Abstract
2,3-Dihydroxybenzoic acid (DHBA) is the only siderophore described for Brucella, and previous studies suggested that DHBA might contribute to the capacity of these organisms to persist in host macrophages. Employing an isogenic siderophore mutant (ΔentC) constructed from virulent Brucella abortus 2308, however, we found that production of DHBA is not required for replication in cultured murine macrophages or for the establishment and maintenance of chronic infection in the BALB/c mouse model.
Iron is an essential micronutrient for bacteria, and the mammalian host represents a severely iron-restricted growth environment (5). Indeed, the capacity of host iron binding proteins to successfully sequester free iron in a form not readily available to invading pathogens is considered to be an important component of innate immunity (25). To overcome this severe iron restriction in the host, some bacterial pathogens produce siderophores (27). These bacterial iron binding compounds have high and specific affinities for iron and can successfully compete for these ions with mammalian iron binding proteins such as transferrin and lactoferrin (19). Studies have demonstrated that siderophores contribute to the virulence of a wide variety of bacterial pathogens, including Vibrio cholerae (10) and Escherichia coli (26). A key mechanism for limiting the replication of intracellular pathogens is through the gamma interferon (IFN-γ)-stimulated reduction of intracellular iron levels within host phagocytes (4). However, the contributions of siderophore production to the virulence of intracellular pathogens are not well defined (27). Such information is particularly relevant to understanding the virulence of Brucella abortus, a zoonotic bacterial pathogen which survives and replicates in IFN-γ-activated macrophages (11).
Published reports suggest that B. abortus produces a single siderophore, 2,3-dihydroxybenzoic acid (DHBA), under conditions of low iron availability (13, 14). Studies have also shown that the addition of exogenous DHBA to B. abortus-infected murine macrophages in culture results in increased numbers of intracellular brucellae recovered from these cells compared to the numbers recovered from macrophages which were not treated with DHBA (12). Increased intracellular replication (or, alternatively, enhanced survival) of intracellular brucellae in response to DHBA supplementation was also observed in cultured murine macrophages activated with IFN-γ. These findings suggest that the production of DHBA could provide intracellular brucellae with an efficient mechanism for acquiring iron in the iron-restricted environment encountered within the phagosome of IFN-γ-activated host macrophages and thereby contribute to bacterial virulence in the host.
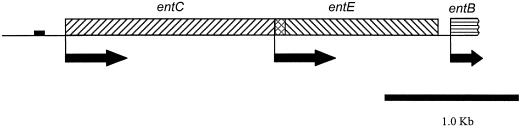
DHBA is synthesized from chorismate, an important precursor in aromatic amino acid and folic acid biosynthesis (18). In E. coli, the conversion of chorismate to DHBA is catalyzed by the products of the entC, entB, and entA genes (17). These genes are clustered in an operon, the expression of which is coordinately regulated in response to environmental iron availability. Homologs of entC, entB, and entA have been described for a variety of gram-negative and gram-positive bacteria, where they also appear to be essential components of catechol siderophore biosynthesis (16). Based on the conserved nature of bacterial DHBA biosynthesis genes, we employed genetic complementation of the E. coli entC mutant SAB11 (2) to clone a portion of the DHBA biosynthesis operon from B. abortus 2308. A bank of pUC9-based recombinant plasmids carrying fragments of genomic DNA from B. abortus 2308, ranging in size from 1 to 20 kb, was introduced into SAB11 via chemical transformation (9), and the resulting transformants were monitored for siderophore production on chrome azurol S agar (CAS) plates (22). By this strategy, a 4.3-kb fragment of genomic DNA from B. abortus 2308 which restored the capacity of E. coli SAB11 to produce a siderophore detectable on CAS plates was identified. Nucleotide sequence analysis (21) was performed on this cloned fragment, and within this region, one complete and one partial open reading frame encoding homologs of previously described bacterial proteins involved in the synthesis of DHBA, isochorismate synthase (EntC) and isochorismate lyase (EntB), were identified (GenBank accession no. U82598). The deduced B. abortus EntC amino acid sequence has 46% identity with the entC gene product of E. coli (18), while the predicted sequence of the 28 N-terminal amino acids of the B. abortus EntB homolog has 32% identity with the corresponding residues of the E. coli EntB (17). As is common with other prokaryotic DHBA biosynthesis operons (16), a gene encoding an EntE homolog (77% predicted amino acid identity with its E. coli counterpart) was found between the B. abortus entC and entB genes (Fig. 1). Other characteristics shared by the B. abortus DHBA operon and those of other gram-negative bacteria include the overlap of entC and entE and the presence of a putative ferric uptake regulator (Fur) binding site upstream of entC, identified by homology with the E. coli consensus sequence (24). In many gram-negative bacteria, siderophore production is repressed in the presence of adequate levels of intracellular iron through the binding of Fur to specific sequences located in the promoter region of iron-regulated genes. The presence of a putative Fur binding site upstream of the B. abortus entC, coupled with the observation that B. abortus produces DHBA only under conditions of iron limitation, suggests that the B. abortus DHBA biosynthesis operon is subject to Fur regulation. Confirmation of this relationship, however, awaits further experimentation. Nevertheless, the genetic organization of the B. abortus ent genes and the strong circumstantial evidence supporting Fur-mediated regulation underscore the similarities between the B. abortus siderophore biosynthesis operon and those described for other gram-negative bacteria. The identification of an entE homolog in B. abortus is intriguing, however. EntE, DHBA-AMP ligase, is not involved in the biosynthesis of DHBA, but rather it participates in the production of the more complex siderophore enterochelin (8), of which DHBA is an integral component. Previous studies indicate that B. abortus does not produce enterochelin (13), and it cannot utilize this compound as a siderophore.
FIG. 1.
Genetic organization of the DHBA biosynthesis genes from B. abortus 2308. Arrows indicate the predicted direction of transcription, and the small closed box represents a putative Fur binding site. The entC and entE open reading frames overlap by 21 amino acids. Only the first 96 nucleotides of the entB open reading frame from B. abortus have been cloned.
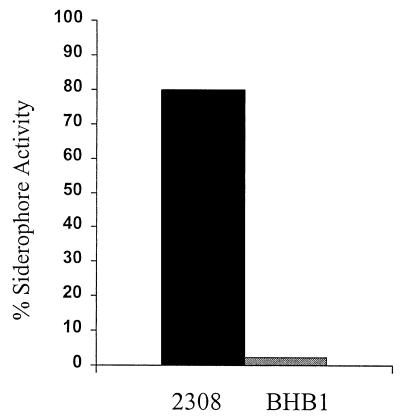
To confirm that the cloned B. abortus entC plays a role in siderophore biosynthesis, a previously described method for gene replacement (6) was used to introduce an entC deletion mutation into B. abortus 2308. A 381-bp fragment internal to the entC coding region in pPS50 was liberated by EcoRV digestion and replaced with the kanamycin resistance gene from TnphoA (15). The gene replacement construct was introduced into 2308 via electroporation, and the resulting transformants were plated onto Schaedler agar supplemented with 5% defibrinated bovine blood (SBA) and 45 μg of kanamycin per ml. Following replica plating onto SBA supplemented with 100 μg of ampicillin per ml, colonies demonstrating kanamycin resistance and sensitivity to ampicillin were retained for further characterization. One of the putative entC mutants was given the designation BHB1. Southern blot analysis (20) employing entC-specific, kanamycin-resistance cassette-specific, and vector-specific probes confirmed the nature of the entC mutation in BHB1 (data not shown). Consistent with the predicted phenotype of a B. abortus entC mutant, no siderophore activity was detected in the supernatants of BHB1 cultures by either the liquid CAS (22) (Fig. 2) or Arnow (1) (data not shown) assay following growth in low-iron minimal medium (13), and BHB1 was more sensitive to growth inhibition on solid medium by the chelators ethylenediaminediacetic acid and 2,2-dipyridyl than 2308.
FIG. 2.
Siderophore activity of supernatants from cultures of B. abortus 2308 and BHB1 (ΔentC) as determined by the liquid CAS assay (23). Supernatants from 48-h cultures grown in low-iron modified minimal medium (14) were mixed with an equal volume of CAS reagent, and the percent siderophore activity was calculated for each supernatant by using the formula [(X−Y)/X] × 100, where X is the absorbance at 630 nm of uninoculated growth medium and Y is the absorbance at 630 nm of the culture supernatant.
Previous studies suggested that DHBA may facilitate survival and replication of brucellae in host macrophages (12). By performing such a function, DHBA might therefore represent a critical virulence determinant. To examine the ability of BHB1 to establish chronic infection in the host, female BALB/c mice (Harlan Sprague Dawley, Indianapolis, Ind.), 7 to 8 weeks of age, were infected intraperitoneally with approximately 5 × 104 CFU of B. abortus 2308 or BHB1 in 100 μl of phosphate-buffered saline by previously described procedures (6). To preclude the possibility that iron loading by the bacterial cells prior to inoculation might mask the true nature of a DBHA requirement in vivo, separate groups of mice were infected in parallel with Brucella cultures grown under either iron-replete or iron-depleted conditions. In these experiments, SBA served as the iron-replete growth medium, while low-iron minimal medium (13) supplemented with 1.5% Noble agar served as the iron-depleted growth medium. At selected times after infection, five mice from each group were euthanatized by halothane overdose, their spleens and livers were removed and homogenized, and the numbers of brucellae per organ were determined by serial dilution and plating on SBA. Statistical comparisons between experimental groups were performed with the two-tailed Student t test (23), and P values less than 0.05 were considered significant. Equivalent spleen (Fig. 3) and liver (data not shown) colonization profiles were observed for BHB1 and 2308 in BALB/c mice over an 18-week period, regardless of whether the inocula were grown under iron-replete (Fig. 3A) or iron-depleted (Fig. 3B) conditions. Previously described methods (7) were also used to examine the capacity of cultured murine resident peritoneal macrophages to control the intracellular replication of BHB1 and 2308. Both strains demonstrated equivalent survival and replication rates within cultured murine macrophages (Fig. 4), and activation of these phagocytes with IFN-γ did not enhance their capacity to eliminate BHB1 beyond their capacity to eliminate 2308 (data not shown).
FIG. 3.
Viable brucellae enumerated from the spleens of mice infected with B. abortus 2308 and BHB1 (ΔentC) cultures grown under either iron-replete (A) or iron-restricted (B) conditions. Each symbol represents the average number of bacteria cultured from the spleens of five mice, and the corresponding error bars represent the standard deviation for each experimental group. p.i., postinfection.
FIG. 4.
Killing of B. abortus 2308 and BHB1 (ΔentC) opsonized with hyperimmune murine serum by resident peritoneal macrophages from BALB/c mice in culture. Data presented are the results of a representative experiment. Percent survival was calculated based on the number of intracellular bacteria enumerated at 24 and 48 h compared to the number of internalized bacteria detected at time zero.
The findings reported here indicate that although DHBA is the sole siderophore described for B. abortus, this compound does not play a critical role in the capacity of B. abortus to establish and maintain chronic spleen and liver infection in the BALB/c mouse model. They also suggest that B. abortus employs another active iron acquisition system or systems to counter the severe iron restriction which it likely encounters in this experimental host. Interestingly, recent studies indicate that BHB1 shows significant attenuation in pregnant goats (3), suggesting that DHBA plays a key role in virulence in ruminants. The nature of the contribution of DHBA to the virulence of B. abortus in ruminants is presently under investigation.
Acknowledgments
This work was supported by a grant from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (9501995) and by the LSUMC Center for Excellence in Cancer Research, Treatment and Education.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the components of 3,4-dihydroxyphenyalanine-tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- 2.Barghouthi S, Payne S M, Arceneaux J E L, Byers B R. Cloning, mutagenesis, and nucleotide sequence of a siderophore biosynthetic gene (amoA) from Aeromonas hydrophila. J Bacteriol. 1991;173:5121–5128. doi: 10.1128/jb.173.16.5121-5128.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellaire B H, Baldwin C L, Elzer P H, Roop R M., II . Proceedings of the 79th Annual Conference of Research Workers in Animal Disease. 1998. Contribution of the Brucella abortus 2,3-dihydroxybenzoic acid (DHBA) biosynthesis operon to virulence in ruminants, abstr. 4; p. 43. [Google Scholar]
- 4.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular replication of Legionella pneumophila by limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earhart C F. Uptake and metabolism of iron and molybdenum. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1075–1090. [Google Scholar]
- 6.Elzer P H, Phillips R W, Kovach M E, Peterson K M, Roop R M., II Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect Immun. 1994;62:4135–4139. doi: 10.1128/iai.62.10.4135-4139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elzer P H, Phillips R W, Robertson G T, Roop R M., II The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect Immun. 1996;64:4838–4841. doi: 10.1128/iai.64.11.4838-4841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehring A M, Bradley K A, Walsh C T. Enterobactin biosynthesis in Escherichia coli: isochorismate lyase is a bifunctional enzyme that is phosphopantetheinylated by EntD and then acylated by EntE using ATP and 2,3-dihydroxybenzoate. Biochemistry. 1997;36:8495–8503. doi: 10.1021/bi970453p. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard B A, Benson R, López-Goñi I, Baldwin C L. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (DHBA) protects brucellae from killing by macrophages. Vet Res. 1997;28:87–92. [PubMed] [Google Scholar]
- 13.López-Goñi I, Moriyón I, Neilands J B. Identification of 2,3-dihydroxybenzoic acid as a Brucella abortus siderophore. Infect Immun. 1992;60:4496–4503. doi: 10.1128/iai.60.11.4496-4503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Goñi I, Moriyón I. Production of 2,3-dihydroxybenzoic acid by Brucella species. Curr Microbiol. 1995;31:291–293. [Google Scholar]
- 15.Manoil C, Beckwith J. TnphoA: a transposon probe for export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massad G, Arceneaux J E L, Byers B R. Diversity of siderophore genes encoding biosynthesis of 2,3-dihydroxybenzoic acid in Aeromonas spp. Biometals. 1994;7:227–236. doi: 10.1007/BF00149553. [DOI] [PubMed] [Google Scholar]
- 17.Nahlik M S, Brickman T J, Ozenberger B A, McIntosh M A. Nucleotide sequence and transcriptional organization of the Escherichia coli enterobactin biosynthesis cistrons entB and entA. J Bacteriol. 1989;171:784–790. doi: 10.1128/jb.171.2.784-790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozenberger B A, Brickman T J, McIntosh M A. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J Bacteriol. 1989;171:775–783. doi: 10.1128/jb.171.2.775-783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 23.Snedecor G W, Cochran W G. Statistical methods. 8th ed. Ames: Iowa State University Press; 1989. [Google Scholar]
- 24.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg E D. Cellular iron metabolism in health and disease. Drug Metab Rev. 1990;22:531–579. doi: 10.3109/03602539008991450. [DOI] [PubMed] [Google Scholar]
- 26.Williams P H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979;26:925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]