Abstract
Purpose
To investigate the interaction of a common monoclonal antibody (bevacizumab) with silicone oils and vitreous substitute hydrogels.
Methods
Protein content (spectrophotometry) and bioactivity (ELISA) of bevacizumab were assessed after direct contact to silicone oil and vitreous substitute hydrogels up to 24 hours. To detect antibody aggregation, particle size was determined by dynamic light scattering. Changes in secondary protein structure were assessed using circular dichroism. Experiments were translated to another antibody, trastuzumab, because its bioactivity can be additionally quantified by its binding to the membrane of HER2-positive cells.
Results
The functionality of bevacizumab gradually decreased during exposure to silicone oils with only 30% of activity remaining after 24 hours. Although circular dichroism did not reveal structural changes, the particle size increased drastically, indicating antibody aggregation for both antibodies. Exposure to silicone oil strongly reduced binding of trastuzumab to HER2-positive cells. The addition of polysorbate, a common stabilizer for antibody formulations, dose-dependently prevented aggregation, with 62% aggregation observed at 0.04% polysorbate compared to only 10% aggregation at 0.5% polysorbate. No aggregation occurred after exposure to vitreous body replacement hydrogels.
Conclusions
Contact to silicone oils induces a major loss of functionality of bevacizumab and trastuzumab. This limits clinical use during silicone oil tamponade. The beneficial effect of adding polysorbate suggests that loss of solubilizer is a likely cause of aggregation. Hydrogels, alternatives for vitreous body replacement, do not impair antibody functionality.
Keywords: silicone oil, anti-VEGF, vitreous body, hydrogels, vitreoretinal surgery
Silicone oils are used as intraocular endotamponades after complex vitreoretinal surgery. Since their introduction by Armaly1 and Cibis et al.2 in 1962, their surgical and rheological properties have been optimized.3–6 The establishment of heavy silicone oils has led to major improvements of these biomaterials including their pharmacological properties.7 Despite the fact that their interaction with concomitantly applied drugs8 is not yet fully understood, the simultaneous intravitreal therapy with anti-VEGF-agents, the most frequently used antibodies, is an established treatment. A current expert consensus supports its use during silicone oil tamponades while at the same time concluding that the evidence that is available is sparse and that very little is known about the interaction of silicone oils and the drug classes in this clinical use.
One major downside of silicone oils is their tendency to emulsify, leading to follow-up complications like glaucoma and adherent silicone oil droplets.9 This emulsification can be aggravated and accelerated by risk factors, such as intraoperative bleeding.10 Previously, it has also been shown that both endogenous proteins10 and therapeutic antibodies,8 such as bevacizumab, strongly decrease the interfacial tension of silicone oil inducing emulsification. This is partially caused by the amphiphilic nature of antibodies but is mainly caused by the addition of polysorbates (e.g., Tween 20 in Avastin [bevacizumab]) that act as solubilizers.8
Additives, like polysorbates, are applied in antibody solutions to impair their degradation and loss of functionality.11–13 More specifically, polysorbates reduce the nonspecific binding of antibodies to other proteinaceous compounds, but the target,11 stabilizing the antibody, may allow for a more effective antibody penetration14 and finally prevent antibody aggregation.12 Based on the known effect of antibody solution on the interfacial tension, it is likely that polysorbates accumulate in the silicone oil-aqueous interphase effectively lowering concentration in the aqueous, hydrophilic phase, which contains the antibodies.
Next to silicone oils, experimental vitreous body replacement hydrogels are currently undergoing preclinical testing.15,16 Because of their low polymer content and hydrophilicity, the interaction with antibody solutions should be similar to the vitreous body preventing aggregation.8
Thus the purpose of this study was to investigate impairment of the functionality of bevacizumab after exposure to lighter- and heavier-than-water silicone oils. A previously established simple model of the posterior segment7 was used to test functionality. The protein content was determined and compared to functionality assays (ELISA). Circular dichroism was applied to detect any secondary structure changes, and dynamic light scattering was used to confirm antibody aggregation. To further test functionality in a cell culture setting, experiments were repeated with trastuzumab and a binding assay to HER2 on SK-Br-3 cells. The results obtained for the silicone oils were compared to a previously published16 third-generation vitreous body replacement hydrogel.
Methods
Material
Avastin (25 mg/mL) and trastuzumab (Herceptin 21 mg/mL) were purchased from Roche Pharma AG (Basel, Switzerland). For bevacizumab, two different stock solutions (prepared at different time points) were used to demonstrate the reproducibility of the results. A complete list of all purchased materials is available in the supplementary methods section.
Methods
In Vitro Posterior Segment Model
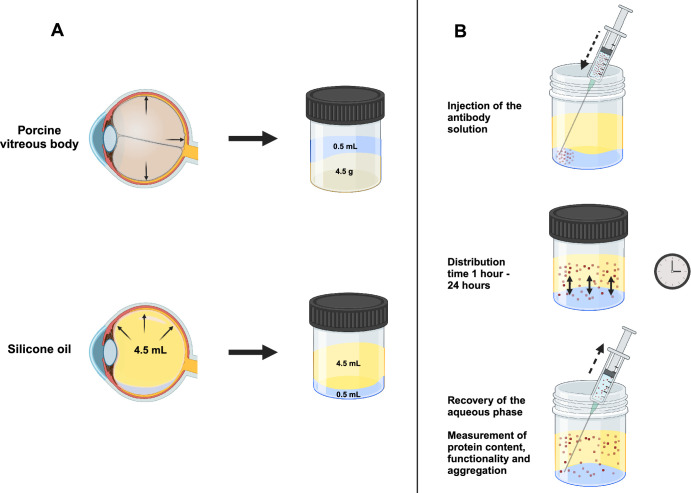
The respective silicone oil or hydrogel 4.5 mL was transferred to a vial. The bevacizumab solution 500 µL was injected and placed on a rocking table at 21°C for one, three, six, 12, and 24 hours. Densiron 68, F4H5, and F6H8 were carried out for 24 hours at 21°C. Additionally, trastuzumab was tested as second antibody in S2000 for 24 hours at 21°C. The aqueous phase was recovered and quantified by Nanodrop measurements (Thermo Scientific NanoDrop 2000 UV-Vis Spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA) and ELISA measurements (ProteoGenix, Schiltigheim, France). Every sample was tested in triplicates. The experimental principle is illustrated in Figure 1.
Figure 1.
The experimental principle of the in vitro posterior segment model. (A) Transfer of the posterior segment of the eye into an in vitro posterior segment model. On top the porcine vitreous body leading to an in vitro model for silicon oils (bottom). (B) Experimental setup of the in vitro posterior segment model. 500 µL of the respective antibody solution were added to the vitreous body substitute (top), incubated (middle), and sampled after the different time points (bottom).
Preparation of Hydrophilic Phases
In clinical practice, a single intravitreal injection dose contains 1.25 mg of bevacizumab in 0.05 mL. This leads to a starting concentration of 2.27 mg/mL, which was prepared from a stock solution (25 mg/mL) which was diluted with BSS or BSS + 0.5% Tween80. For trastuzumab, the stock solution was desalted for Atto-labeling and diluted with BSS to obtain a comparable concentration.
Atto-Labeling
For the labeling of trastuzumab with Atto488-NHS the clinical trastuzumab stock was rebuffered in PBS, and 0.2 M sodium bicarbonate solution was added to reach pH 8.5. Atto488-NHS was dissolved in DMSO (20 mg/mL) and 2 µL were added and mixed for 24 hours. After coupling, purification was performed with a NAP-5 column (Cytiva, Marlborough, MA, US).
Quantification of Functionality and Protein Concentration
To test the functionality and activity of bevacizumab, an ELISA (ProteoGenix, Schiltigheim, France) was performed. A serial five-point calibration in the range of 78 to 1250 ng/mL was performed (R2 = 0.9955). To minimize the influence of storage, all samples were measured immediately after the completion of the experiments. The protein concentrations of the stocks and all test samples were quantified by A280 (IgG) measurement (NanoDrop One; Thermo Scientific, Waltham, Massachusetts, USA).
Quantification of Particle Size
The particle size of the hydrophilic phases before and after incubation in silicone oil was measured in triplicates with a Zetasizer Ultra (Malvern Panalytical Ltd, Malvern, UK) and evaluated by the implemented software (ZS Xplorer 3.31; Malvern Panalytical Ltd). All samples were measured immediately after completion of the experiment to minimize the storage influence.
Structural Determination of Bevacizumab
The structure of bevacizumab before and after 24 hours contact with silicone oil was determined by circular dichroism (J-1700 CD Spectrometer; JASCO Inc, Easton, MD, USA). For this purpose, the samples were diluted to 1 mg/mL with BSS and filled in a 1 mm quartz cuvette (Hellma GmbH & Co. KG, Mühlheim, Germany). The analysis wavelength was between 190 and 260 nm.
Cell Culture and Activity Assay With Trastuzumab
After preparation of the SK-BR-3 cells (details presented in the supplementary methods section), the atto-labeled trastuzumab with and without previous contact to silicone oil was added and incubated for one hour. After a washing procedure and the addition of NucRed Live 647 (details presented in the supplementary methods section), the coverslips were mounted onto microscopic slides and sealed with nail polish. The cells were imaged with a Leica confocal microscope (Leica Camera AG; Leica, Wetzlar, Germany).
Sensitivity Analyses
The experiments in the in vitro posterior segment model with bevacizumab at 24 hours were repeated at 37°C to exclude temperature dependencies of the measurements. Furthermore, to test whether aggregation can be minimized, additional polysorbate (0.5%) was added to the bevacizumab solution before 24 hours’ incubation with Siluron 2000.
Statistical Analyses
Statistical analyses were performed using Prism (GraphPad Software, San Diego, CA, USA). Paired or unpaired t-tests were performed in case of normality.
Results
Determination of Concentration and Activity of Aqueous Phases After Contact to Vitreous Body Substitutes
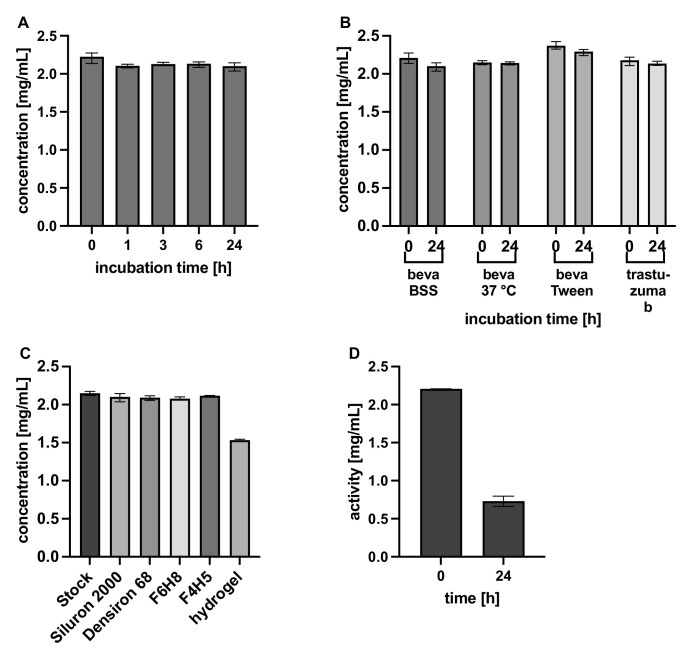
The concentrations of the aqueous phases were determined with Nanodrop and ELISA measurements and are shown in Figure 2. As previously published8 for bevacizumab and trastuzumab, the concentrations were stable in light and heavy silicone oils and the two tested semifluorinated alkanes indicating low solubility in silicone oil. Bevacizumab dissolved in the hydrogel over 24 hours, the concentration decreased to 1.5 mg/mL (Fig. 2C). In contrast the functionality of bevacizumab measured by ELISA (synonymous to its activity) is significantly decreased to 30% after contact with different silicone oils (t-test, P < 0.0001, T = 36.93, df = 7; Fig. 2D). Results of Figures 2C and 2D were repeated using a second stock solution of bevacizumab delivering comparable results (see Supplementary Material).
Figure 2.
Overview of the apparent concentrations of antibody in the aqueous phases. (A) Different incubation times of bevacizumab solution in Siluron 2000 measured with Nanodrop. (B) Variant hydrophilic phases in Siluron 2000 measured with Nanodrop. (C) Bevacizumab in several vitreous body replacements measured with Nanodrop. (D) Activity of bevacizumab in the hydrophilic phase using ELISA after incubation with Siluron 2000 for 24 hours. A–C were measured in triplicates, and error bars show the min-max range; D were six replicates, and error bars are shown as mean ± SD.
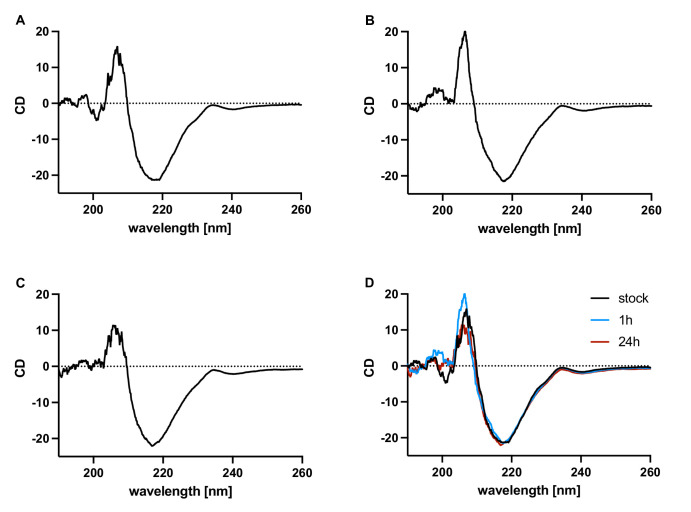
Structural Characterization of Bevacizumab After Incubation in Silicon Oil With Circular Dichroism
Because of the pronounced decrease of activity of bevacizumab after contact with silicone oil, the secondary structure of bevacizumab was investigated using circular dichroism after the incubation with lighter-than-water silicone oil for one hour and 24 hours (Fig. 3). The chromatograms showed the typical β-sheet structures of bevacizumab. No differences between the bevacizumab stock solution (Fig. 3) and the two measurements after incubation in silicone oil for one or 24 hours (Figs. 3B, 3C) were observed, indicating a stable secondary protein structure. Measurements were repeated three times with identical results not suggesting any change of the secondary structure of bevacizumab.
Figure 3.
Circular dichroism studies of bevacizumab. (A) Bevacizumab stock solution. (B) Bevacizumab solution after one hour incubation in Siluron 2000. (C) Bevacizumab solution after 24 hours in Siluron 2000. (D) Overlay of the three spectra.
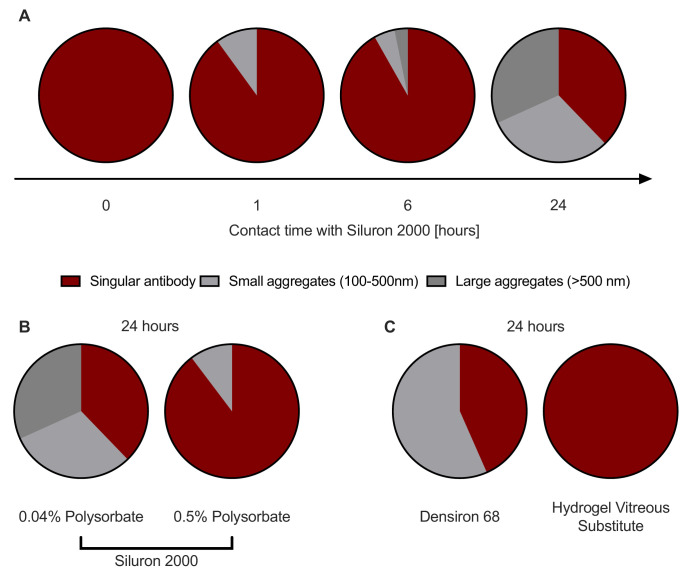
Bevacizumab Forms Aggregates During Contact With Silicone Oil
The particle size of the different aqueous phases of bevacizumab and trastuzumab were measured to verify if the incubation in vitreous body substitutes in particular in silicon oils lead to an aggregation of the antibodies. Figure 4 depicts aggregation formation for bevacizumab. For both stock solutions, the typical particle size for a monoclonal antibody was measured (mean particle size ± SD: 12.8 ± 0.4 nm and 11.4 ± 0.5 nm for bevacizumab and trastuzumab, respectively) (Fig. 4A for bevacizumab, Fig. 5A for trastuzumab). After one hour of contact with Siluron 2000, 10.2% of the bevacizumab already formed aggregates with a size of 165 ± 66 nm (Fig. 4A). This percentage of aggregates increased to 62.9% after 24 hours of contact with Siluron 2000 (t-test, P = 0.0001, T = 6.00, df =10, Fig. 4A). There were smaller aggregates with 228 ± 70 nm and larger aggregates with 2212 ± 676 nm present. Although particle size differed, a similar percentage of the active form was observed after 24 hours of contact with heavier-than-water silicone oil (Fig. 4C). The addition of polysorbate to the bevacizumab solution significantly reduced aggregation but did not completely prevent it (t-test, P = 0.0176, T = 3.089, df = 7, Fig. 4B). The sensitivity test at 37 °C showed an aggregation with 80.9% medium aggregates (104.1 ± 50.5 nm)
Figure 4.
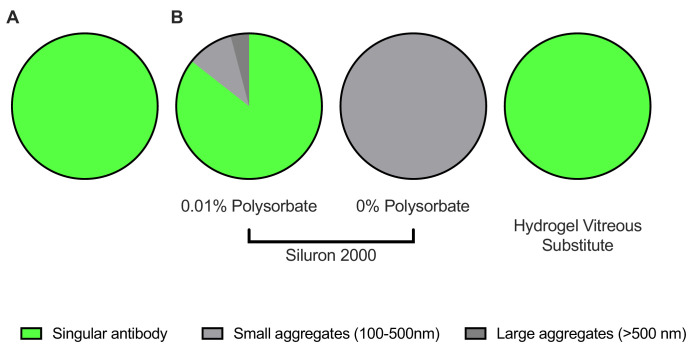
Particle size of bevacizumab solutions. (A) Aggregation begins to occur after one hour of exposure to silicone oil. (B) Increasing the polysorbate concentration in the bevacizumab solution prevents aggregation. (C) Heavier-than-water silicone oils (Densiron 68) also provoke aggregation, in contrast to vitreous substitute hydrogels.
Figure 5.
Particle size of trastuzumab solutions. (A) Measurement of stock solution of trastuzumab. (B) Measurements after 24 hours of contact with vitreous body replacements. When polysorbate was removed, severe aggregation occurred after exposure to Siluron 2000. Again, vitreous body replacement hydrogels did not cause any aggregation.
Trastuzumab Forms Aggregates During Contact With Silicone Oils
For trastuzumab with 0.01% polysorbate, fewer aggregates are formed within 24 hours of contact to light silicone oil (14.4%, Fig. 5B). In contrast, in the trastuzumab sample without any polysorbate the whole sample was aggregated and no particles in the size of the active form were observable after 24 h (Fig. 5B).
Interaction With Vitreous Body Substitute Hydrogels
Finally, no aggregation was observed for either bevacizumab or trastuzumab after extended contact with a third-generation vitreous body replacement hydrogel compared to Siluron 2000 after 24 hours of contact. For both bevacizumab (Fig. 4C) and trastuzumab (Fig. 5B), t-tests were performed (bevacizumab: P = 0.0001, T = 5.9, df = 10; trastuzumab: P = 0.0001, T = 15.3, df = 4).
Activity Assay With Trastuzumab
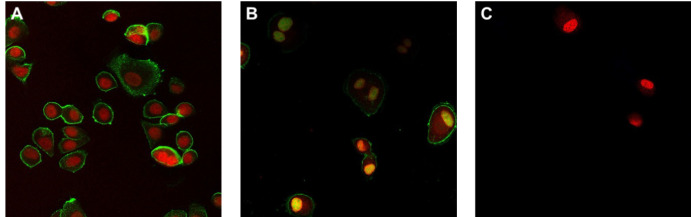
Because bevacizumab binds free VEGF, binding assays to visualize the activity are complex with limited validity. Thus, next to ELISA, the activity of trastuzumab was assessed using a binding assay on HER2-positive SK-Br-3 cells. For this purpose, trastuzumab was labeled with Atto488 and incubated with silicone oil for 24 hours. The binding activity was then compared visually to a positive (stock solution) and negative (only buffer) control performed on the same day (Fig. 6). Imaging showed a clearly reduced binding of trastuzumab after contact with silicone oil indicating a loss of activity and functionality of trastuzumab.
Figure 6.
Binding studies on HER2- positive SK-Br-3 cells with trastuzumab-Atto488. (A) Trastuzumab-Atto488 stock solution: trastuzumab (green) binds to the membrane of all visible cells. Target of trastuzumab is HER-2 expressed on the cell membrane of Br-3 cells. (B) Trastuzumab solution after 24 hours of contact with silicone oil. Less pronounced binding is apparent, indicating a significantly reduced activity. (C) Negative control without trastuzumab. The nucleus is shown in red color (NucRed Live 647).
Discussion
In this study, we assessed the interaction of bevacizumab in clinically applied doses with silicone oil endotamponades and vitreous body replacement hydrogels under development. We found a drastic decrease in bevacizumab functionality after 24 hours of contact with silicone oils because of aggregation. In contrast to silicone oils, no aggregation occurred after exposure to vitreous body replacement hydrogels.
These results for an anti-VEGF agent were confirmed for trastuzumab with a cell-based assay using Atto488-labeled trastuzumab. Bevacizumab and all other anti-VEGF agents bind the free protein and not the receptor. Thus trastuzumab was chosen as control, because it is a commonly used model for membrane-based receptor assays.
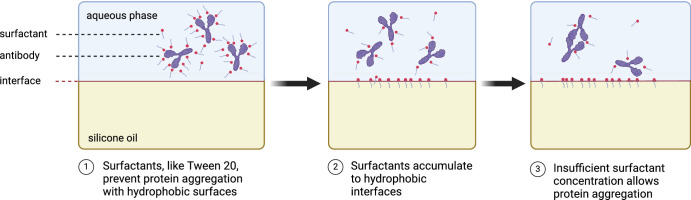
Previously, we showed that protein drugs such as antibodies, are insoluble in silicone oil and significantly lower the interfacial tension between the silicone oil and the aqueous phase.8 Because of the protein content remaining unchanged, it is very likely that these drastic changes of surface tension are mediated by solubilizers in the bevacizumab solution, namely polysorbates. Polysorbates are amphiphilic molecules17 that can accumulate in the interface, effectively lowering the concentration in the aqueous phase. Antibody concentrations applied intravitreally are higher when compared to systemic doses. With an increasing protein concentration, phenomena like aggregation, especially after deprivation of solubilizers can lead to a loss of function (Fig. 7). This is supported by the results on the antibodies’ secondary structure using circular dichroism, which did not detect any conformational changes of the singular antibodies themselves.
Figure 7.
Proposed pathophysiology of the loss of activity of bevacizumab solutions as a consequence of aggregation through polysorbate deprivation.
Aggregation is not only undesirable because of activity loss,18 but also because of decreased solubility and the potential risk of immunogenicity.19 This is especially relevant as the patients undergoing silicone oil endotamponade and intravitreal injections in parallel. In these situations, the blood-retina barrier is already impaired, increasing immunological reactions through an increased leukocyte migration into the retina.20 This may be further aggravated by antibody conglomerates. Previously, we showed that vitreous body replacement hydrogels show an in vitro distribution of bevacizumab similar to the natural, porcine vitreous body.8 In line, no aggregation of bevacizumab occurred after the extended contact to a third-generation vitreous replacement hydrogel previously introduced by Hayashi et al.16
The results gathered of this in vitro study therefore question the evidence level of certain in vivo studies of the silicone oil filled eye that used ELISAs to quantify the amount of bevacizumab. In 2012, Xu et al.21 tested the concentration of bevacizumab in the silicone oil filled eye as compared to the native rabbit eye at a variety of time points by ELISA. They concluded that in the silicone oil–filled eye, lower peak concentration occurs, which is counterintuitive because bevacizumab cannot dissolve in the silicone oil, and one would suspect a high peak concentration and a quicker washout. It is possible that these results were influenced by aggregation and therefore a loss of function influencing the concentrations detected with the ELISA.
Last, the results of this in vitro study should be compared to clinical results of the application of bevacizumab in the silicone oil filled eye. One recent randomized controlled study compared subretinal or intravitreal injection of conbercept (Lumitin) in 13 patients with proliferative diabetic retinopathy that underwent vitrectomy with silicone oil endotamponade. They found that subretinal injection (thus no direct contact to the silicone oil) mainly enhanced the drug concentration measured using ELISA.22 This again indicates that the inactivation of antibody solutions might be provoked by silicone oil. In this clinical study, the application even translated to significant differences in best visual acuity. Furthermore, in another study in five eyes with iris neovascularization, intra-silicone oil injection led to a regression of neovascularization within seven days.23 This is also in line with the data obtained here: Functionality of bevacizumab was still approximately 30%. Given the smaller volume it can dissolve because of the insolubility in silicone oil, it can be expected that concentrations are high and thus an effect is still possible. Apart from the studies mentioned, multiple studies showed variable benefits of different anti-VEGF agents.24–26
Limitations
Of course, this study shows minor limitations. Here, we used an in vitro setup to exclude any confounding factors that may be present in the vitreous cavity to solely analyze the interaction of bevacizumab and silicone oil. In this in vitro model, it is not possible to simulate the movement of the injected hydrophilic volume through the silicone oil. However, it is likely that due to the differences in density, the injected solution will quickly form a greater hydrophilic bubble with the remaining aqueous in the vitreous cavity.
Conclusions
This study describes the interaction of bevacizumab with silicone oils. Although vitreous body replacement hydrogels do not impair functionality, bevacizumab loses approximately 70% of its function in clinically applied doses within 24 hours of contact with silicone oil. The most likely reason for this behavior is aggregation caused by the accumulation of the surfactant polysorbate in the silicone oil aqueous interphase.
Supplementary Material
Acknowledgments
Figures 1 and 7 were created with BioRender.com. We thank Marcos Conde González of the working group of Jun.-Prof. Dr. Franziska Thomas (OCI Heidelberg, Heidelberg University, Germany) for introduction in CD spectroscopy and providing access to a CD spectrometer.
Supported by the Hans Messer Stiftung and the Ernst und Berta Grimmke Foundation. Maximilian Hammer was supported by an Add-on Fellowship of the Joachim Herz Foundation. The Lipoid GmbH is gratefully acknowledged for its endowment to the University of Heidelberg.
Disclosure: S. Wohlfart, None; J. Herth, None; M.B. Böhmann, None; G. Pander, None; L. Herbster, None; W. Mier, None; G.U. Auffarth, None; P. Uhl, None; M. Hammer, None
References
- 1. Armaly MF. Ocular tolerance to silicones. I. Replacement of aqueous and vitreous by silicone fluids. Arch Ophthalmol. 1962; 68: 390–395. [DOI] [PubMed] [Google Scholar]
- 2. Cibis PA, Becker B, Okun E, Canaan S.. The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol. 1962; 68: 590–599. [DOI] [PubMed] [Google Scholar]
- 3. Williams RL, Day M, Garvey MJ, English R, Wong D.. Increasing the extensional viscosity of silicone oil reduces the tendency for emulsification. Retina. 2010; 30: 300–304. [DOI] [PubMed] [Google Scholar]
- 4. Hammer M, Schickhardt SK, Munro D, et al.. The novel use of high-flow polyimide cannulas to improve silicone oil injectability in vitreoretinal surgery. Retina. 2022; 42: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 5. Hammer M, Schickhardt S, Munro D, et al.. New approaches to explanting high-viscosity silicone oil in retinal surgery—polyimide cannulas vs extraction sleeves vs a luer-trocar. Int J Retina Vitreous. 2023; 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammer M, Schickhardt S, Munro DJ, Scheuerle A, Mayer CS, Auffarth GU.. Physicochemical properties of explanted silicone oil after use as an intraocular tamponade. Trans Vis Sci Tech. 2022; 11(2): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammer M, Schickhardt SK, Merz PR, et al.. Intravitreal application: physicochemical properties of drugs dissolved in silicone oils of different density in comparison to the porcine vitreous body. Pharmaceutics. 2022; 14: 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammer M, Herth J, Herbster L, et al.. In vitro physicochemical and pharmacokinetic properties of bevacizumab dissolved in silicone oils compared to hydrogel-substitutes and porcine vitreous bodies. Gels. 2024; 10(8): 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammer M, Britz L, Schickhardt S, et al.. Quantification of straylight induced by silicone oil adherent to intraocular lenses of different materials. Am J Ophthalmol. 2024; 262: 192–198. [DOI] [PubMed] [Google Scholar]
- 10. Nepita I, Repetto R, Pralits JO, et al.. The role of endogenous proteins on the emulsification of silicone oils used in vitreoretinal surgery. BioMed Res Int. 2020; 2020: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garidel P, Kuhn AB, Schäfer LV, Karow-Zwick AR, Blech M.. High-concentration protein formulations: How high is high? Eur J Pharma Biopharm. 2017; 119: 353–360. [DOI] [PubMed] [Google Scholar]
- 12. Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008; 97: 2924–2935. [DOI] [PubMed] [Google Scholar]
- 13. Agarkhed M, O'Dell C, Hsieh MC, Zhang J, Goldstein J, Srivastava A. Effect of Polysorbate 80 Concentration on Thermal and Photostability of a Monoclonal Antibody. AAPS PharmSciTech. 2013; 14(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willingham MC. An alternative fixation-processing method for preembedding ultrastructural immunocytochemistry of cytoplasmic antigens: the GBS (glutaraldehyde-borohydride-saponin) procedure. J Histochem Cytochem. 1983; 31: 791–798. [DOI] [PubMed] [Google Scholar]
- 15. Hammer M, Herth J, Muuss M, et al.. Forward light scattering of first to third generation vitreous body replacement hydrogels after surgical application compared to conventional silicone oils and vitreous body. Gels. 2023; 9: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayashi K, Okamoto F, Hoshi S, et al.. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat Biomed Eng. 2017; 1(3): 0044. [Google Scholar]
- 17. Karjiban RA, Basri M, Rahman MBA, Salleh AB.. Structural properties of nonionic Tween80 micelle in water elucidated by molecular dynamics simulation. APCBEE Procedia. 2012; 3: 287–297. [Google Scholar]
- 18. Joubert MK, Luo Q, Nashed-Samuel Y, Wypych J, Narhi LO.. Classification and characterization of therapeutic antibody aggregates. J Biol Chem. 2011; 286: 25118–25133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Singh S, Zeng DL, King K, Nema S.. Antibody structure, instability, and formulation. J Pharm Sci. 2007; 96: 1–26. [DOI] [PubMed] [Google Scholar]
- 20. Crane IJ, Liversidge J.. Mechanisms of leukocyte migration across the blood–retina barrier. Semin Immunopathol. 2008; 30: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Y, You Y, Du W, et al.. Ocular pharmacokinetics of bevacizumab in vitrectomized eyes with silicone oil tamponade. Invest Ophthalmol Vis Sci. 2012; 53(9): 5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao TT, Yang Y, Jin XL, et al.. Intraocular pharmacokinetics of anti-vascular endothelial growth factor agents by intraoperative subretinal versus intravitreal injection in silicone oil-filled eyes of proliferative diabetic retinopathy: a randomized controlled pilot study. Acta Ophthalmol. 2020; 98(7): e795–e800. [DOI] [PubMed] [Google Scholar]
- 23. Falavarjani KG, Modarres M, Nazari H.. Therapeutic effect of bevacizumab injected into the silicone oil in eyes with neovascular glaucoma after vitrectomy for advanced diabetic retinopathy. Eye. 2010; 24: 717–719. [DOI] [PubMed] [Google Scholar]
- 24. Baek SK, Lee MW, Lee YH.. Effect of intrasilicone bevacizumab injection in diabetic tractional retinal detachment surgery: a retrospective case-control study. JCM. 2020; 9: 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chhablani J, Narayanan R.. Anti-VEGF therapy in a silicone oil-filled myopic eye with choroidal neovascularisation. Case Rep. 2015; 2015(1): bcr2014208663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu J, Khan MA, Shieh WS, et al.. Effect of serial intrasilicone oil bevacizumab injections in eyes with recurrent proliferative vitreoretinopathy retinal detachment. Am J Ophthalmol. 2016; 161: 65–70.e1-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.