Abstract
The activation of C-H bonds is a potent tool for modifying molecular structures in chemistry. This article details the steps involved in a novel ligand bearing a bicyclic [3.3.1]-nonane framework and bissulfoxide moiety. A palladium catalyzed allylic C-H oxidation method enables a direct benzyl-allylic functionalization with the bissulfoxide ligand. Bissulfoixde ligand possesses a rapidly constructed bicyclic [3.3.1] framework and it proved to be effective for enabling both N-and C-alkylation. A total of 13 C-H activation productions were reported with good to excellent yields. This report validated that it is necessary to include bissulfoxide as a ligand for superior reactivities. Naftifine was produced utilizing developed C-H functionalization methodology in good overall yields.
Keywords: C-H activation, bicyclic ligand, sulfoxide, palladium, amino acids
1. Introduction
The field of organic chemistry has witnessed significant advancements, allowing for the achievement of challenging covalent bond formations. In recent years, the area of interest revolving around the direct activation of inert carbon-hydrogen (C-H) bonds has drawn substantial attention. This powerful strategy (C-H activation) enables the transformation of these traditionally unreactive bonds into diverse functional groups, thereby facilitating molecular structure modifications with broad applications across various domains of chemistry. C-H bonds are a ubiquitous feature of many molecules and are often considered to be unreactive. In particular, C(sp3)–H bonds are notoriously difficult to activate compared to other types of C-H bonds. However, C(sp3)–H bonds located in the allylic position are comparatively more reactive. Remarkable strides have been made in this area of research, including the identification of effective metal and ligand complexes for C-H activation. In terms of catalyst selection, it is clear that Pd salts are a rather cost-effective choice with wide accessibility from abundant commercial sources. In addition, Pd-catalyzed C-H functionalization systems generally tolerate a variety of elements, and, therefore, stringent conditions such as oxygen- or moisture-free environments are not required, which enable the Pd-catalyzed system to be much more practical to deploy. These observations have been documented in several notable studies by leading researchers in the field, including Yu, Sanford, Dorta, and Poli [1–4]. Evidentially, there is a continuing need to explore new ligand entities that can enhance the reactivity and the selectivity of C-H activation reactions.
The direct functionalization of allylic alkenes has been the subject of extensive investigation, and, among many approaches, an efficient catalytic system involving the use of Pd metal and sulfoxide ligands was first explored by the Shi and White research groups, who employed a bissulfoxide ligand in palladium-catalyzed allylic amination and alkylation reactions [5–7]. Subsequently, the White group further investigated the usage of sulfoxideoxazoline ligands as an alternative for these transformations [8,9]. Meanwhile, Stambuli and Zhang demonstrated that a palladium sulfide catalyst can effectively catalyze allylic oxidation and oxidative Heck reactions [10,11]. These pioneering works represent significant advancements in the development of novel catalytic systems for direct functionalization of allylic alkenes.
Our group discovered a novel bicyclic [3.3.1] framework for primary amine labeling in 2019 [12]. Drawing inspiration from the Briphos ligand developed by Kim et al. [13], we recognized that the potential of our newly discovered framework may potentially serve as a novel class of geometrically constrained bisulfide/sulfoxide ligands. With the inclusion of a tertiary amine and two sulfide/sulfoxide moieties, we hypothesized that the framework might promote the formation of allylpalladium species while attracting amine nucleophiles to the reaction site [14]. Through extensive ligand screening for C-H activation, DMSO and bissulfoxide were proved as effective promoters for the formation of (σ-allyl)–palladium complexes with allyl compounds. These observations have been documented in several notable studies by researchers including Dorta [3] and White (Figure 1) [5]. In addition, the Dorta and Li groups utilized bissulfoxide ligand in Rh catalyzed addition of arylboronic acid to electron deficient olefins (Figure 1) [15,16]. Along this line, we herein report a direct benzyl-allylic functionalization through Pd-catalyzed C–H activation with a novel bissulfoxide ligand.
Figure 1.

(A) Pd/Rh Thioether/Bissulfoxide Complexes Catalyzed Allylic Oxidation Reactions. (B) Notable Ligands Employed in C-H Activation [8–11,15,16].
2. Material and Methods
Compounds 7, 8 and S3–9 are reported in [5,9,17–22]. All detailed experiment conditions and spectral data are available in Supplementary Materials.
X-ray crystal structure data collection was performed on a Bruker D8 VENTURE X-ray diffractometer with PHOTON 100 CMOS shutterless mode detector equipped with a Mo-target X-ray tube (λ = 0.71073 Å) at T = 100(2) K. Data reduction and integration were performed with the Bruker software package SAINT (version 8.38A). Data were corrected for absorption effects using the empirical methods as implemented in SADABS (version 2016/2). The structure was solved by SHELXT and refined by full-matrix leastsquares procedures using the Bruker SHELXTL (version 2017/1) software package. All non-hydrogen atoms were refined anisotropically [23–26].
3. Results and Discussion
Derived from our protein conjugation study [12], it is revealed that the bicyclic [3.3.1] framework could be rapidly constructed (Scheme 1). Starting from commercially available thiophenol 1, homo-disulfide 2 was furnished upon oxidation of compound 1. In situ reduction of disulfide bond followed by a glycine amination meant bicyclic [3.3.1] nonane motif 3 could be obtained in high yield. Overall, substrate 3 was secured in two steps and features a strikingly geometrically constraint. In addition, the presence of a tertiary amine and sulfide moieties may potentially facilitate the formation of organometallic complexes.
Scheme 1.

Rapid Construction of Bicyclic [3.3.1]-nonane Framework.
Subsequently, we evaluated compound 3 as a potential ligand for C-H functionalization. Unfortunately, despite multiple attempts, the complex of palladium and 3 did not exhibit significant catalytic activity.
Motivated by a recent study [14], we hypothesized that introducing a sulfoxide group to the molecule could enhance its metal chelation ability. Thus, we explored the possibility of oxidizing compound 3 to its sulfoxide form (Table 1). The process of sulfoxide formation presented challenges, primarily due to difficulties of controlling the oxidation state of sulfides. An uncontrolled sulfide oxidation could potentially lead to moieties at different oxidation states. Sulfoxide, sulfone, and a combination of both could be produced as mixtures. Commonly employed oxidants such as Oxone, NaIO4, or PhI(OAc)2 yielded no product. Peroxide-based oxidants were evaluated and mCPBA furnished trace amounts of product in CH2Cl2, but, unfortunately, when other solvents, such as acetone and THF, could not produce any desired oxidation product. Alternatively, DMDO and Sharpless reagents failed to generate correct sulfoxide. We eventually resorted to H2O2, with the assistance from Na2WO4 as a co-oxidant, and the desired sulfoxide was finally furnished in comfortable yield. Furthermore, there is no correct product observed when acetone was replaced by other solvents (HOAc, MeOH, HFIP, and THF). Under the optimized conditions (H2O2, Na2WO4, Acetone), overoxidation was overcome and provided correct disulfide motif with a 51% isolation yield [27].
Table 1.
Sulfoxide Ligand Preparation.

| |||||
|---|---|---|---|---|---|
| Oxidants | Solvents | Additives | Temperature | Time | Yield |
| Oxone (2 eq.) | CH2Cl2 | - | RT | O/N | N.D. |
| NaIO4 (2 eq.) | CH2Cl2 | DDQ (2 eq.) | RT | O/N | N.D. |
| MeOH | - | N.D. | |||
| PhI(OAc)2 (2 eq.) | MeOH | (NH4)2CO3(2eq.) | RT | O/N | N.D. |
| Sharpless reagents a | - | - | 0 °C | O/N | N.D. |
| DMDO (2 eq.) | - | - | 0 °C | O/N | N.D. |
| m-CPBA (2 eq.) | CH2Cl2 | - | 0 °C | 1h | <5% |
| TBHP(0.1 eq.) | 0 °C | 1h | N.D. | ||
| Acetone | - | 0 °C | 1h | N.D. | |
| THF | - | 0 °C | 1h | N.D. | |
| H2O2 (2 eq.) | HOAc | - | RT | O/N | <10% conversion |
| MeOH | - | RT | O/N | N.D. | |
| HFIP | - | RT | O/N | N.D. | |
| THF | Na2WO4·2H2O | RT | O/N | <5% | |
| Acetone:H2O b | Na2WO4·2H2O | 0 °C | 50 min | 51% | |
Room temperature (RT), Overnight (O/N), Not detected (N.D.), 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), tBuOOH (TBHP), Dimethyldioxirane (DMDO). Conditions:
Ti(OiPr)4 (1 eq.), (+)DET (4 eq.), TBHP (2 eq.).
Acetone: H2O = 4:1, Na2WO4·2H2O (1 eq.), H2O2 (6 eq.).
After successfully obtaining the bissulfoxide ligand 4, the subsequent evaluation of bissulfoxide ligand with Palladium catalyst was immediately carried out. Luckily, the bissulfoxide moiety enabled the C-H activation of allyl benzene while utilizing amine as nucleophiles (Scheme 2). Two different bicyclic sulfoxide ligands were explored (4 and see Supplementary Materials compound S2), and a list of palladium catalysts was examined (Pd(PPh3)4, and PdOAc2, Allylpalladium(II) chloride), and other additives were also investigated, including the addition of benzoquinones (BQs) and the usage of bases, and the reaction temperatures were then screened (see Supplementary Materials Section S4.2). After the initial evaluations, we concluded that a combination of ligand 4 and Pd2(dba)3 is a slightly superior complex compared to Pd2(dba)3/S2. The pair furnished the ideal outcomes in the presence of p-toluquinone (TQ) and base. Amination adduct 5a was produced in good yield (71%) and reactions can be carried out within a relatively shorter reaction time (40 h) compared to other reported instances [5]. The electron-withdrawing induction effect of R1 groups seem to contribute to excellent yields of 5b (82%) and 5c (86%), while the methoxy group on R1 position, whose ability to donate electron density might overpower its induction ability, caused the lower yield of 5d (59%). When comparing 5f and 5h, α-substituted aliphatic amine returned a diminished yield of 62% (5f). To achieve successful outcomes in C-H functionalization, electron withdrawing groups, such as tosyl and triflyl, are required to facilitate the transformation. Therefore, it is speculated that the pKa of the amine nucleophile plays a critical role in this process. Triflyl substituted amine nucleophiles provided corresponding C-H activation adducts in acceptable yields (58–72%, 5f–k).
Scheme 2.

Pd-bissulfoxide Catalyzed Allylic C-H Amination. * Purchased from commercial sources.
The absence of the palladium catalyst led to no reaction. In the effort to identify the role of ligand aromatic rings, analogous aliphatic bicyclic scaffold was prepared (S11), but, unfortunately, the oxidation of S11 to its bissulfoxide form was not successful and led to the decomposition of S11, which suggests the possible stabilizing effect of the aromatic ring in the oxidation process. Moreover, the contribution of base is critical, and no product could be obtained when base was not employed, and it could be fine-tuned to either DIPEA or DBU for optimal outcomes. Under the optimized conditions, removal of ligand 4 could generate products with much lower yields (S4.2). The replacement of the carboxylic ester group on the ligand with benzyl group did not impact ligand activity substantially (S4.2). Finally, ligands that possess alternative sulfur oxidation states (bis-sulfide, bis-sulfone, or mono-sulfoxide) failed to catalyze transformations in meaningful yields, and bissulfoxide is required in the ligand framework for an efficient C-H activation.
The application of bissulfoxide ligand was investigated in the alkylative C-H activation settings (Scheme 3). Carbon nucleophile methyl cyanoacetate successfully rendered adduct in excellent yield (6a, 90%). Furthermore, adduct 6b were prepared with benzyl cyanoacetate nucleophile at close to ambient temperature (35 °C/4 h). We were pleased to find out that catalytic loading could be reduced to 2 mol% of palladium and 5 mol% of ligand 4, and that the absence of ligand 4 resulted in poor yields (S4.3). The trisubstituted nucleophiles are preferred, since disubstituted reagents (e.g., cyanoacetate) generated the mixture monoalkylated and di-alkylated products, which complicated the outcome elucidation.
Scheme 3.

Pd-bissulfoxide Catalyzed Allylic C-H Alkylation. * Purchased from a commercial source.
Mechanistically, attempts to identify the palladium and ligand complex were not successful after multiple attempts. Solution 1H NMR could not observe any distinct chemical shift change upon mixing Pd2(dba)3 with ligand at 35 °C. Regardless, despite the presence of TQ and allylbenzene in stoichiometric or catalytic amount, no coordination between Pd and ligand species were observed in NMR spectroscopy. Obtaining a single crystal of catalyst complex failed after multiple experiments. Fortunately, we were able to secure X-ray structures of ligand 4 and S2. Based on X-ray data and the literature precedence [14], the tentative proposed complex is demonstrated in Figure 2. Palladium sulfoxide (Pd-S) complex along with hydrogen bond between sulfoxide and nucleophiles facilitated the C-H functionalization.
Figure 2.
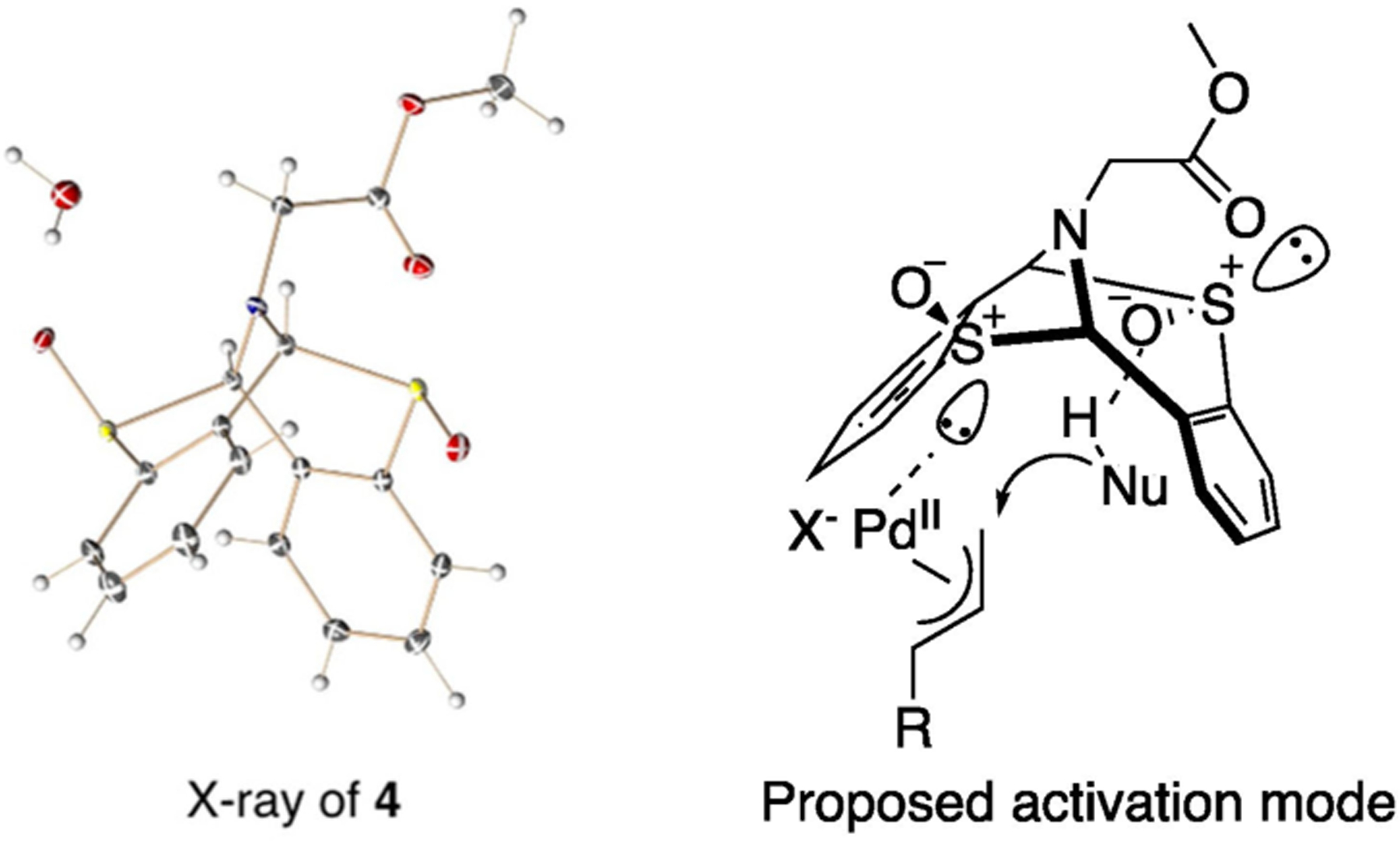
Proposed Hydrogen-bond Activated of Allylic Oxidation.
To illustrate the practicality of C-H functionalization tactic, a therapeutic agent, Naftifine [28] (treatment of fungal infection), was prepared in a concise manner (Scheme 4).Aforementioned adduct 5k (Scheme 2) was successfully reduced to secondary amine 7 in 90% yield via Red-Al reduction. Subsequent N-methylation smoothly provided Naftifine 8 without any issue. Overall, the synthesis of Naftifine was completed in four steps from commercially available material with approximately 30% overall isolation yield boosted by this methodology.
Scheme 4.

Preparation of Naftifine.
4. Conclusions
Overall, in this study, we present a comprehensive investigation of a new class of sulfoxide ligands for C-H functionalization. The utilization of these ligands, featuring a unique [3.3.1] nonane scaffold, offer an intriguing avenue for catalyst activation. We have demonstrated that the combination of sulfoxides with this specific ligand architecture leads to remarkable catalytic outcomes. Despite the apparent structural complexity of the sulfoxide ligand, its chemical synthesis is surprisingly straightforward. By employing commercially available thiophenol, we accomplished the synthesis of the bissulfoxide ligand through a concise sequence of three synthetic steps, resulting in decent overall yields. Notably, compared to conventional sulfoxide ligands, the introduction of the bicyclic sulfoxide ligand leads to notable improvements in reaction kinetics, allowing for shorter reaction times and enhanced product yields. One of the notable advantages of our catalytic system is its compatibility with a wide range of nucleophiles, including both nitrogen-based (N) and carbon-based (C) nucleophiles. This broad substrate scope highlights the versatility and applicability of the developed ligand system. For successful C-H functionalization mediated by the bissulfoxide ligand, careful selection of the palladium source, specifically Pd2(dba)3, and the use of the appropriate additive, such as TQ, are crucial. Furthermore, the role of the base in the reaction system is found to be indispensable and plays a vital role in achieving the desired outcomes. To gain insights into the mechanism of our catalyst system, we propose a tentative activation mode that takes into account the unique features of the sulfoxide ligands and their interactions with the metal catalyst. This proposed mechanism provides a valuable framework for further exploration and refinement of our catalytic system. As a proof of concept, we applied our developed methodology to the rapid synthesis of the therapeutic agent Naftifine. By employing the C-H functionalization tactic in a concise four-step sequence, the success of obtaining this valuable compound highlights the synthetic utility and efficiency of our approach. Our ongoing efforts involve the introduction of chiral polypeptides and other heterocycles into the ligand motif, aiming to expand the range of accessible molecules and potentially enable enantioselective transformations.
Supplementary Material
Acknowledgments:
Support for this work was provided by the National Institute of Health (R35 GM138336) to Q. Zhang. National Science Foundation (CHE 17267240) for NMR purchased is acknowledged.
Funding:
This research was funded by NIH grant number [R35 GM138336].
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Supplementary Materials: The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org4020023/s1. The compounds and spectrum view Figures S1–S54. Table S1. Experimental details. Table S2 Experimental details.
Data Availability Statement:
Complete experimental procedures and characterization data for all new compounds (PDF).
References
- 1.Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liron F; Oble J; Lorion MM; Poli G Direct Allylic Functionalization through Pd-Catalyzed C-H Activation. Eur. J. Org. Chem 2014, 2014, 5863–5883. [Google Scholar]
- 3.Sipos G; Drinkel EE; Dorta R The Emergence of Sulfoxides as Efficient Ligands in Transition Metal Catalysis. Chem. Soc. Rev 2015, 44, 3834–3860. [DOI] [PubMed] [Google Scholar]
- 4.He J; Wasa M; Chan KSL; Shao Q; Yu J-Q Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev 2017, 117, 8754–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed SA; Mazzotti AR; White MC A Catalytic, Brønsted Base Strategy for Intermolecular Allylic C−H Amination. J. Am. Chem. Soc 2009, 131, 11701–11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S; Song C-X; Cai G-X; Wang W-H; Shi Z-J Intra/Intermolecular Direct Allylic Alkylation via Pd(II)-Catalyzed Allylic C−H Activation. J. Am. Chem. Soc 2008, 130, 12901–12903. [DOI] [PubMed] [Google Scholar]
- 7.Howell JM; Liu W; Young AJ; White MC General Allylic C–H Alkylation with Tertiary Nucleophiles. J. Am. Chem. Soc 2014, 136, 5750–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W; Ali SZ; Ammann SE; White MC Asymmetric Allylic C–H Alkylation via Palladium(II)/Cis-ArSOX Catalysis. J. Am. Chem. Soc 2018, 140, 10658–10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma R; White MC C–H to C–N Cross-Coupling of Sulfonamides with Olefins. J. Am. Chem. Soc 2018, 140, 3202–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson WH; Check CT; Proust N; Stambuli JP Allylic Oxidations of Terminal Olefins Using a Palladium Thioether Catalyst. Org. Lett 2010, 12, 824–827. [DOI] [PubMed] [Google Scholar]
- 11.Wu C-Z; He C-Y; Huang Y; Zhang X Thioether-Promoted Direct Olefination of Polyfluoroarenes Catalyzed by Palladium. Org. Lett 2013, 15, 5266–5269. [DOI] [PubMed] [Google Scholar]
- 12.Dai Y; Weng J; George J; Chen H; Lin Q; Wang J; Royzen M; Zhang Q Three-Component Protein Modification Using Mercaptobenzaldehyde Derivatives. Org. Lett 2019, 21, 3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee A; Ahn S; Kang K; Seo M-S; Kim Y; Kim WY; Kim H Bicyclic Bridgehead Phosphoramidite (Briphos) Ligands with Tunable π-Acceptor Ability and Catalytic Activity in the Rhodium-Catalyzed Conjugate Additions. Org. Lett 2014, 16, 5490–5493. [DOI] [PubMed] [Google Scholar]
- 14.Du L; Cao P; Xing J; Lou Y; Jiang L; Li L; Liao J Hydrogen-Bond-Promoted Palladium Catalysis: Allylic Alkylation of Indoles with Unsymmetrical 1,3-Disubstituted Allyl Acetates Using Chiral Bis(Sulfoxide) Phosphine Ligands. Angew. Chem. Int. Ed 2013, 52, 4207–4211. [DOI] [PubMed] [Google Scholar]
- 15.Mariz R; Luan X; Gatti M; Linden A; Dorta R A Chiral Bis-Sulfoxide Ligand in Late-Transition Metal Catalysis; Rhodium-Catalyzed Asymmetric Addition of Arylboronic Acids to Electron-Deficient Olefins. J. Am. Chem. Soc 2008, 130, 2172–2173. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q-A; Dong X; Chen M-W; Wang D-S; Zhou Y-G; Li Y-X Highly Effective and Diastereoselective Synthesis of Axially Chiral Bis-Sulfoxide Ligands via Oxidative Aryl Coupling. Org. Lett 2010, 12, 1928–1931. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi J; Haldar C; Bisht R; Pandey G; Chattopadhyay B Meta Selective C–H Borylation of Sterically Biased and Unbiased Substrates Directed by Electrostatic Interaction. J. Am. Chem. Soc 2021, 143, 7604–7611. [DOI] [PubMed] [Google Scholar]
- 18.Dey S; Schepmann D; Wünsch B Role of the Phenolic OH Moiety of GluN2B-Selective NMDA Antagonists with 3-Benzazepine Scaffold. Bioorganic Med. Chem. Lett 2016, 26, 889–893. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X; Ren J; Tan SM; Tan D; Lee R; Tan C-H An Enantioconvergent Halogenophilic Nucleophilic Substitution (S N 2X) Reaction. Science 2019, 363, 400–404. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnikov PN; Usanov DL; Barablina EA; Maleev VI; Chusov D Atom- and Step-Economical Preparation of Reduced Knoevenagel Adducts Using CO as a Deoxygenative Agent. Org. Lett 2014, 16, 5068–5071. [DOI] [PubMed] [Google Scholar]
- 21.Scattolin T; Deckers K; Schoenebeck F Efficient Synthesis of Trifluoromethyl Amines through a Formal Umpolung Strategy from the Bench-Stable Precursor (Me4N)SCF3. Angew. Chem. Int. Ed 2017, 56, 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisz E; Koston M; Szostak M N -Butylpyrrolidone (NBP) as a Non-Toxic Substitute for NMP in Iron-Catalyzed C(Sp 2)–C(Sp 3) Cross-Coupling of Aryl Chlorides. Green Chem 2021, 23, 7515–7521. [Google Scholar]
- 23.SAINT. Part of Bruker APEX3 Software Package (Version 2016.9–0): Bruker AXS. 2016. Available online: https://www.brukersupport.com/ProductDetail/3177 (accessed on 6 June 2023).
- 24.SADABS. Part of Bruker APEX3 Software Package (Version 2016.9–0): Bruker AXS. 2016. Available online: https://www.brukersupport.com/ProductDetail/3177 (accessed on 6 June 2023).
- 25.SHELXT; Version 2014/5: G. M. Sheldrick. Acta Cryst 2015, A71, 3–8. [Google Scholar]
- 26.XL refinement program version 2016/6: G. M. Sheldrick. Acta Cryst 2015, C71, 3–8. [Google Scholar]
- 27.Das Sato K; Hyodo M; Aoki M; Zheng X-Q; Noyori R Oxidation of Sulfides to Sulfoxides and Sulfones with 30% Hydrogen Peroxide under Organic Solvent- and Halogen-Free Conditions. Tetrahedron 2001, 57, 2469–2476. [Google Scholar]
- 28.Gupta AK; Ryder JE; Cooper EA Naftifine: A Review. J. Cutan. Med. Surg 2008, 12, 51–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Complete experimental procedures and characterization data for all new compounds (PDF).


