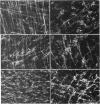
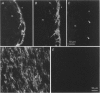
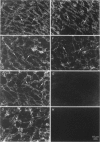
Abstract
1. Interstitial cells of Cajal (ICCs) have been identified as pacemaker cells in the gastrointestinal (GI) tracts of vertebrates. We have studied the development of ICCs in pacemaker regions and the onset of electrical rhythmicity in the gastric antrum, small bowel and proximal colon of the mouse. 2. ICCs, as detected by c-Kit immunofluorescence, were found during embryogenesis in regions of the GI tract that eventually become pacemaker areas. Prior to birth, these cells were organized into well-structured networks, and by the end of the embryonic period the morphology of ICC networks in pacemaker regions appeared very similar to that observed in adult animals. 3. Electrical rhythmicity was recorded prior to birth (by E18) in the proximal GI tract (stomach and jejunum), and this activity developed to adult-like behaviour within a week after birth. In the ileum and proximal colon rhythmicity developed after birth, and adult-like characteristics were apparent within the first week. 4. Post-junctional responses of smooth muscles to neural inputs could be recorded at birth, and stimulation of intrinsic nerves often led to oscillatory activity resembling slow waves for up to several minutes following brief stimuli. Nerve stimulation augmented spontaneous activity in the proximal portions of the GI tract and elicited rhythmic activity temporarily in quiescent tissues of the distal GI tract. 5. ICCs and rhythmicity developed in an apparently normal manner in tissues isolated at birth and placed in organ culture. These data suggest that the tunica muscularis provides a suitable microenvironment for the development of ICCs and rhythmicity without the need for extrinsic stimuli. 6. Treatment of small intestinal tissues taken from embryos at E15 with neutralizing c-Kit antibodies abolished ICC development and the organization of ICCs into networks that typically occurs during the late embryonic period. Treatment of muscles taken from newborn animals with c-Kit antibodies blocked postnatal development of ICCs, disrupted already established and functional ICC networks, and rendered muscles electrically quiescent. 7. In summary, ICC networks develop in the pacemaker regions of the murine GI tract before birth. Development and organization of ICCs of the myenteric plexus region into networks precedes the development of electrical rhythmicity. Post-natal development of electrical rhythmicity is mainly characterized by enhancement of the amplitude and frequency of slow waves. The development of ICCs and electrical rhythmicity persists in vitro. ICCs appear to be necessary for the initiation of electrical rhythmicity. These findings provide further evidence for the pacemaker role of ICCs.
Full text
PDF


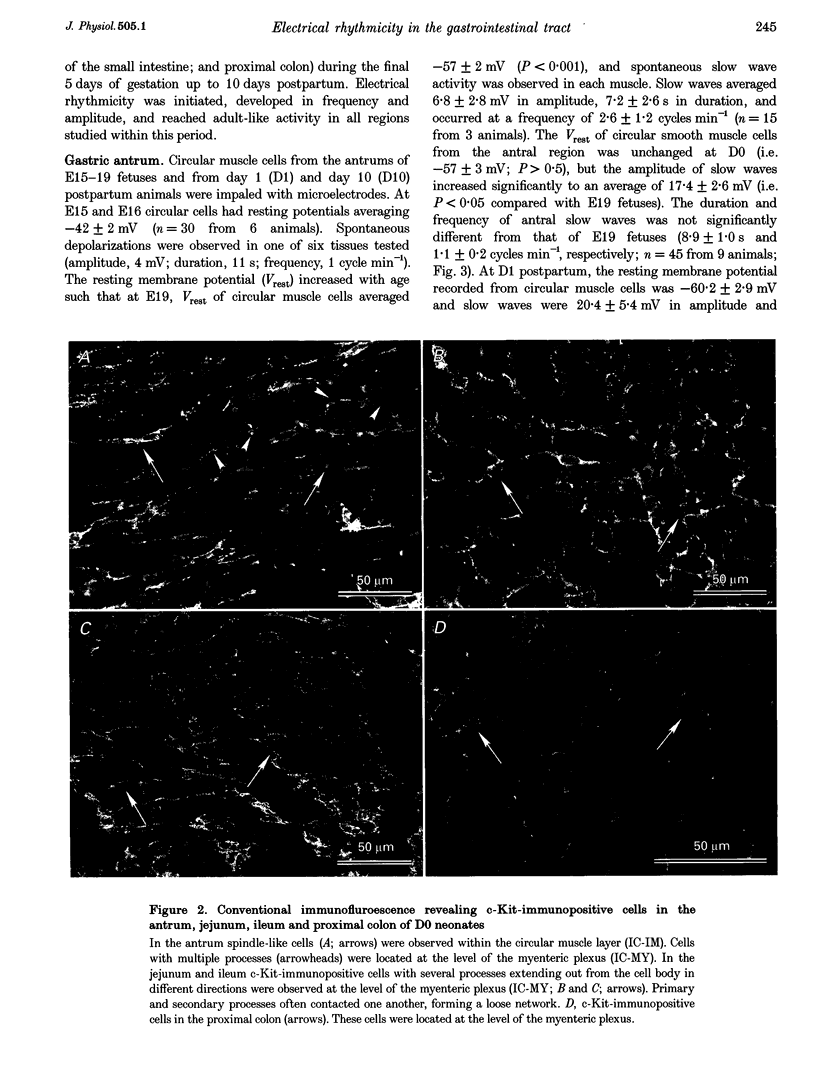
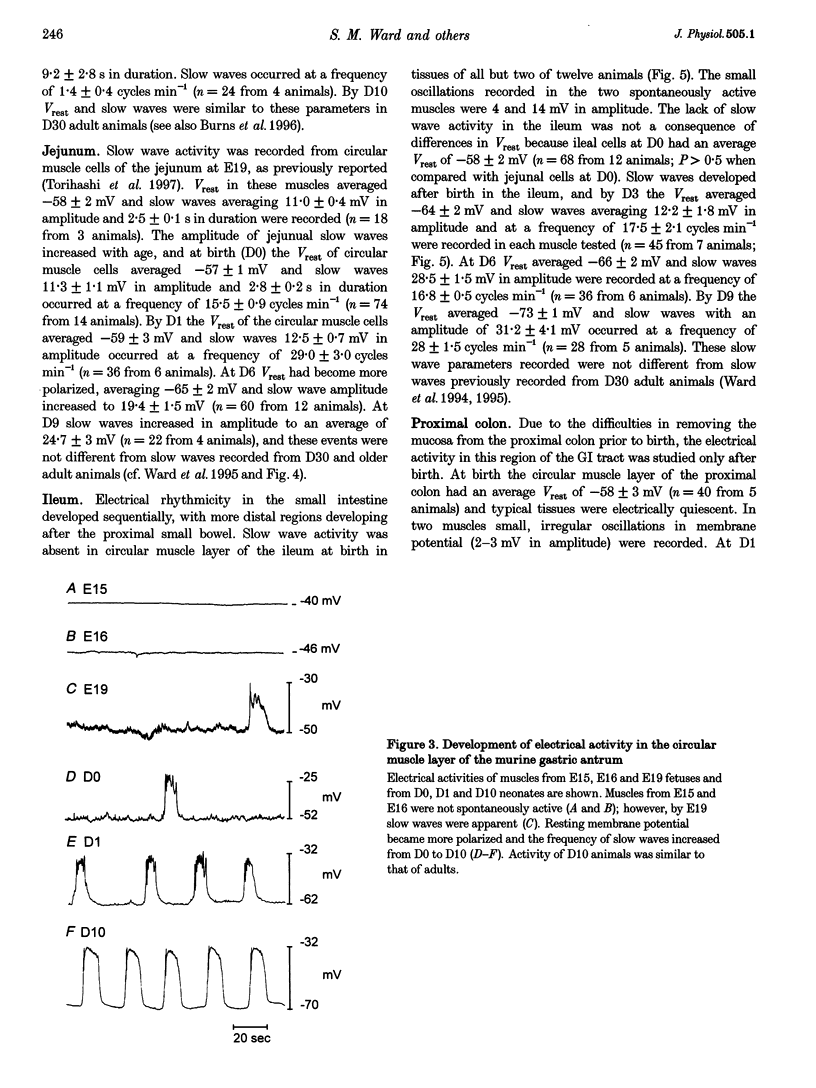
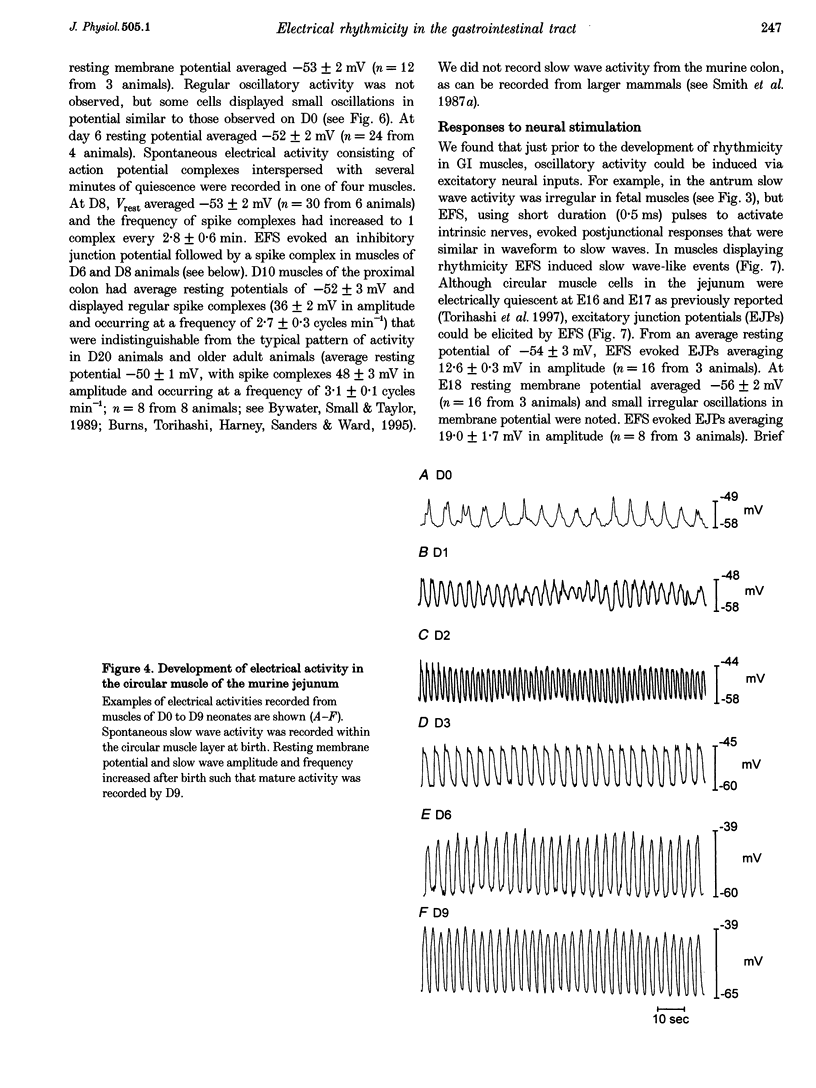
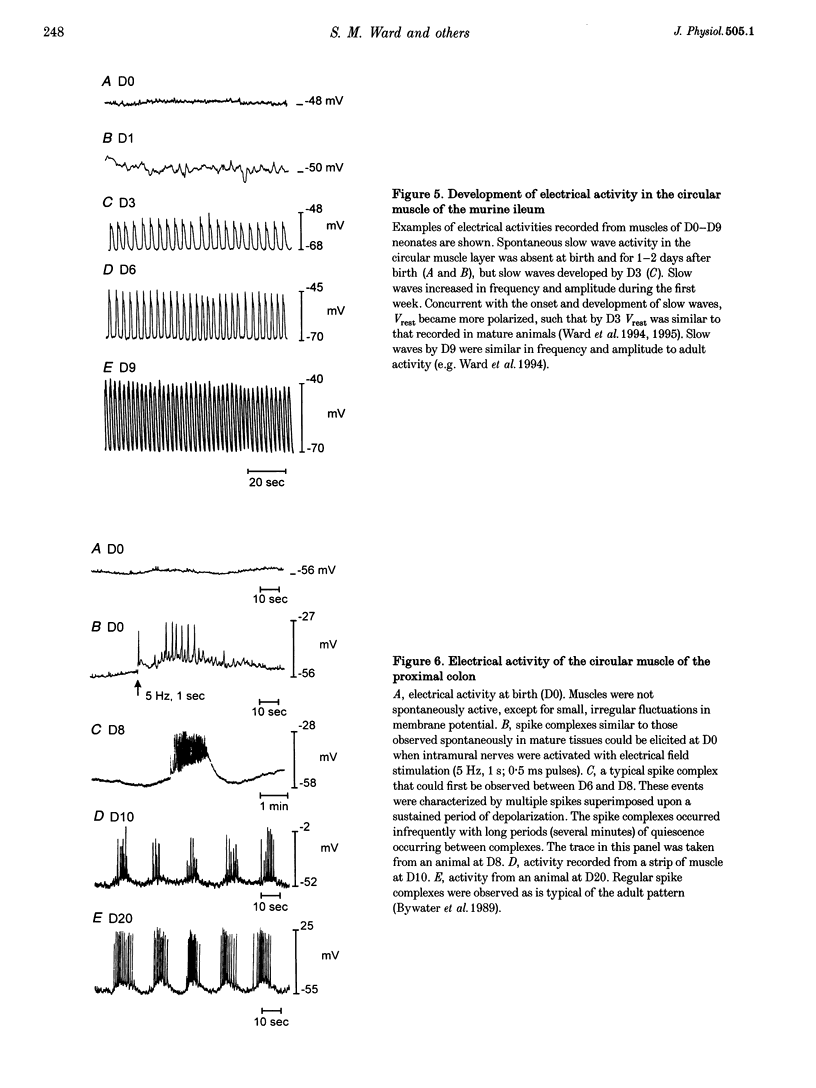
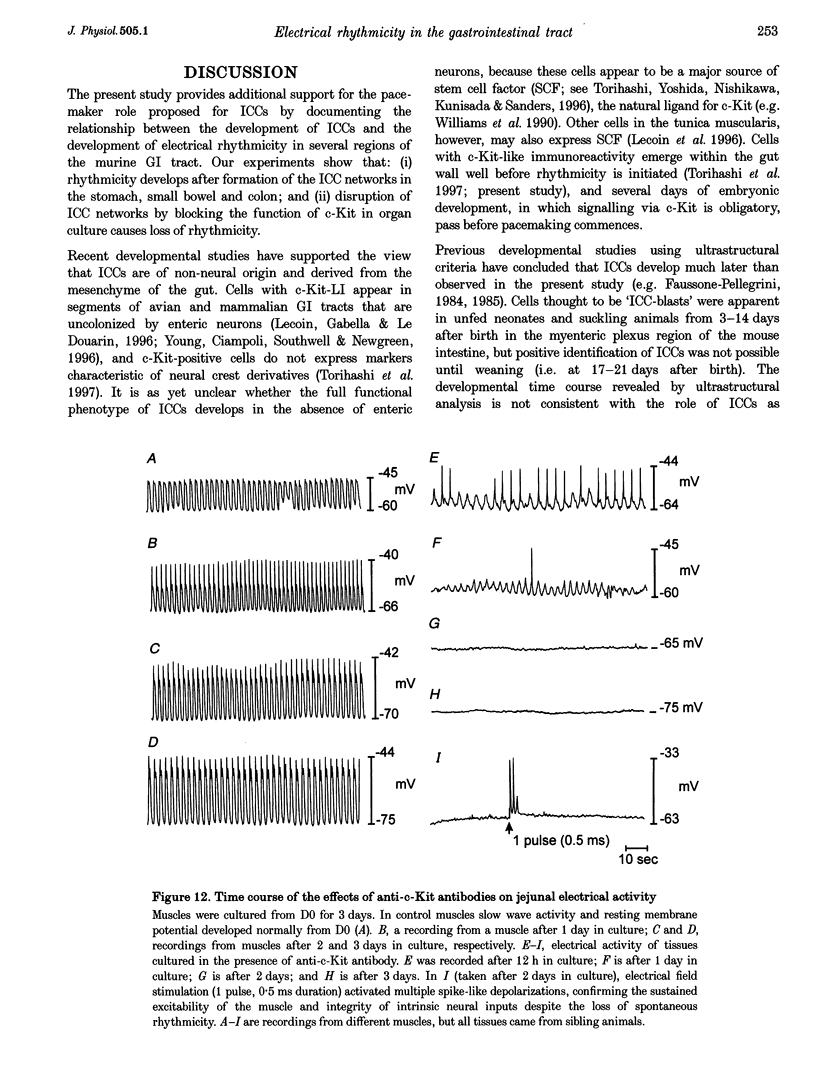
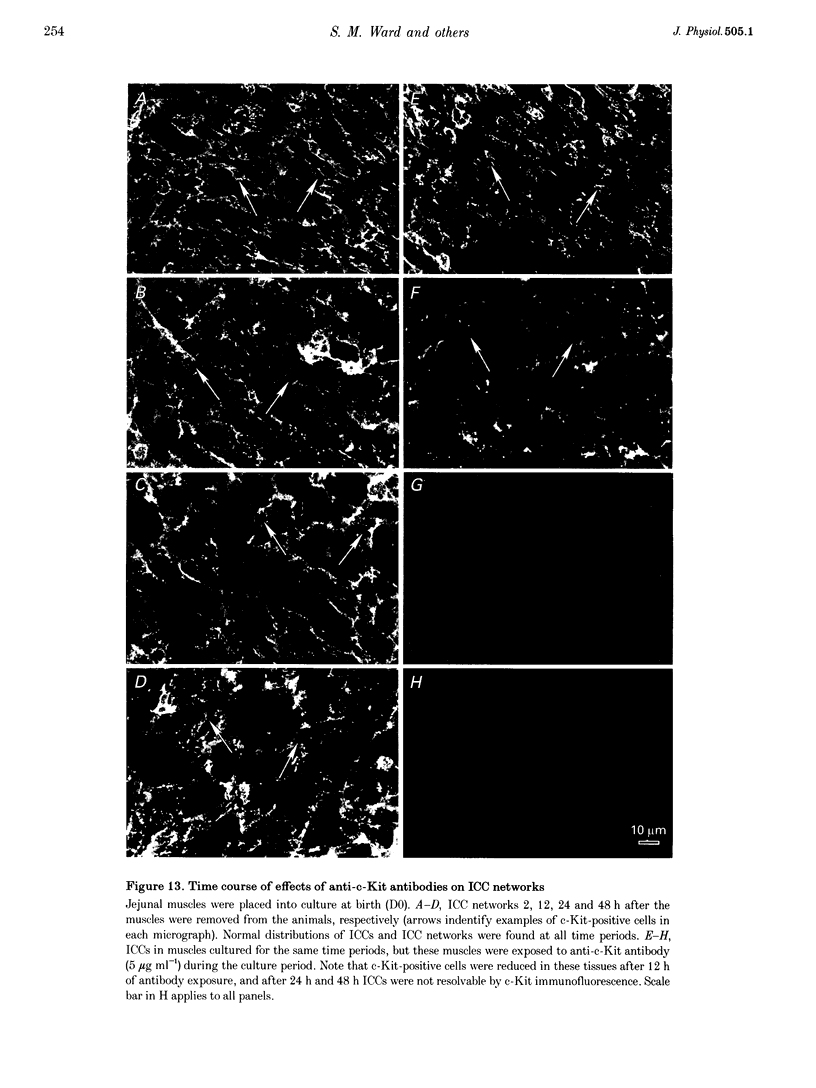
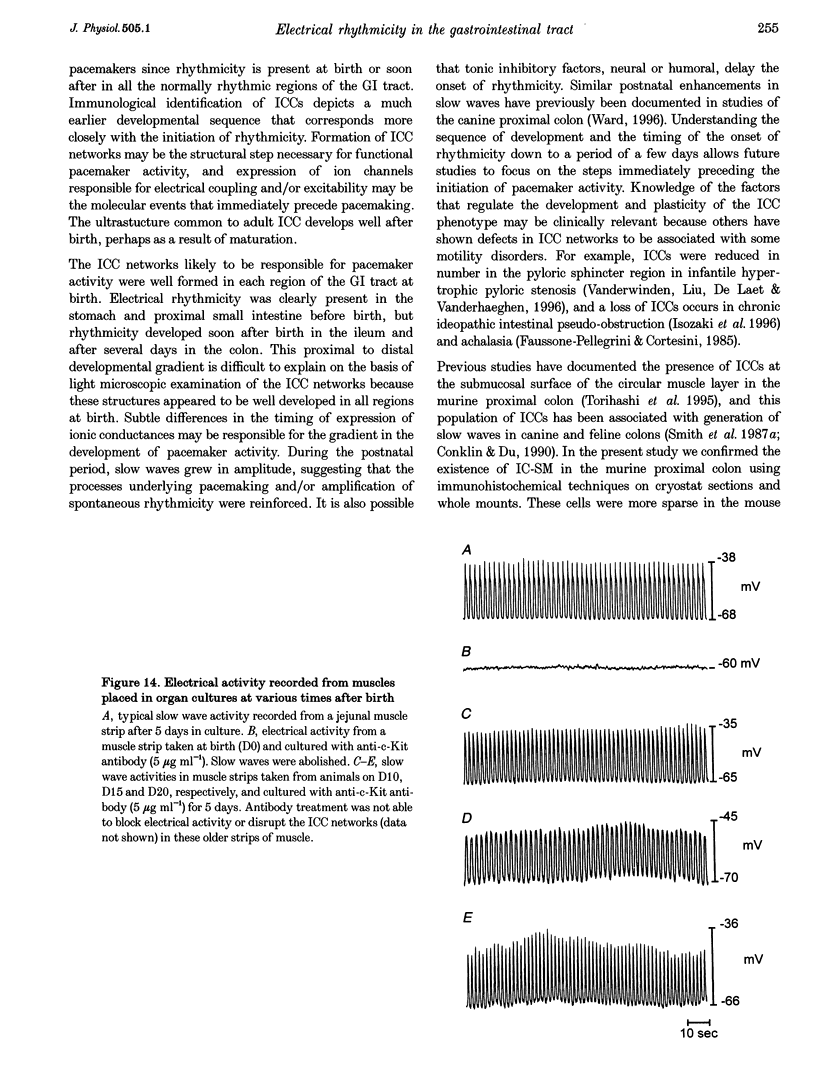



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernex F., De Sepulveda P., Kress C., Elbaz C., Delouis C., Panthier J. J. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996 Oct;122(10):3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- Burns A. J., Lomax A. E., Torihashi S., Sanders K. M., Ward S. M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Small R. C., Taylor G. S. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol. 1989 Jun;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin J. L., Du C. Pathways of slow-wave propagation in proximal colon of cats. Am J Physiol. 1990 Jun;258(6 Pt 1):G894–G903. doi: 10.1152/ajpgi.1990.258.6.G894. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984 Mar;246(3 Pt 1):G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Faussone Pellegrini M. S. Morphogenesis of the special circular muscle layer and of the interstitial cells of Cajal related to the plexus muscularis profundus of mouse intestinal muscle coat. An E.M. study. Anat Embryol (Berl) 1984;169(2):151–158. doi: 10.1007/BF00303144. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini M. S., Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol. 1985 Oct;17(4):673–685. [PubMed] [Google Scholar]
- Faussone-Pellegrini M. S. Cytodifferentiation of the interstitial cells of Cajal related to the myenteric plexus of mouse intestinal muscle coat. An E.M. study from foetal to adult life. Anat Embryol (Berl) 1985;171(2):163–169. doi: 10.1007/BF00341410. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kubota M., Szurszewski J. H. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986 Mar;372:501–520. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Thuneberg L., Klüppel M., Malysz J., Mikkelsen H. B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995 Jan 26;373(6512):347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Langton P., Ward S. M., Carl A., Norell M. A., Sanders K. M. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoin L., Gabella G., Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996 Mar;122(3):725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Lee H. K., Sanders K. M. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993 Jan;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. W., Thuneberg L., Huizinga J. D. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am J Physiol. 1994 Mar;266(3 Pt 1):G485–G496. doi: 10.1152/ajpgi.1994.266.3.G485. [DOI] [PubMed] [Google Scholar]
- Maeda H., Yamagata A., Nishikawa S., Yoshinaga K., Kobayashi S., Nishi K., Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992 Oct;116(2):369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Malysz J., Thuneberg L., Mikkelsen H. B., Huizinga J. D. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996 Sep;271(3 Pt 1):G387–G399. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover N. G., Horowitz N. N., Sanders K. M. Calcium oscillations in freshly dispersed and cultured interstitial cells from canine colon. Am J Physiol. 1992 Mar;262(3 Pt 1):C589–C597. doi: 10.1152/ajpcell.1992.262.3.C589. [DOI] [PubMed] [Google Scholar]
- Sanders K. M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996 Aug;111(2):492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. W., Xue C., Ward S. M., de Vente J., Sanders K. M. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993 Sep;56(2):513–522. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol. 1987 Mar;252(3 Pt 1):C290–C299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987 Feb;252(2 Pt 1):C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Prosser C. L., Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986 Mar;250(3 Pt 1):G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- Torihashi S., Ward S. M., Nishikawa S., Nishi K., Kobayashi S., Sanders K. M. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995 Apr;280(1):97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Torihashi S., Ward S. M., Sanders K. M. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997 Jan;112(1):144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Torihashi S., Yoshida H., Nishikawa S., Kunisada T., Sanders K. M. Enteric neurons express Steel factor-lacZ transgene in the murine gastrointestinal tract. Brain Res. 1996 Nov 4;738(2):323–328. doi: 10.1016/s0006-8993(96)00935-3. [DOI] [PubMed] [Google Scholar]
- Vanderwinden J. M., Liu H., De Laet M. H., Vanderhaeghen J. J. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996 Aug;111(2):279–288. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burke E. P., Sanders K. M. Use of rhodamine 123 to label and lesion interstitial cells of Cajal in canine colonic circular muscle. Anat Embryol (Berl) 1990;182(3):215–224. doi: 10.1007/BF00185515. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burns A. J., Torihashi S., Harney S. C., Sanders K. M. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995 Dec;269(6 Pt 1):C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burns A. J., Torihashi S., Sanders K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994 Oct 1;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Young H. M., Ciampoli D., Southwell B. R., Newgreen D. F. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996 Nov 25;180(1):97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- Young H. M., McConalogue K., Furness J. B., De Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993 Jul;55(2):583–596. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]