Abstract
The production of biofilm is thought to be crucial in the pathogenesis of prosthetic-device infections caused by Staphylococcus epidermidis. An experimental animal model was used to assess the importance of biofilm production, which is mediated by polysaccharide intercellular adhesin/hemagglutinin (PIA/HA), in the pathogenesis of a biomaterial-based infection. Mice were inoculated along the length of a subcutaneously implanted intravenous catheter with either wild-type S. epidermidis 1457 or its isogenic PIA/HA-negative mutant. The wild-type strain was significantly more likely to cause a subcutaneous abscess than the mutant strain (P < 0.01) and was significantly less likely to be eradicated from the inoculation site by host defense (P < 0.05). In addition, the wild-type strain was found to adhere to the implanted catheters more abundantly than the PIA/HA-negative mutant (P < 0.05). The reliability of the adherence assay was assessed by scanning electron microscopy. To exclude contamination or spontaneous infection, bacterial strains recovered from the experimental animals were compared to inoculation strains by analysis of restriction fragment length polymorphism patterns by pulsed-field gel electrophoresis. In vitro binding of the wild-type strain and its isogenic mutant to a fibronectin-coated surface was similar. These results confirm the importance of biofilm production, mediated by PIA/HA, in the pathogenesis of S. epidermidis experimental foreign body infection.
Staphylococcus epidermidis is the most common cause of nosocomial bacteremia and is the principal organism responsible for infections of implanted prosthetic medical devices such as prosthetic heart valves, artificial joints, and cerebrospinal fluid shunts (29, 37). Bacterial adherence appears to be critical in the pathogenesis of biomaterial-based infections. Bacterial adherence and biofilm formation can be arbitrarily divided into early and late phases (17). The early phases of adherence appear to be mediated initially by nonspecific forces such as surface charge and hydrophobicity and somewhat later by specific adhesins such as a proteinaceous autolysin (10) and a polysaccharide adhesin (PSA) (35). The later accumulative phases of adherence, in which organisms adhere to one another and elaborate biofilm, is mediated by polysaccharide intercellular adhesin (PIA) (22). Recent investigation reveals that PIA and the hemagglutinin (HA) of S. epidermidis are closely related if not identical (8, 21).
It is hypothesized that S. epidermidis strains that are deficient in the production of PIA/HA will be less able to colonize intravascular catheters and, therefore, less likely to cause infection. The in vivo milieu, with respect to prosthetic-device infection, is complex and involves interactions between the microbe, the host, and the device. Therefore, in attempting to answer questions regarding the pathogenesis of prosthetic-device infections, it is better to use in vivo models. The goal of this study was to ascertain the relevance of PIA/HA in the pathogenesis of prosthetic device infection in a mouse foreign body infection model.
The bacterial strains used for these studies consisted of S. epidermidis 1457 and a PIA/HA-negative isogenic mutant, S. epidermidis 1457-M10. S. epidermidis 1457 was obtained from a patient with an infected central venous catheter. It adheres to plastic, elaborates biofilm, and is PIA/HA positive (22). S. epidermidis 1457-M10 is a PIA/HA-negative isogenic mutant of S. epidermidis 1457 that was produced by insertion of transposon Tn917 into the icaA gene of the icaADBC gene locus (20, 21).
The mouse foreign body infection model was used to assess the importance of PIA/HA in the pathogenesis of prosthetic-device infection. Seventy-five male Swiss-Albino mice were used in these studies. Animals were divided into five groups of 15. The first two groups of mice were inoculated with 106 CFU of S. epidermidis 1457 or S. epidermidis 1457-M10, respectively. The next two groups were inoculated with 107 CFU of S. epidermidis 1457 or S. epidermidis 1457-M10, respectively. The fifth group of mice was inoculated with 108 CFU of S. epidermidis 1457-M10. The following is a brief description of the experimental procedure. The flanks of anesthetized animals were shaved, and the skin was cleansed with povidone-iodine. By using aseptic technique, a 1-cm segment of 14-gauge Teflon intravenous catheter (QuikCath; Baxter) was implanted into the subcutaneous space. Next, a defined inoculum of S. epidermidis was injected into the catheter bed, and the wound was closed with monofilament suture. On day 7, the animals were sacrificed, and the presence of a subcutaneous abscess was assessed visually. The catheters were aseptically removed, placed in sterile microcentrifuge tubes with 1 ml of phosphate-buffered saline (PBS), and vortexed at high speed for 1 min. The wash fluid was quantitatively cultured on Mueller-Hinton agar plates.
To assess the completeness of the removal of bacteria from the catheters, several catheters were examined by scanning electron microscopy. After removal from the mice and a vortex washing, the catheters were fixed in 2% glutaraldehyde, dehydrated in an ascending concentration series of ethanol baths, and air dried in a vacuum oven at 22°C. Catheters were mounted on aluminum stubs, sputter coated with gold, and examined with a Phillips 515 scanning electron microscope (3).
Bacteria recovered from the catheters were identified as S. epidermidis based on colony morphology, Gram stain characteristics, and coagulase testing. To exclude the possibility of spontaneous infection or contamination, bacterial isolates recovered from 10 of the mice were compared to the parent strains, 1457 and 1457-M10, by pulsed-field gel electrophoresis. Chromosomal DNA was prepared in agarose blocks and digested with the endonuclease SmaI, and restriction fragment length polymorphism patterns were compared by use of a CHEF DRIII pulsed-field gel electrophoresis system (Bio-Rad, Hercules, Calif.) as previously described (30). Electrophoresis conditions were as follows: 6 V/cm, 14°C, switch time 1 to 30 s, 18 h, 120° angle.
To compare the adherence of S. epidermidis 1457 and its isogenic mutant 1457-M10 to immobilized fibronectin and fibrinogen, bacteria were grown in Trypticase soy broth (Becton Dickinson, Cockeysville, Md.) for 18 h at 37°C with agitation. Cells were harvested by centrifugation and resuspended in PBS. The bacterial cell concentration was determined by plate count. Microtiter plates (PS U-96; Greiner, Nürtingen, Germany) were coated with 150 μl per well of human fibronectin (Boehringer Mannheim, Germany) or human fibrinogen (Sigma, Deisenhofen, Germany) in PBS for 16 h at 4°C at concentrations of 0.1, 1, and 10 μg/ml. Plates were washed three times with wash buffer (PBS containing 0.05% Tween 20 and 0.05% NaN3) and blocked with 3% bovine serum albumin in PBS containing 0.05% NaN3 for 2 h at 37°C. After being washed, 100 μl of the bacterial suspensions at various concentrations was added in triplicate to the wells of coated microtiter plates, which were then incubated for 1 h at 37°C. After another washing, the attached cells were detected by enzyme-linked immunosorbent assay (ELISA) with rabbit anti-S. epidermidis 5179 antiserum and alkaline phosphatase-coupled anti-rabbit immunoglobulin G (Sigma) as described previously (18, 21).
A one-way analysis of variance was performed to assess the difference in the overall formation of abscesses, sterilization of catheters, and numbers of adherent bacteria in the experimental animals. The Bonferroni multiple comparison test was used to compare specific groups of animals. Data from the catheter adherence studies, expressed as CFU/catheter, were log normalized prior to analysis. All statistical tests were performed with GraphPad Prism 2.0 (San Diego, Calif.).
Formation of subcutaneous abscesses.
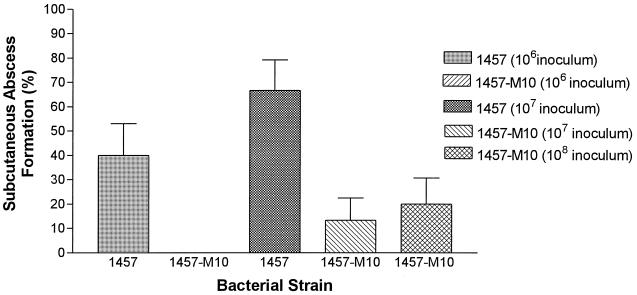
Results were as follows: 6 of 15 versus 0 of 15 mice developed grossly apparent subcutaneous abscesses when challenged with 106 CFU of S. epidermidis 1457 or S. epidermidis 1457-M10, respectively (P < 0.05); 10 of 15 versus 2 of 15 mice developed subcutaneous abscesses when inoculated with 107 CFU of S. epidermidis 1457 or S. epidermidis 1457-M10, respectively (P < 0.01); and 3 of 15 mice inoculated with 108 CFU of S. epidermidis 1457-M10 developed subcutaneous abscesses. The frequency of abscess formation observed in the group of animals inoculated with 108 CFU of S. epidermidis 1457-M10 was significantly less than that observed in the group of animals inoculated with 107 CFU of S. epidermidis 1457 (P < 0.05). Although twice as many animals inoculated with 106 CFU of S. epidermidis 1457 developed subcutaneous abscesses than animals inoculated with 108 CFU of S. epidermidis 1457-M10, this difference did not reach statistical significance. These results are summarized in Fig. 1. Photographs of representative animals are shown in Fig. 2.
FIG. 1.
Subcutaneous abscess formation by S. epidermidis 1457 and isogenic PIA/HA-negative mutant 1457-M10 in the mouse foreign body infection model. Visually apparent abscesses developed in 40 and 67% of animals inoculated with 106 and 107 CFU, respectively, of the wild-type S. epidermidis 1457 compared to 0 and 13% of animals inoculated with equal numbers of S. epidermidis 1457-M10. Of animals inoculated with 108 CFU of S. epidermidis 1457-M10, 20% developed a subcutaneous abscess. Bars represent the mean abscess formation, and the lines represent the standard error of the mean.
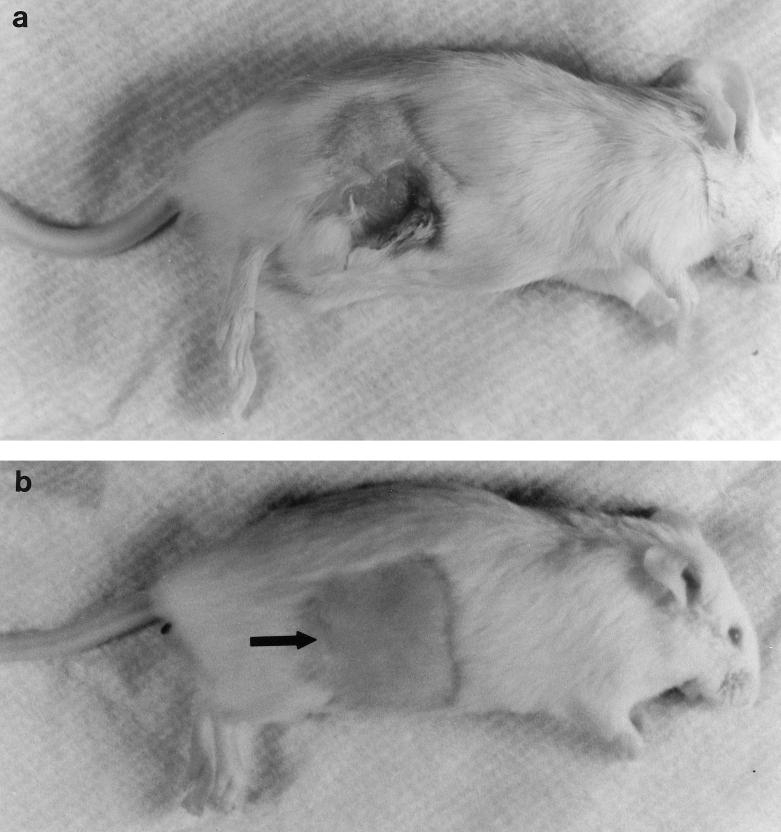
FIG. 2.
Appearance of representative animals on day 7 in the foreign body infection models that were inoculated with 107 CFU of S. epidermidis 1457 (a) and 107 CFU of the PIA/HA-negative mutant S. epidermidis 1457-M10 (b). An obvious subcutaneous abscess is evident in the strain 1457-infected mouse. The surgical site (arrow) is well healed in the strain 1457-M10-infected mouse, and there are no signs of inflammation or abscess formation.
Bacterial adherence.
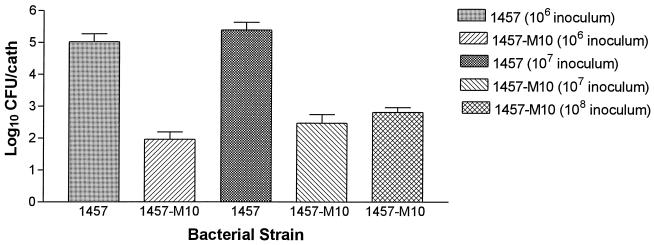
As presented in Fig. 3, the number of bacteria recovered from catheters of animals infected with the wild-type strain was significantly greater than the number of bacteria recovered from the catheters of animals infected with the PIA/HA-negative mutant strain. At the 106 CFU inoculum level, a mean of 1.04 × 105 CFU was recovered from the explanted catheters of mice challenged with S. epidermidis 1457 compared to a mean of 91 CFU per catheter in mice challenged with S. epidermidis 1457-M10 (P < 0.001). At the 107 inoculum level a mean of 2.46 × 105 CFU was recovered from the explanted catheters of mice challenged with S. epidermidis 1457 compared to a mean of 294 CFU per catheter in mice challenged with S. epidermidis 1457-M10 (P < 0.05). In addition, there was a significant difference in recovered bacteria when the mice challenged with 106 CFU of S. epidermidis 1457 were compared with the mice challenged with 107 CFU of S. epidermidis 1457-M10 (P < 0.01). Although mice challenged with 108 CFU of S. epidermidis 1457-M10 had fewer bacteria recovered from their catheters (mean of 643 CFU) than mice challenged with either 106 or 107 CFU of S. epidermidis 1457, these differences were not statistically significant.
FIG. 3.
Recovery of S. epidermidis 1457 and its isogenic PIA/HA-negative mutant 1457-M10 from implanted subcutaneous catheter segments in the mouse foreign body infection model. There were significantly greater numbers of strain 1457 adherent to the catheters compared to strain 1457-M10 at both the 106 and 107 CFU inoculum levels. Although there were fewer bacteria recovered from the catheters of mice inoculated with 108 CFU of S. epidermidis 1457-M10 than from the catheters of mice inoculated with either 107 or 106 CFU of S. epidermidis 1457, these differences did not reach statistical significance. Bars represent the mean of log-transformed adherence values, and the lines represent the standard error of the mean. cath, catheter.
Sterilization of infected subcutaneous catheters.
At the time of catheter removal sterile catheters were observed in 27% of animals challenged with 106 CFU of S. epidermidis 1457 versus 87% of animals challenged with an equal number of S. epidermidis 1457-M10 (P < 0.001). Sterile catheters were recovered from 13% of the animals challenged with 107 CFU of S. epidermidis 1457 versus 73% of the animals challenged with an equal number of S. epidermidis 1457-M10 (P < 0.05). In addition, there was a significantly greater number of mice with sterile catheters when animals challenged 107 CFU of S. epidermidis 1457-M10 were compared with animals challenged with 106 CFU of S. epidermidis 1457 (P < 0.01). Although catheters from mice challenged with 108 CFU of S. epidermidis 1457-M10 were more likely to be sterile (40%) at the time of removal than catheters from mice challenged with either 106 or 107 CFU of S. epidermidis 1457, these differences were not statistically significant.
To ensure that the vortex washing process resulted in the complete removal of bacteria, several catheters were examined by scanning electron microscopy after the wash procedure. Catheters removed from mice infected with strain 1457 revealed a few fibrinous strands of biofilm but no adherent bacteria (photographs not shown). Scanning electron microscopy of catheters infected with the 1457-M10 mutant strain revealed no biofilm or adherent bacteria (photographs not shown). These observations confirmed that the wash process had dislodged the adherent bacteria.
Bacterial strains recovered from 10 of the catheters at the time of removal were compared to 1457 and 1457-M10 stock strains by analysis of the restriction fragment length polymorphism pattern of SmaI-digested chromosomal DNA by pulsed-field gel electrophoresis. The isolates recovered from the mice were identical to the stock strains (gel not shown).
Fibronectin and fibrinogen binding.
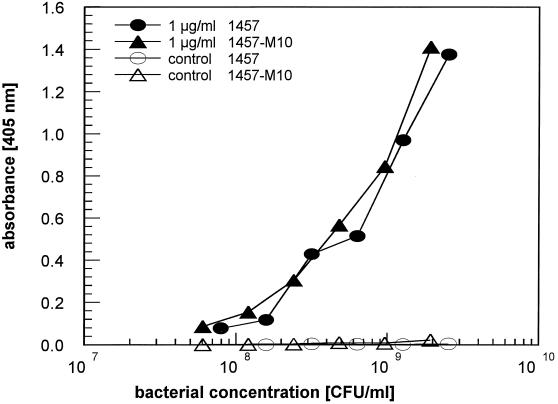
The attachment of wild-type S. epidermidis 1457 and its isogenic mutant 1457-M10 to fibronectin- or fibrinogen-coated microtiter wells was analyzed. Almost identical inoculum-dependent binding of wild-type and mutant cells was observed with wells coated with 1-μg/ml (Fig. 4) and 10-μg/ml concentrations of fibronectin (data not shown). Binding of cells to wells coated with 0.1 μg of fibronectin (data not shown) per ml and to uncoated control wells (Fig. 4) was below the level of detection up to a concentration of 5 × 109 CFU/ml. Significant binding of bacterial cells to wells coated with 10 μg of fibrinogen per ml tested in parallel could not be detected (data not shown).
FIG. 4.
Binding of S. epidermidis 1457 and its isogenic PIA/HA-negative mutant 1457-M10 to immobilized fibronectin. Bacterial suspensions of both strains were applied to microtiter wells coated with 1 μg of fibronectin per ml and to uncoated control wells. Attached cells were detected by ELISA. A representative experiment is shown.
It has long been suspected that biofilm, also known as exopolysaccharide, glycocalyx, or slime, is important in the pathogenesis of infections due to S. epidermidis. Bayston and Penny first suggested the clinical importance of S. epidermidis biofilm in relation to infected cerebral spinal fluid shunts (2). Electron microscopy studies showed that biofilm was not evident in the early stages of adherence but was produced in the later accumulative stages of adherence (28). A number of investigators observed that the majority of clinically significant strains of S. epidermidis elaborated biofilm as opposed to contaminants or skin isolates (4, 14). However, others were unable to confirm the importance of biofilm in epidemiologic or immunologic studies (6, 15). Efforts to biochemically characterize biofilm were also confusing (7). Similarly, conflicting data regarding the importance of biofilm arose from studies utilizing the mouse foreign body model. Christensen and coworkers found that a biofilm-producing strain of S. epidermidis caused three times more infections than a non-biofilm-producing strain (5). Conversely, Patrick and colleagues observed that although biofilm-producing S. epidermidis strains adhered to catheters in greater number than non-biofilm-producing strains, they were less likely to cause a subcutaneous abscess (25). All of the early investigation regarding putative virulence factors of S. epidermidis was limited by one or more of the following factors: imprecise species identification, the possibility of spontaneous phenotypic variation, and the lack of genetic techniques and systems to create isogenic mutants.
More recently, these technical hurdles have been cleared. Several teams of investigators have successfully perfected the ability to transform S. epidermidis, and a number of specific factors relating to biofilm have been described. PIA, described by Mack and colleagues, is a polysaccharide that is elaborated by the majority of clinically significant strains of S. epidermidis and whose expression is dependent on glucose in the growth medium (19, 22). Biochemical characterization and nuclear magnetic resonance spectra show that PIA is composed of the two similar polysaccharide components I and II, consisting primarily of N-acetyl-d-glucosaminyl residues, in a ratio of 7:1 (18). Isogenic PIA-negative, Tn917 mutants are capable of initial attachment to plastic but are unable to form multilayer macrocolonies (20). PIA is synthesized by gene products of the icaADBC locus, which is organized in an operon structure (9, 12). The HA of S. epidermidis has been demonstrated to be present in the majority of clinically relevant strains of S. epidermidis and is primarily composed of carbohydrates (31, 32). HA also appears to play a role in adherence (16, 31). Recently, phenotypic and genotypic studies have demonstrated that PIA and HA are closely related or identical (8, 21). In addition, a 140-kDa extracellular protein has been demonstrated that appears to play a role in cellular accumulation (13).
Bacterial adherence to biomaterials is a complex process and appears to proceed in stages. The initial stages of adherence are influenced by several adhesins. PSA, described by Pier and coworkers, is a complex mixture of monosaccharides that blocks the adherence of PSA-producing strains of S. epidermidis to plastic (35). In animal models, antibody directed against PSA is protective and PSA-negative isogenic transposon mutants are less virulent (33, 34). Recently, data were reported that indicate the ica locus may also encode production of PSA (23). Genetic methods have also been used to characterize a 148-kDa proteinaceous autolysin that is important in the initial adherence of S. epidermidis to biomaterials (10, 11). Investigation is proceeding in a number of laboratories to determine the relative importance and interaction between the various adhesins that act in various stages of adherence.
The decreased ability of the PIA/HA-negative mutant strain to produce abscesses in the experimental animals is quite interesting in light of what is known of the structure of PIA and other bacterial polysaccharides. It has been demonstrated by Tzianabos and colleagues that the capsular polysaccharide complex from Bacteroides fragilis promotes the formation of abscesses in a rat model because of repeating positively charged amino groups and negatively charged carboxyl or phosphate groups (36). These investigators were also able to induce the capsular polysaccharide of Salmonella typhi to produce abscesses by introducing alternately charged groups (36). Although the structures of many bacterial polysaccharides have been studied, very few possess oppositely charged groups. Previous analysis of S. epidermidis PIA demonstrates that it possesses this trait, which offers an explanation for the increased ability of the wild-type strain to produce abscesses in our experimental model (18).
Shortly after insertion, prosthetic medical devices are coated by a host-derived glycoproteinaceous conditioning film. Staphylococcus aureus is known to possess specific adhesins for a variety of host matrix proteins (27). The role of host-derived matrix proteins in S. epidermidis adherence is less clear, and the relationship between biofilm formation and adherence to host matrix proteins is controversial. Some investigators have observed that coating polymers with matrix proteins such as fibrinogen or fibronectin increases the adherence of S. epidermidis (1, 38), while others have not (24, 26). Baldassarri et al. hypothesized that biofilm may serve to mask bacterial adhesins (1). We observed that coating plastic with fibronectin, but not fibrinogen, resulted in an increase in bacterial adherence. However, there was no difference in adherence between the wild-type and biofilm-negative mutant strains. These data reinforce the specific nature of the Tn917 insertional mutation and argue against PIA masking other potential adhesins.
In light of the previous pitfalls encountered in evaluating the pathogenic significance of biofilm, a number of precautions were taken to ensure the reliability of the data obtained from this study. First, only strains that were genetically well characterized were utilized. A clinically relevant strain which produced a central-venous-catheter-related infection in a human was used as the wild-type strain. This strain was compared to its isogenic PIA/HA-negative mutant. Second, electron microscopy was used to analyze the catheters to ensure that adherent bacteria were completely removed from the catheters by the wash process. Third, to rule out contamination, molecular typing studies were performed to ensure that the bacterial strains recovered from the animals were identical to the stock strains that were inoculated.
This work clearly demonstrates the importance of PIA/HA in the pathogenesis of prosthetic-device infections as reflected in the mouse foreign body model. This model most closely resembles subcutaneous tunnel tract infection in patients with surgically implanted central venous catheters such as the Hickman or Broviac catheters. Additional studies are necessary to ascertain the interactions and relative importance of the various adhesins. Hopefully, this will suggest novel methods of prevention and treatment of S. epidermidis prosthetic-device infections.
Acknowledgments
This work was supported by a grant-in-aid from the American Heart Association, 96006810 (M.E.R.), and by a grant from the Deutsche Forschungsgemeinschaft (D.M.).
REFERENCES
- 1.Baldassarri L, Donelli G, Gelosia A, Simpson A W, Christensen G D. Expression of slime interferes with in vitro detection of host protein receptors of Staphylococcus epidermidis. Infect Immun. 1997;65:1522–1526. doi: 10.1128/iai.65.4.1522-1526.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayston R, Penny S R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonization of Holter shunts. Dev Med Child Neurol. 1972;14(Suppl. 27):25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- 3.Cano M, Suzuki T, Cohen S M. Application of scanning electron microscopy and X-ray analysis to urinary tract cancer in animals and humans. Scanning Microsc. 1993;7:363–370. [PubMed] [Google Scholar]
- 4.Christensen G D, Parisi J T, Bisno A L, Simpson W A, Beachey E H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983;18:258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Mitoma F, Harding G K M, Hoban D J, Roberts R S, Low D E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987;156:555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- 7.Drewry D T, Galbraith L, Wilkinson B J, Wilkinson S G. Staphylococcal slime: a cautionary tale. J Clin Microbiol. 1990;28:1292–1296. doi: 10.1128/jcm.28.6.1292-1296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fey P, Ulphani J, Heilmann C, Gotz F, Mack D, Rupp M E. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Polysaccharide intercellular adhesin (PIA) mediates hemagglutination (HA) in Staphylococcus epidermidis, abstr. B-40; p. 241. [Google Scholar]
- 9.Gerke C, Krafts A, Süssmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18594. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 12.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishak M A, Dieter H M, Groschel G L, Mandell G L, Wenzel R P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985;22:1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristinsson K G, Hastings J G M, Spencer R C. The role of extracellular slime in opsonophagocytosis of Staphylococcus epidermidis. J Med Microbiol. 1988;27:207–213. doi: 10.1099/00222615-27-3-207. [DOI] [PubMed] [Google Scholar]
- 16.Limoges J, Han J, Rupp M E. Abstracts of the 95th General Meeting of American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Differential adherence assay profiles and scatchard analysis of hemagglutination-positive and -negative strains of Staphylococcus epidermidis, abstr. B-436; p. 241. [Google Scholar]
- 17.Mack, D. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect., in press. [DOI] [PubMed]
- 18.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 20.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack D, Riedewald J, Rohde H, Magnus T, Feucht R H, Elsner H A, Laufs R, Rupp M E. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect Immun. 1999;67:1004–1008. doi: 10.1128/iai.67.2.1004-1008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenney D, Hubner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller E, Takeda S, Goldmann D A, Pier G B. Blood proteins do not promote adherence of coagulase-negative staphylococci to biomaterials. Infect Immun. 1991;59:3323–3326. doi: 10.1128/iai.59.9.3323-3326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrick C C, Plaunt M R, Hetherington S V, May S M. Role of the Staphylococcus epidermidis slime layer in experimental tunnel tract infections. Infect Immun. 1992;60:1363–1367. doi: 10.1128/iai.60.4.1363-1367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual A, Fleer A, Westerdaal N A, Verhoef J. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur J Clin Microbiol. 1986;5:518–522. doi: 10.1007/BF02017694. [DOI] [PubMed] [Google Scholar]
- 27.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 28.Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–245. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 30.Rupp M E, Han J, Goering R V. Repeated recovery of Staphylococcus saprophyticus from the urogenital tracts of women: persistence vs. recurrence. Infect Dis Obstet Gynecol. 1995;2:218–222. doi: 10.1155/S1064744995000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupp M E, Archer G L. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect Immun. 1992;60:4322–4327. doi: 10.1128/iai.60.10.4322-4327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupp M E, Sloot N, Meyer H-G W, Han J, Gatermann S. Characterization of the hemagglutinin of Staphylococcus epidermidis. J Infect Dis. 1995;172:1509–1518. doi: 10.1093/infdis/172.6.1509. [DOI] [PubMed] [Google Scholar]
- 33.Shiro H, Muller E, Gutierrez N, Boisot S, Grout M, Tosteson T D, Goldmann D, Pier G B. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J Infect Dis. 1994;169:1042–1049. doi: 10.1093/infdis/169.5.1042. [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Pier G B, Kojima Y, Tojo M, Muller E, Tosteson T, Goldmann D A. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation. 1991;84:2539–2546. doi: 10.1161/01.cir.84.6.2539. [DOI] [PubMed] [Google Scholar]
- 35.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- 36.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services, Public Health Service. National nosocomial infectious surveillance (NNIS) report. Data summary from October 1986–April 1996, issued May 1996. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 38.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger U E, Lew D P, Waldvogel F A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]