxically fell to below detectable levels. During subsequent prolonged application of IAA, high-energy phosphates steadily decreased. On the other hand, after 50 min CN application, [PCr] and [ATP] decreased to approximately 30 and 80% of the control, respectively, while [Pi] increased by 2.6-fold. 5. In the presence of either CN or IAA, spontaneous mechanical and electrical activities were reduced or eliminated, although amounts of high-energy phosphates sufficient to contract smooth muscle remained. It can be postulated that some mechanism(s) related to energy metabolism, but not including ATP-sensitive K+ channels, plays an important role in generating spontaneous activity in guinea-pig stomach smooth muscle. During metabolic inhibition the energy metabolism-dependent mechanism(s) would preserve high-energy phosphates, and consequently cell viability, by stopping spontaneous activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A., Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. J Physiol. 1985 Mar;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey I. A., Williams S. R., Radda G. K., Gadian D. G. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem J. 1981 Apr 15;196(1):171–178. doi: 10.1042/bj1960171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993 Oct;110(2):573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol. 1993 Oct;110(2):583–590. doi: 10.1111/j.1476-5381.1993.tb13850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J. High-energy phosphate metabolism in vascular smooth muscle. Annu Rev Physiol. 1985;47:629–643. doi: 10.1146/annurev.ph.47.030185.003213. [DOI] [PubMed] [Google Scholar]
- Chihara S., Tomita T. Mechanical and electrical responses to alpha-adrenoceptor activation in the circular muscle of guinea-pig stomach. Br J Pharmacol. 1987 Aug;91(4):789–798. doi: 10.1111/j.1476-5381.1987.tb11277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. F., Dillon P. F. Phosphocreatine and creatine kinase in energetic metabolism of the porcine carotid artery. J Vasc Res. 1995 Jan-Feb;32(1):24–30. doi: 10.1159/000159074. [DOI] [PubMed] [Google Scholar]
- Clark J. F., Kemp G. J., Radda G. K. The creatine kinase equilibrium, free [ADP] and myosin ATPase in vascular smooth muscle cross-bridges. J Theor Biol. 1995 Mar 21;173(2):207–211. doi: 10.1006/jtbi.1995.0056. [DOI] [PubMed] [Google Scholar]
- Clark J. F., Khuchua Z., Kuznetsov A. V., Vassil'eva E., Boehm E., Radda G. K., Saks V. Actions of the creatine analogue beta-guanidinopropionic acid on rat heart mitochondria. Biochem J. 1994 May 15;300(Pt 1):211–216. doi: 10.1042/bj3000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Romanek R. R., Orlowski J., Grinstein S. ATP dependence of Na+/H+ exchange. Nucleotide specificity and assessment of the role of phospholipids. J Gen Physiol. 1997 Feb;109(2):117–128. doi: 10.1085/jgp.109.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Eisner D. A., Allen D. G. Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol. 1992 Aug;454:467–490. doi: 10.1113/jphysiol.1992.sp019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. D., Raeymaekers L., Paul R. J. Comparison of endogenous and exogenous sources of ATP in fueling Ca2+ uptake in smooth muscle plasma membrane vesicles. J Gen Physiol. 1992 Jan;99(1):21–40. doi: 10.1085/jgp.99.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin C. D., Wiseman R. W., Kushmerick M. J. Tension responses of sheep aorta to simultaneous decreases in phosphocreatine, inorganic phosphate and ATP. J Physiol. 1992 Dec;458:139–150. doi: 10.1113/jphysiol.1992.sp019410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. C., Wray S., Eisner D. A. Effects of metabolic inhibition and changes of intracellular pH on potassium permeability and contraction of rat uterus. J Physiol. 1993 Jun;465:43–56. doi: 10.1113/jphysiol.1993.sp019665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand P., Vogel H. J. Phosphagens and intracellular pH in intact rabbit smooth muscle studied by 31P-NMR. Am J Physiol. 1985 Mar;248(3 Pt 1):C320–C329. doi: 10.1152/ajpcell.1985.248.3.C320. [DOI] [PubMed] [Google Scholar]
- Huang S. M., Chowdhury J. U., Kobayashi K., Tomita T. Inhibitory effects of cyanide on mechanical and electrical activities in the circular muscle of gastric antrum of guinea-pig stomach. Jpn J Physiol. 1993;43(2):229–238. doi: 10.2170/jjphysiol.43.229. [DOI] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Shibata S. Relaxing and metabolic inhibitory action of X537A (Lasalocid) on the taenia of the guinea-pig caecum. J Physiol. 1982 Dec;333:293–304. doi: 10.1113/jphysiol.1982.sp014454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- Kajioka S., Kitamura K., Kuriyama H. Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991 Dec;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama N., Huang S. M., Tomita T., Brading A. F. Effects of cromakalim on the electrical slow wave in the circular muscle of guinea-pig gastric antrum. Br J Pharmacol. 1993 Aug;109(4):1097–1100. doi: 10.1111/j.1476-5381.1993.tb13735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Dillon P. F., Meyer R. A., Brown T. R., Krisanda J. M., Sweeney H. L. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J Biol Chem. 1986 Nov 5;261(31):14420–14429. [PubMed] [Google Scholar]
- Nakayama S., Hachisuka T., Itoh K., Matsumoto T., Tomita T. Phosphomonoesters in the guinea-pig taenia caeci: pH-dependency of the phosphomonoester peaks in 31P-NMR. Jpn J Physiol. 1995;45(3):411–422. doi: 10.2170/jjphysiol.45.411. [DOI] [PubMed] [Google Scholar]
- Nakayama S., Nomura H. Mechanisms of intracellular Mg2+ regulation affected by amiloride and ouabain in the guinea-pig taenia caeci. J Physiol. 1995 Oct 1;488(Pt 1):1–12. doi: 10.1113/jphysiol.1995.sp020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Nomura H., Tomita T. Intracellular-free magnesium in the smooth muscle of guinea pig taenia caeci: a concomitant analysis for magnesium and pH upon sodium removal. J Gen Physiol. 1994 May;103(5):833–851. doi: 10.1085/jgp.103.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Seo Y., Takai A., Tomita T., Watari H. Phosphorous compounds studied by 31P nuclear magnetic resonance spectroscopy in the taenia of guinea-pig caecum. J Physiol. 1988 Aug;402:565–578. doi: 10.1113/jphysiol.1988.sp017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Depletion of intracellular free Mg2+ in Mg2(+)- and Ca2(+)-free solution in the taenia isolated from guinea-pig caecum. J Physiol. 1990 Feb;421:363–378. doi: 10.1113/jphysiol.1990.sp017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Regulation of intracellular free magnesium concentration in the taenia of guinea-pig caecum. J Physiol. 1991 Apr;435:559–572. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975 Dec;253(2):505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. J. Functional compartmentalization of oxidative and glycolytic metabolism in vascular smooth muscle. Am J Physiol. 1983 May;244(5):C399–C409. doi: 10.1152/ajpcell.1983.244.5.C399. [DOI] [PubMed] [Google Scholar]
- Taggart M. J., Wray S. The effect of metabolic inhibition on rat uterine intracellular pH and its role in contractile failure. Pflugers Arch. 1995 May;430(1):125–131. doi: 10.1007/BF00373847. [DOI] [PubMed] [Google Scholar]
- Tsugeno M., Huang S. M., Pang Y. W., Chowdhury J. U., Tomita T. Effects of phosphodiesterase inhibitors on spontaneous electrical activity (slow waves) in the guinea-pig gastric muscle. J Physiol. 1995 Jun 1;485(Pt 2):493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. J Physiol. 1990 Apr;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]



